一种2‑苯基‑1,2‑二氢萘‑1‑醇外消旋化合物及其衍生物的合成方法与流程
本发明涉及二氢化萘结构单元中间体的合成技术领域,尤其涉及一种2-苯基-1,2-二氢萘-1-醇外消旋化合物及其衍生物的合成方法,以及通过该方法合成新的2-苯基-1,2-二氢萘-1-醇外消旋化合物或其衍生物。
背景技术:
四氢化萘结构单元是许多具有生物活性的天然产物及药物分子中常见的骨架之一,在许多肾上腺激素、抗生素以及中国紫杉醇中的异紫杉脂素、去氧鬼臼素葡萄糖酯苷等化合物中都含有四氢化萘基本结构。这类化合物具有许多特殊的生理活性和药理活性,如抗抑郁、消炎止痛、抗帕金森、抗癌等。相应的合成研究也因此受到化学家们的广泛关注,过渡金属催化氧(氮)杂二环烯烃的不对称开环反应是一种合成此类手性药物中间体的一种方法,它可以通过一步开环反应构建两个手性碳中心。在手性过渡金属配合物的催化下,氧(氮)杂二环烯烃与亲核试剂发生开环反应,可以得到一种高对映立体选择性、具有两个手性中心的二氢化萘骨架单元的开环产物。该产物具有四种立体化学结构构型,分别为1,2-(r,r)、1,2-(s,s)、1,2-(r,s)和1,2-(s,r)。由于此类开环反应具有催化剂用量少、产率高和对映选择性高等特点,化学家们对此产生了浓厚的兴趣,并取得了很好的研究结果。过渡金属ni、cu、ru、rh、ir、pt和pd催化氧(氮)杂二环烯烃的开环反应,具有较好的区域选择性、对映选择性和手性诱导立体控制,其中,常用的亲核试剂主要包括有机锌试剂、有机铝试剂、有机锂试剂、有机硼酸试剂、格利雅试剂、炔烃试剂等。但是,尽管目前已合成出多种有效的过渡金属配合物催化剂应用于氧(氮)杂二环烯烃开环反应的研究,但没有任何一种催化剂能适用于所有底物与不同的亲核试剂的开环反应。因此,研究高立体化学选择性、高对映选择性、高催化活性的过渡金属配合物催化剂,实现高效立体选择性的开环反应,探索出更适合的反应条件,仍然是将来需要研究的重点课题。
技术实现要素:
本发明针对现有技术使用氧杂苯并降冰片烯与芳基亚磺酸钠合成外消旋化合物时存在产率低的问题,提供一种使用氧杂苯并降冰片烯与芳基亚磺酸钠可高效合成2-苯基-1,2-二氢萘-1-醇外消旋化合物及其衍生物的方法。
本发明的另一个目的是提供通过上述方法合成新的2-苯基-1,2-二氢萘-1-醇外消旋化合物或其衍生物。
为实现上述目的,本发明采用以下技术方案。
一种2-苯基-1,2-二氢萘-1-醇外消旋化合物及其衍生物的合成方法,包括以下步骤:
s1、在氮气或惰性气体的气氛保护下,将pt(cod)cl2和有机配体置于有机溶剂中络合5-40min,形成催化体系;所述pt(cod)cl2与有机配体的物质的量之比为1:1-4。
优选的,在步骤s1中,还包括向有机溶剂中加入含银盐添加剂,所述的pt(cod)cl2、有机配体和含银盐添加剂在有机溶剂中反应5-40min,形成催化体系。
具体的,所述含银盐添加剂为agsbf6或agbf4。
具体的,所述有机配体为pph3、dppf、p(c6h5ch3)3、p(c6h3f2)3、(s)-binap、(s)-tol-binap、(s)-segphos或(s)-dtbm-segphos。
具体的,所述有机溶剂为ch3oh、i-proh、ch3ch2oh或1,4-dioxane。
s2、向催化体系中加入反应物氧杂苯并降冰片烯和芳基亚磺酸钠,得反应体系,使反应体系在50-80℃下反应;所述氧杂苯并降冰片烯与芳基亚磺酸钠的物质的量之比为1:1-3,所述氧杂苯并降冰片烯与pt(cod)cl2的物质的量之比为100:2.5-10。
所述氧杂苯并降冰片烯的结构如下:
其中,r1为h或卤素,r2为h或烷氧基。
具体的,所述卤素为br;所述烷氧基为och3。
