一种不对称alfa-C-H烯基化反应合成轴手性芳基共轭二烯的方法及其产品
一种不对称alfa-c-h烯基化反应合成轴手性芳基共轭二烯的方法及其产品
技术领域
1.本发明涉及一种轴手性芳基二烯的制备方法,具体为一种不对称alfa-c-h烯基化反应合成轴手性芳基共轭二烯的方法及其产品。
背景技术:
2.众所周知,共轭二烯结构是合成有机化学中的重要组成部分,是天然产物和各种生物活性分子中的关键结构基序。共轭二烯也常作为配体广泛用于过渡金属催化的反应中(org.chem.front.,2015,2,73
–
89;acs catal.,2017,7,833
–
847)。目前报导了多种制备共轭二烯化合物的方法,包括:羰基烯化、烯烃复分解、 heck反应、适当烷烃的消除和炔烃的插入偶联,这些方法在多取代烯烃的高效 e/z选择性合成方面取得了一些进展(acc.chem.res.,2008,41,1474-1485)。随着能源的枯竭和环境问题的日益严峻,发展高效、高选择性的1,3二烯化合物的合成方法显得尤为迫切。
3.轴手性化合物在天然产物和药物中无处不在,并已广泛用作不对称合成中的通用手性配体或催化剂。与深入研究的轴手性联芳基化合物不同,手性苯乙烯在取代的烯烃和芳环之间表现出手性,尽管这种类型的轴手性化合物在1940s被 adams等人意识到并深入研究(j.am.chem.soc.,1940,62,53
–
56),但由于其灵活的结构,旋转能垒相对较低,不对称合成较为困难,导致其受到的关注度大大降低。近年来,由于手性烯烃不仅可以用作全合成的合成子(angew.chem.int.ed. engl.;2009,48,5633
–
5637),还可以作为手性催化剂或配体应用于不对称合成中 (angew.chem.int.ed.engl.;2008,47,4482
–
4502),轴手性苯乙烯得到了蓬勃发展。谭斌课题组(nat.commun.,2017,8,15238)和阎海龙课题组(j.am.chem.soc., 2018,140,7056
–
7060)报道了有机分子不对称催化策略合成轴手性苯乙烯的方法。最近,不对称c-h官能团化策略也用于轴手性苯乙烯的构建。史炳锋课题组先后报道了pd催化芳基c-h官能化合成轴手性苯乙烯的策略,(angew.chem.int. ed.,2020,59,6576
–
6580;chem.,2020,6,497
–
511)为轴手性苯乙烯的合成提供了新的技术手段。王细胜课题组报道了通过pd催化的不对称芳基c-h官能化合成轴手性苯乙烯型羧酸(chem.sci.,2021,12,3726
–
3732)。但是,不对称烯基c-h 官能化合成轴手性苯乙烯的方法还报导很少。史炳锋课题组在2021年报道的通过pd催化硫醚导向的烯基碳氢烯基化合成轴手性苯基共轭二烯(j.am.chem. soc.,2021,143,12335-12344),反应涉及苯乙烯β位的不对称c-h烯基化反应,经历了六元金属杂环中间体。但是,应用的硫醚导向基不容易化学修饰,且用到的手性磷酸配体价格昂贵,在合成应用上有较大的限制。
4.发明人之前报道了一种钯催化下的手性瞬态导向基控制的轴手性芳基共轭二烯化合物的合成,反应以简单的邻烯基-苯甲醛为原料,以n,n-二异丙基保护的l-叔亮氨酸为配体,醋酸/dmso为混合溶剂,通过苯乙烯beta位不对称烯基 c-h烯基化反应,经历七元endo-金属杂环中间体,得到轴手性苯甲醛,产率最高达到89%,ee值最高达到》99/1(org.chem.front.,2022,9,2109)。然而,当该反应条件应用于苯乙烯alpha位不对称烯基
c-h烯基化反应时,产率和选择性却很低。
[0005][0006]
在此,本发明提出一种钯催化下的手性瞬态导向基控制的轴手性芳基共轭二烯化合物的合成,反应以简单的邻烯基-苯甲醛为原料,以简单的l-叔亮氨酸为配体,通过苯乙烯alpha位不对称烯基c-h烯基化反应,经历六元exo-金属杂环中间体,得到轴手性苯乙烯。与先前的方法相比,本发明应用廉价易得的l-叔亮氨酸作为配体,三氟醋酸/dmso为混合溶剂,得到的手性芳基醛具有广泛的化学转化选择性,并且反应条件温和,适用于克级合成。本方法得到的轴手性苯甲醛可经氧化得到轴手性苯甲酸,该化合物可作为钴催化不对称c-h烷基化反应的手性配体,er值高达90:10。
[0007]
技术实现要素:
[0008]
本发明的目的在于针对现有技术的不足,提供一种轴手性芳基1,3-二烯的合成方法。
[0009]
为实现上述目的,本发明为合成式(1)所示的化合物采用如下技术方案:
[0010][0011]
式(1)中r1为c
1~4
烷基或c
6~10
芳基;r2为酯基、苯基或酰胺基;r为甲基或 c6芳基。
[0012]
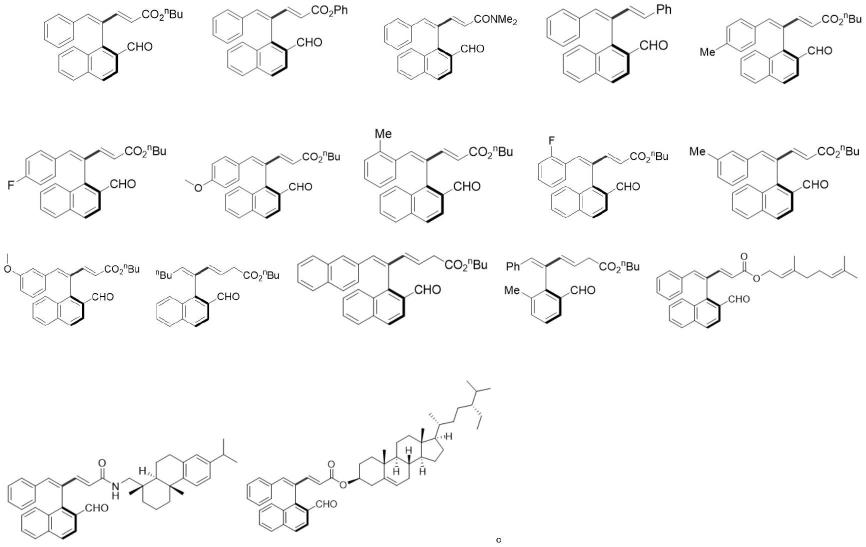
作为优选,式(1)中r1为正丁基、苯基、萘基、4-甲基取代苯基、4-氟取代苯基、4-甲氧基取代苯基、2-甲基取代苯基、2-氟取代苯基、3-甲基取代苯基、 3-甲氧基取代苯基;r2为正丁酯基、叔丁酯基、苯酯基、n,n-二甲基甲酰胺基、苯基、香叶酯基、脱氢枞酰胺基、β-谷
甾酯基;r为甲基或苯基。
[0013]
更为优选,轴手性芳基1,3-二烯的化学结构式具体如下之一:
[0014][0015][0016]
本发明在相对温和的条件下,采用简单过渡金属盐作为催化剂,无需外源氧化剂,从简单原料苯乙烯来制备1,3-二烯化合物,反应式如下:
[0017][0018]
在反应容器中加入过渡金属盐催化剂、配体l3、氧化剂、助氧化剂、酸类、有机溶剂,然后依次向溶液中加入邻烯基-苯甲醛、烯基衍生物。再将反应容器在氧气下密封并加热至一定温度下搅拌反应一段时间,冷却后将混合物直接应用于快速柱色谱(pe/ea),反应液经直接柱层析分离后得到。
[0019]
所述配体l3的结构式如下:
[0020]
作为优选,所述氧化剂为二氧化锰,助氧化剂为苯醌,酸类为磷酸二苄酯。
[0021]
作为优选,所述邻烯基-苯甲醛、烯基衍生物、过渡金属盐催化剂、配体、氧化剂、助氧化剂、酸类的物质的量之比为1:4:0.15:0.45:1.5:1:2。
[0022]
作为优选,所述有机溶剂为乙酸、二甲基亚砜、三氟乙酸、三氟乙醇、叔丁醇、六氟异丙醇中的一种或多种。
[0023]
作为优选,所述过渡金属盐催化剂为钯盐,更为优选为醋酸钯或氯化钯。
[0024]
作为优选,每mol邻烯基-苯甲醛加入5~10l有机溶剂。
[0025]
作为优选,所述加热反应温度为25-40℃,反应时间40-48小时。
[0026]
作为优选,所述后处理为:将反应液浓缩后装柱,剩余物用300目硅胶进行柱层析分离,洗脱剂为体积比1:9的乙酸乙酯与石油醚混合液,收集含目标化合物的洗脱剂,浓缩干燥得到相应目标产物。
[0027]
本发明制备得到的类化合物都是全新的化合物,未曾报道过,属于全新的合成方法。
[0028]
与现有技术相比,本发明有益效果主要体现在:
[0029]
(1)本发明提供了一种高效合成新型轴手性芳基共轭二烯化合物的方法,得到的产物都是全新且未见报道的。
[0030]
(2)本发明的合成方法,操作简单,反应条件温和,反应收率高且对映选择性优异,最高达到了92%,89%ee。
[0031]
(3)本发明的合成方法中采用了苯乙烯的不对称alpha-c-h烯基化反应合成轴手性芳基共轭二烯的方法,操作简单有效,经济效益更高,生产环保压力更小。
[0032]
(4)本发明底物适用范围广泛,产率较高(67~92%),对映选择性优异(高达 99%ee),应用范围广泛,具有非常好的经济价值。
[0033]
(5)本发明的合成原料经济易得,适用于1,3-二烯芳环化合物的高效制备,具有广泛的应用前景,在制备方法上是现有方法的一种有效补充。
具体实施方式
[0034]
下面结合具体实施例对本发明进行进一步描述,但本发明的保护范围并不仅限于此。
[0035]
以下实施例所述的柱层析分离皆采用300目硅胶进行柱层析分离,洗脱剂为体积比1:9的乙酸乙酯与石油醚混合液,收集含目标化合物的洗脱剂,浓缩干燥得到式(1)所示的化合物。
[0036]
实施例1:(2e,4z)-4-(2-甲酰基萘基-1-基)-5-苯基-2,4-戊二烯酸正丁酯
[0037][0038]
向螺旋盖小瓶中装入醋酸钯(3.4mg,0.015mmol),配体l3(5.9mg,0.045 mmol),二氧化锰(13.1mg,0.15mmol),苯醌(10.9mg,0.1mmol),磷酸二苄酯(48.0 mg,0.2mmol),三氟乙醇(0.5ml)和二甲基亚砜(0.5ml)。然后依次向溶液中加入 (z)-1-苯乙烯基-2-萘甲醛(25.9mg,0.1mmol)和丙烯酸正丁酯(51.3mg,0.4mmol)。将小瓶在氧气下密封并加热至40℃并搅拌48小时。冷却后,将混合物直接应用于快速柱色谱(pe/ea),反应液经直接柱层析分离后得到淡黄色液体(30.2mg,收率78%,ee值93%)。1h nmr(500mhz,cdcl3)δ10.11(s,1h),8.08(d,j=8.5 hz,1h),8.01(d,j=8.5hz,1h),7.97
–
7.94(m,2h),7.91(d,j=8.5hz,1h),7.65
ꢀ–
7.62(m,1h),7.49
–
7.46(m,2h),7.11
–
7.08(m,1h),7.01(t,j=7.5hz,2h),6.74 (d,j=7.5hz,2h),5.14(d,j=15.5hz,1h),4.12
–
4.03(m,2h),1.60
–
1.54(m, 2h),1.37
–
1.29(m,2h),0.89(t,j=7.5hz,3h);
13
c nmr(125mhz,cdcl3)δ 190.66,165.78,148.23,141.50,140.30,135.55,133.49,131.32,129.84,129.48, 128.78,128.43,128.23,128.09,127.65,127.59,126.74,125.21,121.61,120.56, 63.47,29.61,18.07,12.65;hrms(esi):
m/zforc
26h24
o3na[m+na]
+
:407.1618,found:407.1613;ftir(kbr,cm-1
):3442.06,3383.18,2957.01,2354.21,1689.72,1656.07,1636.45,1557.94,1541.12,1510.28,1406.54,1398.13,1033.64;opt.rot.[α]
20
d=-101.9(c=0.8,chcl3);hplcdaicelchiralpakia-hcolumn,n-hexane/i-proh(98/2),0.5ml/min,254nm,15.526min(majorenantiomer),16.693min(minorenantiomer).
[0039]
实施例2:(2e,4z)-4-(2-甲酰基萘基-1-基)-5-苯基-2,4-戊二烯酸苯酯的制备
[0040][0041]
向螺旋盖小瓶中装入醋酸钯(3.4mg,0.015mmol),配体l3(5.9mg,0.045mmol),二氧化锰(13.1mg,0.15mmol),苯醌(10.9mg,0.1mmol),磷酸二苄酯(48.0mg,0.2mmol),三氟乙醇(0.5ml)和二甲基亚砜(0.5ml)。然后依次向溶液中加入(z)-1-苯乙烯基-2-萘甲醛(25.9mg,0.1mmol)和丙烯酸苯酯(59.3mg,0.4mmol)。将小瓶在氧气下密封并加热至40℃并搅拌48小时。冷却后,将混合物直接应用于快速柱色谱(pe/ea),反应液经直接柱层析分离后得到白色液体(37.1mg,收率92%,ee值89%)。1hnmr(500mhz,cdcl3)δ10.17(s,1h),8.14(d,j=12.5hz,1h),8.12(d,j=6.0hz,1h)8.04(d,j=8.5hz,1h),7.97(t,j=8.5hz,2h),7.66(t,j=7.5hz,1h),7.55(s,1h),7.52(t,j=7.5hz,1h),7.34(t,j=8.0hz,2h),7.19(t,j=7.5hz,1h),7.12(t,j=7.5hz,1h),7.06
–
7.02(m,4h),6.78(d,j=8.0hz,2h),5.32(d,j=15.5hz,1h);
13
cnmr(125mhz,cdcl3)δ190.57,164.10,150.04,149.54,142.50,139.99,135.60,133.36,131.21,129.79,129.52,128.95,128.54,128.39,128.37,127.75,127.67,126.88,125.14,124.76,121.71,120.44,119.50;hrms(esi):m/zforc
28h20
o3k[m+k]
+
:443.1044,found:443.1050;ftir(kbr,cm-1
):3848.60,3744.86,3624.30,3565.42,3442.06,3419.63,2357.01,1734.58,1686.92,1656.07,1557.94,1538.32,1504.67,1403.74,1019.63,806.54;opt.rot.[α]
20
d=-83.8(c=1.0,chcl3);hplcdaicelchiralpakia-hcolumn,n-hexane/i-proh(95/5),1.0ml/min,254nm,12.395min(majorenantiomer),13.416min(minorenantiomer).
[0042]
实施例3:(2e,4z)-4-(2-甲酰基萘基-1-基)-n,n-二甲基5-苯基-2,4-二烯胺的制备
[0043][0044]
向螺旋盖小瓶中装入醋酸钯(3.4mg,0.015mmol),配体l3(5.9mg,0.045mmol),二氧化锰(13.1mg,0.15mmol),苯醌(10.9mg,0.1mmol),磷酸二苄酯(48.0mg,0.2mmol),三氟乙醇(0.5ml)和二甲基亚砜(0.5ml)。然后依次向溶液中加入(z)-1-苯乙烯基-2-萘甲醛(25.9mg,0.1mmol)和n,n-二甲基丙烯酰胺(39.7mg,0.4mmol)。将小瓶在氧气下密封并加热至40℃并搅拌48小时。冷却后,将混合物直接应用于快速柱色谱(pe/ea),反应液经直接柱层析分离后得到淡黄色液体(31.9mg,收率90%,ee值88%,m.p.=54.0℃)。1hnmr(500mhz,cdcl3)δ10.12(s,1h),8.09(d,j=9.0hz,1h),8.00(d,j=8.5hz,1h),7.97(d,j=
8.0hz,1h),7.96(s,1h),7.94(d,j=9.0hz,1h),7.63(t,j=7.5hz,1h),7.49
–
7.46(m,2h),7.07(t,j=7.5hz,1h),7.00(t,j=7.5hz,2h),6.73(d,j=8.0hz,2h),5.56(d,j=15.0hz,1h),2.92(s,3h),2.61(s,3h);
13
cnmr(125mhz,cdcl3)δ190.96,165.26,146.19,141.04,140.32,135.46,133.76,131.62,129.93,129.49,128.61,128.42,128.05,127.72,127.54,127.52,126.67,125.51,121.46,120.08,36.03,34.71;hrms(esi):m/zforc
24h21
no2k[m+k]
+
:394.1204,found:394.1201;ftir(kbr,cm-1
):3851.40,3739.25,3649.53,3629.91,3565.42,2348.60,1737.38,1684.11,1650.47,1563.55,1541.12,1510.28,1398.13,1386.92,1028.04;opt.rot.[α]
20
d=-72.7(c=1.0,chcl3);hplcdaicelchiralpakod-hcolumn,n-hexane/i-proh(90/10),1.0ml/min,254nm,16.816min(majorenantiomer),19.483min(minorenantiomer).