所述芳基亚磺酸钠盐为c6h5so2na、4-fc6h4so2na、4-clc6h4so2na、4-brc6h4so2na、3-clc6h4so2na、2-clc6h4so2na、4-h3cc6h4so2na、4-o2nc6h4so2na、4-f3cc6h4so2na、2,5-(ch3)2c6h4so2na、2,4,6-(ch3)3c6h4so2na、1-萘亚磺酸钠或2-萘亚磺酸钠。
s3、反应至反应体系中的反应物消失或浓度不再变化时,将反应体系冷却至室温,然后分离提纯产物,收集2-苯基-1,2-二氢萘-1-醇外消旋化合物或其衍生物。
具体的,步骤s3中,反应体系冷却至室温后,先真空浓缩除去溶剂,残留物再通过柱层析色谱法纯化,收集2-苯基-1,2-二氢萘-1-醇外消旋化合物或其衍生物。
通过以上方法合成了新的2-苯基-1,2-二氢萘-1-醇外消旋化合物或其衍生物,所述化合物的结构如下:
与现有技术相比,本发明的有益效果是:本发明以pt(cod)cl2与有机配体形成的过渡金属配合物作为催化剂,催化氧杂苯并降冰片烯与芳基亚磺酸钠反应,可高效合成2-苯基-1,2-二氢萘-1-醇外消旋化合物或其衍生物,且反应条件温和,在50-80℃下即可完成反应。当以适量的pph3作为有机配体与pt(cod)cl2形成过渡金属配合物催化剂且同时使用添加剂agsbf6,并以ch3oh为溶剂时,反应时间短,且合成2-苯基-1,2-二氢萘-1-醇外消旋化合物的产率可高达92%。
附图说明
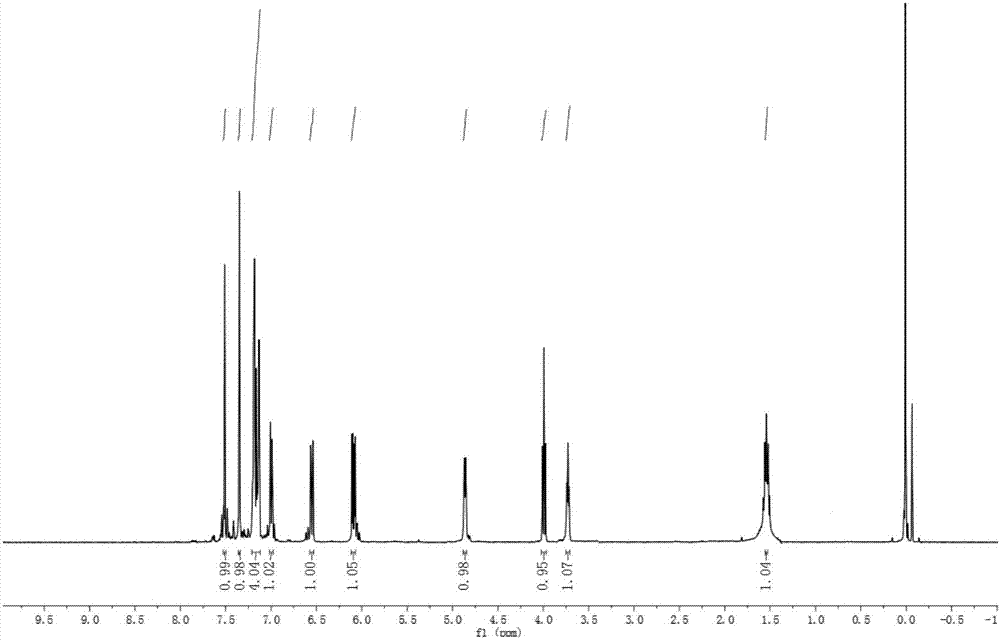
图1为化合物3ce的核磁共振氢谱;
图2为化合物3ce的核磁共振碳谱;
图3为化合物3cf的核磁共振氢谱;
图4为化合物3cf的核磁共振碳谱;
图5为化合物4dc的核磁共振氢谱;
图6为化合物4dc的核磁共振碳谱。
具体实施方式
为了更充分的理解本发明的技术内容,下面结合具体实施例对本发明的技术方案作进一步介绍和说明。
实施例1-57
实施例1-57提供的2-苯基-1,2-二氢萘-1-醇外消旋化合物或其衍生物的合成方法,具体步骤如下:
(1)在室温及氮气或惰性气体的气氛保护下,先将铂催化剂和有机配体置于有机溶剂中,若使用添加剂,则向有机溶剂中加入所用添加剂,混合物在室温下络合5-40min,形成催化体系。
(2)向催化体系中加入反应物氧杂苯并降冰片烯和芳基亚磺酸钠,得反应体系,使反应体系在一定温度下反应。
(3)反应至反应体系中的氧杂苯并降冰片烯消失或浓度不再变化时,将反应体系冷却至室温,然后先真空浓缩除去溶剂,再通过柱层析色谱法纯化,收集2-苯基-1,2-二氢萘-1-醇外消旋化合物或其衍生物。
实施例1-57的反应条件及产量和产率如下表1和表2所示。
表1实施例1-57的反应条件
表1中反应物1指氧杂苯并降冰片烯,反应物2指芳基亚磺酸钠;反应物1和反应物2所包含的具体化合物的结构式如下表3所示。
表2实施例1-57的反应条件及产量和产率
表2中各实施例合成的2-苯基-1,2-二氢萘-1-醇外消旋化合物或其衍生物的结构式如下表4所示。
表3反应物1和反应物2所包含的具体化合物的结构式
表4实施例29-57合成的2-苯基-1,2-二氢萘-1-醇外消旋化合物或其衍生物的结构式
由上述实施例合成的化合物3ce、3cf和4dc的核磁共振氢谱和核磁共振碳谱如图1-6所示。
由实施例1-57合成的上述化合物的性状、产量、产率、熔点、硅胶柱层析的比移值rf、核磁共振氢谱、核磁共振碳谱和质谱数据分别如下所示。
化合物3aa:(1s*,2r*)-2-phenyl-1,2-dihydronaphthalen-1-ol
无色油状(35.9mg,81%).rf=0.25(ethylacetate/petroleumether1:20,v/v).1hnmr(500mhz,cdcl3):δ7.28–7.19(m,6h),7.19–7.15(m,2h),7.11–7.07(m,1h),6.63(dd,j=9.6,2.0hz,1h),6.05(dd,j=9.6,4.0hz,1h),4.85(t,j=6.7hz,1h),3.79(ddd,j=6.0,4.0,2.1hz,1h),1.43(d,j=7.9hz,1h).13c{1h}nmr(125mhz,cdcl3)δ137.8,136.1,132.7,129.7,129.3,128.7,128.4,128.3,128.1,127.5,126.8,126.4,71.4,47.4.ms(ei)m/z:[m-3h]–calcdforc16h11o,219.08;found219.04.