[0045]
实施例4:1-((1z,3e)-1,3-二苯基-1,3-二烯-2-基)-2-萘甲醛
[0046][0047]
向螺旋盖小瓶中装入醋酸钯(3.4mg,0.015mmol),配体l3(5.9mg,0.045mmol),二氧化锰(13.1mg,0.15mmol),苯醌(10.9mg,0.1mmol),磷酸二苄酯(48.0mg,0.2mmol),三氟乙醇(0.5ml)和二甲基亚砜(0.5ml)。然后依次向溶液中加入(z)-1-苯乙烯基-2-萘甲醛(25.9mg,0.1mmol)和苯乙烯(41.7mg,0.4mmol)。将小瓶在氧气下密封并加热至40℃并搅拌48小时。冷却后,将混合物直接应用于快速柱色谱(pe/ea),反应液经直接柱层析分离后得到黄色油状液体(25.3mg,收率71%,ee值94%)。1hnmr(500mhz,cdcl3)δ10.19(s,1h),8.11(d,j=8.5hz,1h),8.04(d,j=8.5hz,1h),8.01(d,j=8.5hz,1h),7.96(d,j=8.0hz,1h),7.62(t,j=7.5hz,1h),7.49
–
7.44(m,2h),7.27
–
7.16(m,6h),7.20
–
7.16(m,1h),7.03
–
6.96(m,3h),6.68(d,j=7.5hz,2h),5.79(d,j=16.0hz,1h);
13
cnmr(125mhz,cdcl3)δ191.40,142.04,135.66,135.49,134.97,134.54,133.46,133.38,131.78,130.33,129.64,128.25,128.01,127.80,127.56,127.48,127.38,126.89,126.69,126.49,125.72,125.50,121.45;hrms(esi):m/zforc
27h20
ok[m+k]
+
:399.1146,found:399.1145;ftir(kbr,cm-1
):3245.79,2359.81,1698.13,1650.47,1541.12,1451.40;opt.rot.[α]
20
d=-25.7(c=0.16,chcl3);hplcdaicelchiralpakia-hcolumn,n-hexane/i-proh(98/2),1.0ml/min,254nm,13.965min(majorenantiomer),16.944min(minorenantiomer).
[0048]
实施例5:(2e,4z)-4-(2-甲酰基萘基-1-基)-5-(4-甲基苯基)-2,4-戊二烯酸正丁酯的制备
[0049][0050]
向螺旋盖小瓶中装入醋酸钯(3.4mg,0.015mmol),配体l3(5.9mg,0.045mmol),二氧化锰(13.1mg,0.15mmol),苯醌(10.9mg,0.1mmol),磷酸二苄酯(48.0mg,0.2mmol),三氟乙醇(0.5ml)和二甲基亚砜(0.5ml)。然后依次向溶液中加入(z)-1-(4-甲基苯乙烯基)-2-萘甲醛(27.3mg,0.1mmol)和丙烯酸正丁酯(51.3mg,0.4mmol)。将小瓶在氧气下密封并加
热至40℃并搅拌48小时。冷却后,将混合物直接应用于快速柱色谱(pe/ea),反应液经直接柱层析分离后得到黄色油状液体(32.7mg,收率82%,ee值83%)。1hnmr(500mhz,cdcl3)δ10.10(s,1h),8.08(d,j=8.5hz,1h),8.00(d,j=8.5hz,1h),7.96(d,j=4.0hz,1h),7.94(d,j=3.0hz,1h),7.91(d,j=8.5hz,1h),7.65
–
7.62(m,1h),7.48
–
7.47(m,1h),6.82(d,j=8.0hz,2h),6.63(d,j=8.0hz,2h),5.11(d,j=15.5hz,1h),4.10
–
4.04(m,2h),2.16(s,3h),1.59
–
1.53(m,2h),1.35
–
1.31(m,2h),0.89(t,j=7.5hz,3h);
13
cnmr(125mhz,cdcl3)δ190.80,165.89,148.46,141.61,140.61,138.52,135.57,130.77,130.24,129.85,129.48,128.83,128.40,128.38,128.14,127.62,126.69,125.23,121.58,119.93,63.41,29.62,20.17,18.07,12.65;hrms(esi):m/zforc
27h26
o3na[m+na]
+
:421.1774,found:421.1762;ftir(kbr,cm-1
):3259.81,2357.01,1656.07,1636.45,1400.93;opt.rot.[α]
20
d=-81.9(c=0.498,chcl3);hplcdaicelchiralpakia-hcolumn,n-hexane/i-proh(99/1),1.0ml/min,254nm,21.062min(majorenantiomer),23.744min(minorenantiomer).
[0051]
实施例6:(2e,4z)-4-(2-甲酰基萘基-1-基)-5-(4-氟苯基)-2,4-戊二烯酸正丁酯的制备
[0052][0053]
向螺旋盖小瓶中装入醋酸钯(3.4mg,0.015mmol),配体l3(5.9mg,0.045mmol),二氧化锰(13.1mg,0.15mmol),苯醌(10.9mg,0.1mmol),磷酸二苄酯(48.0mg,0.2mmol),三氟乙醇(0.5ml)和二甲基亚砜(0.5ml)。然后依次向溶液中加入(z)-1-(4-氟苯乙烯基)-2-萘甲醛(27.7mg,0.1mmol)和丙烯酸正丁酯(51.3mg,0.4mmol)。将小瓶在氧气下密封并加热至40℃并搅拌48小时。冷却后,将混合物直接应用于快速柱色谱(pe/ea),反应液经直接柱层析分离后得到淡黄色油状液体(36.5mg,收率91%,ee值94%)。1hnmr(500mhz,cdcl3)δ10.10(s,1h),8.09(d,j=8.5hz,1h),8.02(d,j=8.5hz,1h),7.97
–
7.92(m,2h),7.89(d,j=8.5hz,1h),7.67
–
7.64(m,1h),7.51
–
7.47(m,1h),7.44(s,1h),6.72(s,2h),6.71(d,j=1.5hz,2h),5.14(d,j=15.5hz,1h),4.12
–
4.04(m,2h),1.59
–
1.54(m,2h),1.37
–
1.29(m,2h),0.89(t,j=7.5hz,3h);
13
cnmr(125mhz,cdcl3)δ190.52,165.74,161.72(d,j
cf
=251.3hz),148.01,139.97(d,j
cf
=12.5hz),131.01(d,j
cf
=2.5hz),130.59(d,j
cf
=7.5hz),129.79(d,j
cf
=2.5hz),129.73,129.54,128.45(d,j
cf
=21.3hz),127.73,126.85,125.06,121.67,120.62,114.89,114.71,63.50,29.60,18.07,12.64;
19
fnmr(471mhz,cdcl3)δ-110.32;hrms(esi):m/zforc
26h23
fo3na[m+na]
+
:425.1523,found:425.1501;ftir(kbr,cm-1
):3226.17,2357.01,1656.07,1636.45,1457.01,1389.72;opt.rot.[α]
20
d=-352.9(c=0.12,chcl3);hplcdaicelchiralpakia-hcolumn,n-hexane/i-proh(98/2),1.0ml/min,254nm,17.686min(majorenantiomer),19.934min(minorenantiomer).
[0054]
实施例7:(2e,4z)-4-(2-甲酰基萘基-1-基)-5-(4-甲氧基苯基)-2,4-戊二烯酸正丁酯的制备
[0055][0056]
向螺旋盖小瓶中装入醋酸钯(3.4mg,0.015mmol),配体l3(5.9mg,0.045mmol),二氧化锰(13.1mg,0.15mmol),苯醌(10.9mg,0.1mmol),磷酸二苄酯(48.0mg,0.2mmol),三氟乙醇(0.5ml)和二甲基亚砜(0.5ml)。然后依次向溶液中加入(z)-1-(4-甲氧基苯乙烯基)-2-萘甲醛(28.9mg,0.1mmol)和丙烯酸正丁酯(51.3mg,0.4mmol)。将小瓶在氧气下密封并加热至40℃并搅拌48小时。冷却后,将混合物直接应用于快速柱色谱(pe/ea),反应液经直接柱层析分离后得到淡黄色油状液体(34.9mg,收率84%,ee值68%)。1hnmr(500mhz,cdcl3)δ10.10(s,1h),8.09(d,j=8.5hz,1h),8.00(d,j=8.5hz,1h),7.96
–
7.90(m,3h),7.65
–
7.62(m,1h),7.49
–
7.45(m,1h),7.42(s,1h),6.67(d,j=9.0hz,2h),6.54(d,j=9.0hz,2h),5.06(s,1h),4.11
–
4.03(m,2h),3.65(s,3h),1.59
–
1.53(m,2h),1.36
–
1.29(m,2h),0.89(t,j=7.5hz,3h);
13
cnmr(125mhz,cdcl3)δ190.92,165.99,159.21,148.64,141.23,140.77,135.62,130.54,129.85,129.57,128.78,128.43,128.12,127.63,126.69,126.30,125.23,121.62,119.18,113.16,63.36,54.14,29.63,18.08,12.65;hrms(esi):m/zforc
27h26
o4na[m+na]
+
:437.1723,found:437.1703;ftir(kbr,cm-1
):3251.40,2362.62,1653.27,1633.64,1454.21;opt.rot.[α]
20
d=-111.3(c=0.25,chcl3);hplcdaicelchiralpakod-hcolumn,n-hexane/i-proh(98/2),0.5ml/min,254nm,25.607min(majorenantiomer),27.576min(minorenantiomer).
[0057]
实施例8:(2e,4z)-4-(2-甲酰基萘基-1-基)-5-(2-甲基苯基)-2,4-戊二烯酸正丁酯的制备
[0058][0059]
向螺旋盖小瓶中装入醋酸钯(3.4mg,0.015mmol),配体l3(5.9mg,0.045mmol),二氧化锰(13.1mg,0.15mmol),苯醌(10.9mg,0.1mmol),磷酸二苄酯(48.0mg,0.2mmol),三氟乙醇(0.5ml)和二甲基亚砜(0.5ml)。然后依次向溶液中加入(z)-1-(2-甲基苯乙烯基)-2-萘甲醛(27.3mg,0.1mmol)和丙烯酸正丁酯(51.3mg,0.4mmol)。将小瓶在氧气下密封并加热至40℃并搅拌72小时。冷却后,将混合物直接应用于快速柱色谱(pe/ea),反应液经直接柱层析分离后得到淡黄色油状液体(33.2mg,收率83%,ee值97%)。1hnmr(500mhz,cdcl3)δ10.10(d,j=0.5hz,1h),8.01
–
7.98(m,2h),7.94
–
7.91(m,3h),7.71(s,1h),7.64
–
7.61(m,1h),7.53
–
7.49(m,1h),7.09(d,j=7.5hz,1h),6.96(t,j=7.0hz,1h),6.55(t,j=7.5hz,1h),6.29(d,j=8.0hz,1h),5.16(d,j=15.5hz,1h),4.13
–
4.04(m,2h),2.47(s,3h),1.60
–
1.54(m,2h),1.37
–
1.29(m,2h),0.89(t,j=7.5hz,3h);
13
cnmr(125mhz,cdcl3)δ190.61,165.91,148.09,140.14,139.67,136.49,135.32,132.28,131.84,130.36,129.65,129.48,128.23,128.01,127.82,127.69,126.81,126.62,125.43,124.70,121.51,120.57,63.51,29.61,19.23,18.06,12.65;hrms(esi):m/zforc
27h26
o3na[m+na]
+
:421.1774,found:421.1761;ftir(kbr,cm-1
):3203.74,2354.21,1650.47,1628.04,
1398.13;opt.rot.[α]
20
d=-116.3(c=0.544, chcl3);hplc daicel chiralpak ic-h column,n-hexane/i-proh(92/8),1.0 ml/min,254nm,14.602min(major enantiomer),16.477min(minor enantiomer).