化合物3ab:(1s*,2r*)-2-(4-fluorophenyl)-1,2-dihydronaphthalen-1-ol
白色固体(34.6mg,72%yield).mp85–86℃.rf=0.13(ethylacetate/petroleumether1:20,v/v).1hnmr(600mhz,cdcl3)δ7.29–7.15(m,6h),6.94(t,j=8.6hz,2h),6.65(dd,j=9.6,1.8hz,1h),6.04(dd,j=9.6,4.1hz,1h),4.87(d,j=6.0hz,1h),3.80–3.78(m,1h),1.18(d,j=6.4hz,1h).13c{1h}nmr(150mhz,cdcl3)δ163.3(d,1jc-f=243.8hz),161.7,136.3,133.6(d,4jc-f=3hz),132.8,131.1,129.9,128.7,128.6,128.5,126.7(d,3jc-f=15hz),115.8,115.6(d,2jc-f=21.0hz),71.6,46.8.19fnmr(376mhz,cdcl3)δ-115.4.ms(ei)m/z:[m-3h]–calcdforc16h10fo,237.07;found237.23.
化合物3ac:(1s*,2r*)-2-(4-chlorophenyl)-1,2-dihydronaphthalen-1-ol
白色固体(44.2mg,87%yield).mp113–114℃.rf=0.13(ethylacetate/petroleumether1:20,v/v).1hnmr(600mhz,cdcl3)δ7.26–7.16(m,5h),7.13–7.08(m,3h),6.63(d,j=9.6hz,1h),6.00(dd,j=9.6,4.0hz,1h),4.83(d,j=4.0hz,1h),3.75(s,1h),1.41(s,1h).13c{1h}nmr(150mhz,cdcl3)δ136.3,136.0,133.2,132.5,130.7,129.3,128.7,128.5,128.5,128.2,126.6,126.5,71.3,46.7.ms(ei)m/z:[m+na]+calcdforc16h13clona,279.07;found279.13.
化合物3ad:(1s*,2r*)-2-(4-bromophenyl)-1,2-dihydronaphthalen-1-ol
无色油状(37.1mg,62%yield).rf=0.13onsilicagel(ethylacetate/petroleumether1:20,v/v).1hnmr(400mhz,cdcl3)δ7.46–7.39(m,2h),7.35–7.25(m,3h),7.19–7.15(m,1h),7.13(d,j=8.3hz,2h),6.71(dd,j=9.6,1.9hz,1h),6.07(dd,j=9.6,4.1hz,1h),4.91(d,j=5.9hz,1h),3.84–3.79(m,1h),1.52(s,1h).13c{1h}nmr(100mhz,cdcl3)δ136.8,135.9,132.4,131.6,131.0,129.1,128.5,128.4,128.2,126.5,126.5,121.3,71.2,46.8.ms(ei)m/z:[m–3h]-calcdforc16h10bro,297.00;found297.46.
化合物3ae:(1s*,2r*)-2-(3-chlorophenyl)-1,2-dihydronaphthalen-1-ol
无色油状(46.1mg,90%yield).rf=0.21(ethylacetate/petroleumether1:20,v/v).1hnmr(500mhz,cdcl3)δ7.29–7.15(m,6h),6.94(t,j=8.6hz,2h),6.65(dd,j=9.6,1.8hz,1h),6.04(dd,j=9.6,4.1hz,1h),4.87(d,j=6.0hz,1h),3.80–3.78(m,1h),1.18(t,j=6.4hz,1h).13c{1h}nmr(150mhz,cdcl3)13cnmr(125mhz,cdcl3)δ139.9,135.4,133.9,131.9,129.3,129.0,128.4,128.1,128.1,127.8,127.1,127.0,126.3,126.1,70.8,46.7.ms(ei)m/z:[m–3h]-calcdforc16h10clo,253.04;found253.70.
化合物3af:(1s*,2r*)-2-(2-chlorophenyl)-1,2-dihydronaphthalen-1-ol
无色油状(42.5mg,83%yield).rf=0.19(ethylacetate/petroleumether1:20,v/v).1hnmr(500mhz,cdcl3)δ7.36(dd,j=7.4,1.9hz,1h),7.34–7.25(m,3h),7.25–7.14(m,4h),7.13(d,j=7.4hz,1h),6.67(dd,j=9.6,2.7hz,1h),5.97(dd,j=9.6,2.5hz,1h),4.82(d,j=5.0hz,1h),4.42(dt,j=5.3,2.8hz,1h),1.48(s,1h).13c{1h}nmr(125mhz,cdcl3)δ137.5,135.8,134.8,132.8,131.6,130.2,129.7,129.5,129.1,129.0,128.7,128.6,127.6,127.4,69.9,44.7.ms(ei)m/z:[m-3h]–calcdforc16h10clo,253.04;found253.13.
化合物3ag:(1s*,2r*)-2-(4-methylphenyl)-1,2-dihydronaphthalen-1-ol
无色油状(39.1mg,83%yield).rf=0.20(ethylacetate/petroleumether1:20,v/v).1hnmr(600mhz,cdcl3)δ7.25(d,j=7.3hz,1h),7.20(t,j=7.3hz,1h),7.16–7.13(m,1h),7.08–7.01(m,5h),6.60(d,j=9.6hz,1h),6.02(dd,j=9.6,4.1hz,1h),4.83(d,j=5.6hz,1h),3.74(s,1h),2.23(s,3h),1.40(s,1h).13c{1h}nmr(150mhz,cdcl3)δ136.7,135.8,134.0,132.3,129.5,129.0,128.8,127.9,127.7,127.60,126.3,126.0,71.0,46.5,20.7.ms(ei)m/z:[m-3h]–calcdforc17h13o,233.10;found233.16.
化合物3ah:(1s*,2r*)-2-(4-nitrophenyl)-1,2-dihydronaphthalen-1-ol
浅黄色固体(21.3mg,40%yield).mp122.3–123.6℃.rf=0.3(ethylacetate/petroleumether1:5,v/v).1hnmr(600mhz,cdcl3)δ8.12(d,j=8.7hz,2h),7.41(d,j=8.7hz,2h),7.28(dt,j=24.6,6.2hz,3h),7.17(d,j=6.9hz,1h),6.73(dd,j=9.6,2.0hz,1h),6.04(dd,j=9.6,3.8hz,1h),4.90(d,j=5.2hz,1h),3.92(dd,j=6.8,4.7hz,1h),1.54(s,1h).13c{1h}nmr(150mhz,cdcl3)δ147.3,146.5,135.6,132.1,130.2,129.0,128.8,128.5,128.1,126.8,126.7,123.6,71.2,58.5,47.3.ms(ei)m/z:[m–3h]–calcdforc16h10no3,264.08;found264.05.
化合物3ai:(1s*,2r*)-2-(4-trifluoromethylphenyl)-1,2-dihydronaphthalen-1-ol
白色固体(38.9mg,67%yield).mp112–114℃.rf=0.11(ethylacetate/petroleumether1:20,v/v).1hnmr(400mhz,cdcl3)δ7.49(d,j=8.0hz,2h),7.31(d,j=8.0hz,2h),7.27–7.17(m,3h),7.12(d,j=7.2hz,1h),6.67(dd,j=9.6,1.5hz,1h),6.02(dd,j=9.6,3.8hz,1h),4.85(d,j=5.7hz,1h),3.84(s,1h),1.46(s,1h).13c{1h}nmr(100mhz,cdcl3)δ143.1,136.4,133.0,130.3,130.2,129.5(q,2jc-f=30.0hz),129.4,129.3,129.0,127.4,127.3(q,1jc-f=271.3hz),126.1,126.0(q,3jc-f=3.7hz),72.0,47.9.19fnmr(376mhz,cdcl3)δ-62.51.ms(ei)m/z:[m–3h]–calcdforc17h10f3o,287.06;found287.08.