[0060]
实施例9:(2e,4z)-4-(2-甲酰基萘基-1-基)-5-(2-氟苯基)-2,4-戊二烯酸正丁酯的制备
[0061][0062]
向螺旋盖小瓶中装入醋酸钯(3.4mg,0.015mmol),配体l3(5.9mg,0.045 mmol),二氧化锰(13.1mg,0.15mmol),苯醌(10.9mg,0.1mmol),磷酸二苄酯(48.0 mg,0.2mmol),三氟乙醇(0.5ml)和二甲基亚砜(0.5ml)。然后依次向溶液中加入 (z)-1-(2-氟苯乙烯基)-2-萘甲醛(27.7mg,0.4mmol)和丙烯酸正丁酯(51.3mg,0.4 mmol)。将小瓶在氧气下密封并加热至40℃并搅拌72小时。冷却后,将混合物直接应用于快速柱色谱(pe/ea),反应液经直接柱层析分离后得到淡黄色油状液体(30.2mg,收率77%,ee值99%)。1h nmr(500mhz,cdcl3)δ10.12(s,1h), 8.07(d,j=8.5hz,1h),8.01
–
7.97(m,2h),7.95(d,j=8.0hz,1h),7.91(d,j= 8.5hz,1h),7.76(s,1h),7.66
–
7.63(m,1h),7.52
–
7.49(m,1h),7.09
–
7.05(m,1h), 7.00
–
6.96(m,1h),6.51(t,j=7.5hz,1h),6.16(td,j=8.0,1.5hz,1h),5.18(d,j =15.5hz,1h),4.13
–
4.05(m,2h),1.60
–
1.55(m,2h),1.37
–
1.30(m,2h),0.90(t, j=7.5hz,3h);
13
c nmr(125mhz,cdcl3)δ190.49,165.62,160.08(d,j
cf
= 251.3hz),147.83,139.79,135.46,132.90(d,j
cf
=2.5hz),132.32(d,j
cf
=6.3hz), 129.81,129.72,129.65,129.58,128.47,128.35,127.70,127.49(d,j
cf
=2.5hz), 126.84,125.16,122.97(d,j
cf
=3.8hz),121.60,121.36,114.65(d,j
cf
=21.3hz), 63.56,29.60,18.08,12.65;
19
f nmr(471mhz,cdcl3)δ-114.98;hrms(esi): m/z for c
26h23
fo3na[m+na]
+
:425.1523,found:425.1554;ftir(kbr,cm-1
): 3217.76,2357.01,1658.88,1636.45,1462.6,1162.62;opt.rot.[α]
20
d=-77.8(c= 0.512,chcl3);hplc daicel chiralpak ia-h column,n-hexane/i-proh(99/1),1.0 ml/min,254nm,15.198min(major enantiomer),17.346min(minor enantiomer).
[0063]
实施例10:(2e,4z)-4-(2-甲酰基萘基-1-基)-5-(3-甲基苯基)-2,4-戊二烯酸正丁酯的制备
[0064][0065]
向螺旋盖小瓶中装入醋酸钯(3.4mg,0.015mmol),配体l3(5.9mg,0.045 mmol),二氧化锰(13.1mg,0.15mmol),苯醌(10.9mg,0.1mmol),磷酸二苄酯(48.0 mg,0.2mmol),三氟乙醇(0.5ml)和二甲基亚砜(0.5ml)。然后依次向溶液中加入 (z)-1-(3-甲基苯乙烯基)-2-萘甲醛(27.3mg,0.1mmol)和丙烯酸正丁酯(51.3mg, 0.4mmol)。将小瓶在氧气下密封并加热至40℃并搅拌48小时。冷却后,将混合物直接应用于快速柱色谱(pe/ea),反应液经直接柱层析分离后得到黄色油状液体(31.7mg,收率80%,ee值89%)。1h nmr(500mhz,cdcl3)δ
10.10(s,1h),8.08(d,j=8.5hz,1h),8.01(d,j=8.5hz,1h),7.97
–
7.94(m,2h),7.91(d,j=8.5hz,1h),7.65
–
7.62(m,1h),7.49
–
7.46(m,1h),7.44(s,1h),6.91(d,j=7.5hz,1h),6.85(t,j=7.5hz,1h),6.63(s,1h),6.43(d,j=8.0hz,1h),5.14(d,j=15.5hz,1h),4.12
–
4.03(m,2h),2.05(s,3h),1.60
–
1.54(m,2h),1.37
–
1.29(m,2h),0.89(t,j=7.5hz,3h);
13
cnmr(125mhz,cdcl3)δ190.74,165.85,148.33,141.75,140.51,137.09,135.53,133.43,131.11,130.08,129.88,129.48,128.93,128.39,128.12,127.59,127.44,126.70,125.52,125.24,121.55,120.35,63.45,29.61,20.15,18.07,12.65;hrms(esi):m/zforc
27h26
o3na[m+na]
+
:421.1774,found:421.1759;ftir(kbr,cm-1
):3320.56,2357.01,1650.47,1633.64,1395.33;opt.rot.[α]
20
d=-143.8(c=0.44,chcl3);hplcdaicelchiralpakia-hcolumn,n-hexane/i-proh(98/2),1.0ml/min,254nm,12.303min(majorenantiomer),14.067min(minorenantiomer).