化合物3aj:(1s*,2r*)-2-(2,5-dimethylphenyl)-1,2-dihydronaphthalen-1-ol
无色油状(39.0mg,78%yield).rf=0.27(ethylacetate/petroleumether1:20,v/v).1hnmr(400mhz,cdcl3)δ7.24(dd,j=9.8,7.3hz,2h),7.17(d,j=7.2hz,1h),7.10(d,j=7.3hz,1h),7.03(d,j=6.9hz,2h),6.92(d,j=7.3hz,1h),6.63(dd,j=9.6,2.5hz,1h),5.98(dd,j=9.6,2.7hz,1h),4.66(d,j=5.0hz,1h),4.07(dt,j=5.3,2.8hz,1h),2.28(s,3h),2.19(s,3h),1.48(s,1h).13c{1h}nmr(100mhz,cdcl3)δ136.1,135.2,134.7,132.8,131.9,130.0,129.9,129.4,128.1,127.4,127.3,127.2,127.1,126.0,68.8,42.8,20.3,18.7.ms(ei)m/z:[m-3h]–calcdforc18h15o,247.11;found247.21.
化合物3al:(1s*,2r*)-1',2'-dihydro-[1,2']binaphthalenyl-1'-ol
无色油状(29.9mg,55%yield).rf=0.22(ethylacetate/petroleumether1:20,v/v).1hnmr(600mhz,cdcl3)δ8.05(d,j=8.4hz,1h),7.82–7.79(m,1h),7.70(t,j=7.8hz,1h),7.47–7.35(m,4h),7.25(ddd,j=7.2,6.4,2.4hz,2h),7.19–7.16(m,1h),7.14–7.12(m,1h),6.70(dt,j=7.8,3.9hz,1h),6.10(dd,j=9.6,2.5hz,1h),4.82(d,j=4.9hz,1h),4.70(s,1h),1.42(s,1h).13c{1h}nmr(150mhz,cdcl3)δ135.5,135.1,134.4,132.9,132.2,130.6,129.5,129.1,128.6,128.4,128.3,128.2,127.3,127.1,126.7,126.0,125.9,123.4,70.6,43.1.ms(ei)m/z:[m+ch3]+calcdforc21h19o,287.15;found287.06.
化合物3am:(1s*,2r*)-1,2-dihydro-[2,2'-binaphthalen]-1-ol
无色油状(26.1mg,48%yield).rf=0.22(ethylacetate/petroleumether1:20,v/v).1hnmr(600mhz,cdcl3)δ7.82–7.76(m,4h),7.48–7.43(m,2h),7.36–7.30(m,3h),7.24(ddd,j=22.5,14.4,6.8hz,2h),6.77(dd,j=9.6,2.0hz,1h),6.22(dd,j=9.6,4.0hz,1h),5.01(d,j=5.9hz,1h),4.04(ddd,j=6.0,4.0,2.1hz,1h),1.53(s,1h).13c{1h}nmr(150mhz,cdcl3)δ136.1,135.3,133.4,132.8,132.7,129.5,128.4,128.4,128.3,128.1,127.8,127.6,127.3,126.7,126.5,126.1,125.8,71.3,47.5.ms(ei)m/z:[m–3h]–calcdforc20h13o,269.10;found268.88.
化合物4ba:1,4-dimethoxy-6-phenylnaphthalene
白色固体(50.2mg,95%yield).mp87.2–87.8℃.rf=0.2(ethylacetate/petroleumether1:10,v/v).1hnmr(600mhz,cdcl3)δ8.40(d,j=1.8hz,1h),8.23(d,j=8.7hz,1h),7.72(ddd,j=8.3,7.5,1.4hz,3h),7.45–7.42(m,2h),7.34–7.31(m,1h),6.69–6.65(m,2h),3.94(d,j=1.8hz,6h).13c{1h}nmr(150mhz,cdcl3)δ150.3,150.1,141.9,139.1,129.3,128.1,127.8,127.2,126.0,125.9,123.0,120.4,104.2,103.9,56.4,56.3.ms(ei)m/z:[m–ch3]–calcdforc17h13o2,249.08;found248.52.
化合物4bg:1,4-dimethoxy-6-(p-tolyl)naphthalene
白色固体(56.8mg,90%yield).mp113.2–114.4℃.rf=0.23(ethylacetate/petroleumether1:10,v/v).1hnmr(400mhz,cdcl3)δ8.44(d,j=1.6hz,1h),8.27(d,j=8.7hz,1h),7.78(dd,j=8.7,1.9hz,1h),7.68(d,j=8.1hz,2h),7.30(d,j=7.9hz,2h),6.71(q,j=8.3hz,2h),3.98(t,j=3.4hz,6h),2.43(s,3h).13c{1h}nmr(100mhz,cdcl3)δ150.1,149.9,138.8,138.7,137.4,129.9,127.7,127.0,125.6,122.8,119.8,104.0,103.5,56.13,21.5.hrms(esi-iontrap)m/z:[m+h]+calcdforc19h19o2,279.1386;found279.1375.