[0066]
实施例11:(2e,4z)-4-(2-甲酰基萘基-1-基)-5-(3-甲氧基苯基)-2,4-戊二烯酸正丁酯的制备
[0067][0068]
向螺旋盖小瓶中装入醋酸钯(3.4mg,0.015mmol),配体l3(5.9mg,0.045mmol),二氧化锰(13.1mg,0.15mmol),苯醌(10.9mg,0.1mmol),磷酸二苄酯(48.0mg,0.2mmol),三氟乙醇(0.5ml)和二甲基亚砜(0.5ml)。然后依次向溶液中加入(z)-1-(3-甲氧基苯乙烯基)-2-萘甲醛(28.9mg,0.1mmol)和丙烯酸正丁酯(51.3mg,0.4mmol)。将小瓶在氧气下密封并加热至40℃并搅拌48小时。冷却后,将混合物直接应用于快速柱色谱(pe/ea),反应液经直接柱层析分离后得到黄色油状液体(37.2mg,收率90%,ee值87%)。1hnmr(500mhz,cdcl3)δ10.11(s,1h),8.09(d,j=8.5hz,1h),8.00(d,j=8.5hz,1h),7.96(d,j=9.5hz,1h),7.94
–
7.92(m,2h),7.66
–
7.63(m,1h),7.51
–
7.45(m,1h),7.45(s,1h),6.98(t,j=16.0hz,1h),6.65(dd,j=8.0,2.0hz,1h),6.51(d,j=7.5hz,1h),6.11
–
6.10(m,1h),5.18(d,j=15.5hz,1h),4.12
–
4.04(m,2h),3.19(s,3h),1.60
–
1.54(m,2h),1.37
–
1.30(m,2h),0.89(t,j=7.5hz,3h);
13
cnmr(125mhz,cdcl3)δ190.61,165.76,158.25,148.11,141.34,140.38,135.51,134.70,131.49,129.95,129.58,128.51,128.15,127.57,126.84,125.27,122.14,121.63,120.71,115.09,112.28,63.49,53.50,29.61,18.08,12.65;hrms(esi):m/zforc
27h26
o4na[m+na]
+
:437.1723,found:437.1701;ftir(kbr,cm-1
):3234.58,2357.01,1650.47,1633.64,1392.52;opt.rot.[α]
20
d=-130.9(c=0.498,chcl3);hplcdaicelchiralpakic-hcolumn,n-hexane/i-proh(90/10),1.0ml/min,254nm,19.065min(majorenantiomer),27.883min(minorenantiomer).
[0069]
实施例12:(3e,5z)-5-(2-甲酰基萘基-1-基)-3,5-癸二烯酸正丁酯的制备
[0070][0071]
向螺旋盖小瓶中装入醋酸钯(3.4mg,0.015mmol),配体l3(5.9mg,0.045mmol),二
氧化锰(13.1mg,0.15mmol),苯醌(10.9mg,0.1mmol),磷酸二苄酯(48.0mg,0.2mmol),三氟乙醇(0.5ml)和二甲基亚砜(0.5ml)。然后依次向溶液中加入(z)-1-(1-己烯)-2-萘甲醛(23.9mg,0.1mmol)和1-丁烯酸正丁酯(56.9mg,0.4mmol)。将小瓶在氧气下密封并加热至40℃并搅拌72小时。冷却后,将混合物直接应用于快速柱色谱(pe/ea),反应液经直接柱层析分离后得到淡黄色油状液体(23.5mg,收率65%,ee值85%)。1hnmr(500mhz,cdcl3)δ10.11(s,1h),8.05(d,j=8.5hz,1h),7.94
–
7.91(m,2h),7.85(d,j=8.5hz,1h),7.77(d,j=15.5hz,1h),7.65
–
7.62(m,1h),7.53
–
7.50(m,1h),6.68(t,j=7.5hz,1h),5.06(d,j=15.5hz,1h),4.10
–
4.01(m,2h),1.79(q,j=7.5hz,2h),1.58
–
1.52(m,2h),1.34
–
1.28(m,4h),1.18
–
1.11(m,2h),0.88(t,j=7.5hz,3h),0.72(t,j=7.5hz,3h);
13
cnmr(125mhz,cdcl3)δ191.12,165.96,146.89,146.00,140.14,135.41,132.35,130.06,129.77,128.16,127.80,127.52,126.29,125.37,121.14,119.45,76.20,63.36,29.61,28.94,21.26,18.06,12.67,12.63;hrms(esi):m/zforc
25h30
o3na[m+na]
+
:401.2087,found:401.2100;ftir(kbr,cm-1
):3226.17,2354.21,1653.27,1633.64,1538.32,1457.01;opt.rot.[α]
20
d=-37.5(c=0.21,chcl3);hplcdaicelchiralpakod-hcolumn,n-hexane/i-proh(98/2),1.0ml/min,254nm,6.518min(majorenantiomer),7.693min(minorenantiomer).
[0072]
实施例13:(3e,5z)-5-(2-甲酰基萘基-1-基)-6-萘基-3,5-己二烯酸正丁酯的制备
[0073][0074]
向螺旋盖小瓶中装入醋酸钯(3.4mg,0.015mmol),配体l3(5.9mg,0.045mmol),二氧化锰(13.1mg,0.15mmol),苯醌(10.9mg,0.1mmol),磷酸二苄酯(48.0mg,0.2mmol),三氟乙醇(0.5ml)和二甲基亚砜(0.5ml)。然后依次向溶液中加入(z)-1-(2-(萘基-2-基)烯基)-2-萘甲醛(30.1mg,0.1mmol)和1-丁烯酸正丁酯(56.9mg,0.4mmol)。将小瓶在氧气下密封并加热至40℃并搅拌96小时。冷却后,将混合物直接应用于快速柱色谱(pe/ea),反应液经直接柱层析分离后得到淡黄色油状液体(35.0mg,收率81%,ee值85%)。1hnmr(500mhz,cdcl3)δ10.15(s,1h),8.11(d,j=8.5hz,1h),8.05
–
8.00(m,2h),7.96(t,j=8.4hz,2h),7.65
–
7.61(m,2h),7.60
–
7.59(m,1h),7.49
–
7.45(m,2h),7.39
–
7.33(m,4h),6.65(dd,j=8.5,2.0hz,1h),5.20(d,j=15.5hz,1h),4.15
–
4.02(m,2h),1.62
–
1.55(m,2h),1.38
–
1.30(m,2h),0.90(t,j=7.5hz,3h);
13
cnmr(125mhz,cdcl3)δ190.68,165.83,148.29,141.59,140.40,135.57,132.09,131.91,131.52,131.12,130.00,129.97,129.70,128.48,128.27,127.65,127.40,127.20,126.79,126.37,126.13,125.41,125.23,124.75,121.63,120.51,63.48,29.62,18.08,12.66;hrms(esi):m/zforc
31h28
o3k[m+k]
+
:487.1670,found:487.1654;ftir(kbr,cm-1
):3172.90,2359.81,1656.07,1507.48,1386.92;opt.rot.[α]
20
d=-171.3(c=0.592,chcl3);hplcdaicelchiralpakic-hcolumn,n-hexane/i-proh(90/10),1.0ml/min,254nm,19.763min(majorenantiomer),23.790min(minorenantiomer).