化合物3bc:
(1s*,2r*)-2-(4-chlorophenyl)-5,8-dimethoxy-1,2-dihydronaphthalen-1-ol
白色固体(50.8mg,80%yield).mp115.6–117.2℃.rf=0.3(ethylacetate/petroleumether1:5,v/v).1hnmr(600mhz,cdcl3)δ7.28(d,j=9.2hz,4h),7.01(dd,j=9.8,3.2hz,1h),6.74(q,j=9.0hz,2h),6.00–5.97(m,1h),4.98(d,j=4.2hz,1h),3.75(d,j=8.3hz,6h),3.69–3.66(m,1h),1.15(p,j=9.0hz,1h).13c{1h}nmr(150mhz,cdcl3)δ149.6,148.7,138.1,131.7,129.5,127.5,127.4,123.2,121.3,121.2,110.5,110.0,63.3,55.2,55.1,45.6.ms(ei)m/z:[m-3h]-calcdforc18h14clo3,313.06;found313.14.
化合物3bd:
(1s*,2r*)-2-(4-bromophenyl)-5,8-dimethoxy-1,2-dihydronaphthalen-1-ol
白色固体(28.8mg,40%yield).mp91.6–93.8℃.rf=0.29(ethylacetate/petroleumether1:5,v/v).1hnmr(600mhz,cdcl3)δ7.45–7.41(m,2h),7.24–7.21(m,2h),7.03–6.99(m,1h),6.77–6.72(m,2h),5.98(ddd,j=9.8,2.2,1.6hz,1h),4.99(d,j=4.1hz,1h),3.75(d,j=8.1hz,6h),3.67–3.65(m,1h),1.53(s,1h).13c{1h}nmr(150mhz,cdcl3)δ150.6,149.7,139.6,131.5,130.9,128.3,124.2,122.3,122.2,120.8,111.5,111.0,64.2,56.2,56.1,46.7.ms(ei)m/z:[m-h]–calcdforc18h16bro3,359.02;found359.01.
化合物3be:
(1s*,2r*)-2-(3-chlorophenyl)-5,8-dimethoxy-1,2-dihydronaphthalen-1-ol
无色油状(57.9mg,92%yield).rf=0.24(ethylacetate/petroleumether1:5,v/v).1hnmr(600mhz,cdcl3)δ7.36(s,1h),7.24(d,j=5.2hz,2h),7.22–7.19(m,1h),7.02(dd,j=9.8,3.2hz,1h),6.75(q,j=8.9hz,2h),6.02–5.99(m,1h),5.02–5.00(m,1h),3.75(d,j=5.9hz,6h),3.69–3.67(m,1h),1.53(s,1h).13c{1h}nmr(150mhz,cdcl3)δ150.9,150.0,143.0,134.6,130.0,129.6,128.3,127.6,127.4,124.6,122.7,122.5,111.8,111.4,64.5,56.5,56.4,47.3.ms(ei)m/z:[m+na]+calcdforc18h17clo3na,339.08;found338.84.
化合物3bf:
(1s*,2r*)-2-(2-chlorophenyl)-5,8-dimethoxy-1,2-dihydronaphthalen-1-ol
白色固体(55.4mg,92%yield).mp98–99℃.rf=0.3(ethylacetate/petroleumether1:5,v/v).1hnmr(600mhz,cdcl3)δ7.50(dd,j=7.6,1.5hz,1h),7.42(dd,j=7.9,1.0hz,1h),7.30(td,j=7.5,1.0hz,1h),7.25–7.22(m,1h),7.11(dd,j=9.8,3.2hz,1h),6.82(q,j=9.0hz,2h),6.07–6.04(m,1h),5.23(d,j=4.2hz,1h),4.35–4.33(m,1h),3.83(d,j=4.8hz,6h),1.59(d,j=33.7hz,1h).13c{1h}nmr(150mhz,cdcl3)δ150.8,149.6,137.9,134.0,131.4,129.4,128.6,128.2,126.8,124.1,122.2,122.1,111.5,111.0,61.6,56.2,56.1,43.8.ms(ei)m/z:[m–3h]–calcdforc18h14clo3,313.06;found313.14.
化合物3ca:(1s*,2r*)-6,7-dibromo-2-phenyl-1,2-dihydronaphthalen-1-ol
无色油状(68.2mg,90%yield).rf=0.29(ethylacetate/petroleumether1:10,v/v).1hnmr(600mhz,cdcl3)δ7.51(s,1h),7.33(d,j=3.8hz,1h),7.23(tt,j=4.7,3.6hz,3h),7.12–7.09(m,2h),6.53(dd,j=9.6,1.5hz,1h),6.14(dd,j=9.6,4.8hz,1h),4.90(t,j=7.0hz,1h),3.76(ddd,j=6.5,4.8,1.6hz,1h),1.44(d,j=8.6hz,1h).13c{1h}nmr(150mhz,cdcl3)δ137.1,135.6,133.5,131.8,131.4,130.8,129.3,128.9,127.9,126.4,124.0,123.6,70.3,46.7.ms(ei)m/z:[m–h]–calcdforc16h11br2o,376.93;found376.94.
化合物3cg:
(1s*,2r*)-6,7-dibromo-2-(4-methylphenyl)-1,2-dihydronaphthalen-1-ol
白色固体(62.9mg,80%yield).mp108–109℃.rf=0.24(ethylacetate/petroleumether1:10,v/v).1hnmr(500mhz,cdcl3)δ7.72(d,j=4.5hz,1h),7.45–7.39(m,1h),7.16(dd,j=7.7,1.8hz,4h),6.60–6.51(m,1h),6.09(td,j=9.4,3.3hz,1h),4.79–4.73(m,1h),3.75–3.68(m,1h),2.37(s,3h),2.06(t,j=9.3hz,1h).13c{1h}nmr(125mhz,cdcl3)δ137.3,137.1,136.6,133.4,132.4,131.2,130.8,129.7,128.3,125.9,124.0,123.5,73.6,49.5,21.1.ms(ei)m/z:[m-ch3]-calcdforc16h11br2o,376.91;found376.87.
化合物3cc:
(1s*,2r*)-6,7-dibromo-2-(4-chlorophenyl)-1,2-dihydronaphthalen-1-ol
无色油状(69.87mg,85%yield).rf=0.3(ethylacetate/petroleumether1:10,v/v).1hnmr(400mhz,cdcl3)δ7.51(d,j=9.5hz,1h),7.32(d,j=3.4hz,1h),7.02(t,j=6.0hz,2h),6.97(d,j=8.1hz,2h),6.50(dd,j=9.7,1.1hz,1h),6.11(dd,j=9.7,4.9hz,1h),4.87(d,j=6.7hz,1h),3.73–3.69(m,1h),1.54–1.47(m,1h).13c{1h}nmr(100mhz,cdcl3)δ137.7,137.3,133.6,132.2,132.0,131.4,130.7,129.6,129.2,126.2,123.9,123.5,70.2,46.2.ms(ei)m/z:[m–h]–calcdforc16h10br2clo,410.88;found410.81.
化合物3ce:
(1s*,2r*)-6,7-dibromo-2-(3-chlorophenyl)-1,2-dihydronaphthalen-1-ol
无色油状(71.9mg,87%yield).rf=0.32(ethylacetate/petroleumether1:10,v/v).1hnmr(400mhz,cdcl3)δ7.52(d,j=4.5hz,1h),7.35(s,1h),7.21–7.12(m,4h),7.02–6.98(m,1h),6.55(dd,j=9.7,1.5hz,1h),6.09(dd,j=9.7,4.5hz,1h),4.86(d,j=6.3hz,1h),3.99(t,j=6.7hz,1h),3.73(dd,j=7.8,3.1hz,1h),1.54(t,j=4.6hz,1h).13c{1h}nmr(100mhz,cdcl3)δ138.3,136.7,134.6,133.1,131.5,131.0,130.9,130.0,129.5,128.0,127.3,127.0,124.3,123.8,70.1,46.5.hrms(esi-iontrap)m/z:[m–3h]–calcdforc16h8br2clo,408.8628;found408.8627.
化合物3cf:
(1s*,2r*)-6,7-dibromo-2-(2-chlorophenyl)-1,2-dihydronaphthalen-1-ol
无色油状(64.4mg,78%yield).rf=0.32onsilicagel(ethylacetate/petroleumether1:10,v/v).1hnmr(400mhz,cdcl3)δ7.55(s,1h),7.37(s,1h),7.21–7.14(m,4h),6.57(dd,j=9.7,2.3hz,1h),6.06(dd,j=9.7,3.5hz,1h),4.85(d,j=5.3hz,1h),4.38(dt,j=5.7,2.9hz,1h),1.53(dd,j=14.8,6.3hz,1h).13c{1h}nmr(100mhz,cdcl3)δ135.8,135.3,134.3,132.8,132.6,131.2,131.0,130.7,129.7,128.8,127.2,126.6,124.6,123.5,68.4,43.3.hrms(esi-iontrap)m/z:[m-3h]–calcdforc16h8br2clo,408.8628;found408.8632.
化合物4da:6,7-dimethoxy-2-phenyl-naphthalene
白色固体(49.6mg,88%yield).mp121–122℃.rf=0.35(ethylacetate/petroleumether1:10,v/v).1hnmr(500mhz,cdcl3)δ7.82(d,j=1.5hz,1h),7.66(d,j=8.4hz,1h),7.63–7.60(m,2h),7.52(dd,j=8.4,1.8hz,1h),7.38(dd,j=10.6,4.8hz,2h),7.29–7.24(m,1h),7.07(d,j=19.6hz,2h),3.93(d,j=1.2hz,6h).13c{1h}nmr(125mhz,cdcl3)δ149.9,149.6,141.4,137.0,129.5,128.8,128.4,127.3,127.1,126.8,124.4,123.9,106.6,106.1,55.9.ms(ei)m/z:[m-h]–calcdforc18h15o2,263.11;found263.00.
化合物4dg:6,7-dimethoxy-2-(4-methylphenyl)-naphthalene
白色固体(47.8mg,86%yield).mp136.2–137.3℃.rf=0.36(ethylacetate/petroleumether1:10,v/v).1hnmr(600mhz,cdcl3)δ7.80(d,j=1.4hz,1h),7.65(d,j=8.4hz,1h),7.51(ddd,j=7.1,4.2,1.8hz,3h),7.20(d,j=7.9hz,2h),7.09(s,1h),7.05(s,1h),3.93(d,j=2.2hz,6h),2.33(s,3h).13c{1h}nmr(150mhz,cdcl3)δ150.4,150.1,139.1,137.5,137.4,130.1,130.1,128.8,127.7,127.3,124.6,124.4,107.1,106.7,56.5,21.7.hrms(esi-iontrap)m/z:[m+h]+calcdforc19h19o2279.1386;found,279.1375.
化合物4dc:6,7-dimethoxy-2-(4-chlorophenyl)-naphthalene
白色固体(47.1mg,79%yield).mp139–140℃.rf=0.15(ethylacetate/petroleumether1:10,v/v).1hnmr(600mhz,cdcl3)δ8.34(d,j=1.7hz,1h),8.17(d,j=8.7hz,1h),7.68(dd,j=8.7,1.8hz,1h),7.58(d,j=8.1hz,2h),7.20(d,j=8.0hz,2h),6.65–6.60(m,2h),3.90(d,j=2.7hz,6h).13c{1h}nmr(150mhz,cdcl3)δ150.6,150.4,140.5,136.4,133.8,130.1,129.6,129.2,129.1,127.6,125.0,124.2,107.2,106.7,56.6,56.5.hrms(esi-iontrap)m/z:[m+3h]+calcdforc18h18clo2,301.0998;found301.1002.
化合物4di:6,7-dimethoxy-2-(4-trifluoromethylphenyl)-naphthalene
白色固体(36.5mg,55%yield).mp113–114℃.rf=0.3(ethylacetate/petroleumether1:10,v/v).1hnmr(400mhz,cdcl3)δ7.82(s,1h),7.72–7.60(m,6h),7.49(dd,j=8.4,1.7hz,1h),7.08(d,j=15.6hz,2h),3.93(s,6h).13c{1h}nmr(100mhz,cdcl3)δ149.7(q,2jc-f=30hz),144.6,135.1,129.3,129.1(q,3jc-f=315hz),128.6,127.1(q,3jc-f=18hz),126.8,125.5,125.4(q,4jc-f=4hz),124.5,123.4,123.2,106.3,105.7,55.6.19fnmr(376mhz,cdcl3)δ-62.30.hrms(esi-iontrap)m/z:[m+h]+calcdforc19h16f3o2,333.1103;found333.1094.
化合物3ea:(1s*,2r*)-2-phenyl-1,2-dihydrotriphenylen-1-ol
白色固体(26.4mg,41%yield).mp156–158℃.rf=0.23(ethylacetate/petroleumether1:10,v/v).1hnmr(500mhz,cdcl3):δ8.70–8.64(m,2h),8.27–8.22(m,1h),8.21–8.14(m,1h),7.64–7.50(m,5h),7.48–7.45(m,2h),7.42–7.37(m,2h),7.34–7.28(m,1h),6.41(ddd,j=9.8,2.3,1.5hz,1h),5.36(t,j=4.7hz,1h),3.98–3.89(m,1h),1.60(d,j=5.7hz,1h).13c{1h}nmr(125mhz,cdcl3):δ140.3,131.1,131.0,130.8,130.1,129.4,129.0,128.9,128.8,127.6,127.5,127.2,127.1,126.8,126.6,124.3,124.2,124.0,123.3,123.2,67.8,48.2.ms(ei)m/z:[m–3h]–calcdforc24h15o,319.11;found319.86.
以上所述仅以实施例来进一步说明本发明的技术内容,以便于读者更容易理解,但不代表本发明的实施方式仅限于此,任何依本发明所做的技术延伸或再创造,均受本发明的保护。
- 还没有人留言评论。精彩留言会获得点赞!