[0075]
实施例14:(3e,5z)-5-(2-甲酰基-6-甲基苯基)-6-苯基-3,5-己二烯酸正丁酯的
制备
[0076][0077]
向螺旋盖小瓶中装入醋酸钯(3.4mg,0.015mmol),配体l3(5.9mg,0.045mmol),二氧化锰(13.1mg,0.15mmol),苯醌(10.9mg,0.1mmol),磷酸二苄酯(48.0mg,0.2mmol),三氟乙醇(0.5ml)和二甲基亚砜(0.5ml)。然后依次向溶液中加入(z)-3-甲基-2-苯乙烯基苯甲醛(22.3mg,0.1mmol)和1-丁烯酸正丁酯(56.9mg,0.4mmol)。将小瓶在氧气下密封并加热至40℃并搅拌96小时。冷却后,将混合物直接应用于快速柱色谱(pe/ea),反应液经直接柱层析分离后得到淡黄色液体(31.7mg,收率91%,ee值95%)。1hnmr(500mhz,cdcl3)δ9.91(s,1h),7.92(d,j=8.0hz,1h),7.79(d,j=16.0hz,1h),7.58(d,j=8.0hz,1h),7.49(t,j=7.5hz,1h),7.21(s,1h),7.19
–
7.16(m,1h),7.15
–
7.11(m,2h),6.85
–
6.83(m,2h),5.25(s,1h),4.15
–
4.10(m,2h),2.14(s,3h),1.63
–
1.60(m,2h),1.40
–
1.36(m,2h),0.93(t,j=7.5hz,3h);
13
cnmr(125mhz,cdcl3)δ9.91(s,1h),7.92(d,j=8.0hz,1h),7.79(d,j=16.0hz,1h),7.58(d,j=8.0hz,1h),7.49(t,j=7.5hz,1h),7.21(s,1h),7.19
–
7.16(m,1h),7.15
–
7.11(m,2h),6.85
–
6.83(m,2h),5.25(s,1h),4.15
–
4.10(m,2h),2.14(s,3h),1.63
–
1.60(m,2h),1.40
–
1.36(m,2h),0.93(t,j=7.5hz,3h);hrms(esi):m/zforc
24h26
o3na[m+na]
+
:385.1774,found:385.1753;ftir(kbr,cm-1
):3245.79,2357.01,1653.27,1633.64,1457.01,1033.64;opt.rot.[α]
20
d=+1.8(c=0.498,chcl3);hplcdaicelchiralpakod-hcolumn,n-hexane/i-proh(99/1),1.0ml/min,254nm,14.244min(majorenantiomer),16.127min(minorenantiomer).
[0078]
实施例15:(2e,4z)-4-(2-甲酰基萘基-1-基)-5-苯基-2,4-戊二烯酸香叶酯的制备
[0079][0080]
向螺旋盖小瓶中装入醋酸钯(3.4mg,0.015mmol),配体l3(5.9mg,0.045mmol),二氧化锰(13.1mg,0.15mmol),苯醌(10.9mg,0.1mmol),磷酸二苄酯(48.0mg,0.2mmol),三氟乙醇(0.5ml)和二甲基亚砜(0.5ml)。然后依次向溶液中加入(z)-1-苯乙烯基-2-萘甲醛(25.9mg,0.1mmol)和丙烯酸香叶酯(83.4mg,0.4mmol)。将小瓶在氧气下密封并加热至40℃并搅拌48小时。冷却后,将混合物直接应用于快速柱色谱(pe/ea),反应液经直接柱层析分离后得到棕色油状液体(39.2mg,收率84%,ee值88%)。1hnmr(500mhz,cdcl3)δ10.10(s,1h),8.07(d,j=8.5hz,1h),8.00(d,j=8.5hz,1h),7.98
–
7.94(m,2h),7.91(d,j=8.5hz,1h),7.64
–
7.62(m,1h),7.49
–
7.46(m,2h),7.09(t,j=7.5hz,1h),7.01(t,j=7.5hz,2h),6.75(d,j=7.5hz,2h),7.30
–
7.27(m,1h),5.15(d,j=15.5hz,1h),5.06
–
5.03(m,1h),4.60(d,j=7.0hz,2h),2.09
–
2.04(m,2h),2.02
–
1.99(m,2h),1.66(d,j=6.5hz,6h),1.57(s,3h);
13
cnmr(125mhz,cdcl3)δ190.64,165.71,148.33,141.66,141.52,140.29,135.55,133.50,131.34,130.80,129.83,129.46,128.79,128.43,128.21,128.10,127.65,127.60,126.73,125.21,122.66,121.60,120.56,116.90,60.46,38.49,25.21,
24.64,16.65,15.44;hrms(esi):m/zforc
32h32
o3k[m+k]
+
:503.1983,found:503.1976;ftir(kbr,cm-1
):3273.83,2359.81,2328.97,1656.07,1633.64,1504.67,1466.62;opt.rot.[α]
20
d=-147.9(c=0.276,chcl3);hplcdaicelchiralpakia-hcolumn,n-hexane/i-proh(98/2),1.0ml/min,254nm,12.494min(majorenantiomer),13.771min(minorenantiomer).
[0081]
实施例16:(2e,4z)-4-(2-甲酰基萘基-1-基)-5-苯基-2,4-戊二烯酸脱氢枞酰胺的制备
[0082][0083]
向螺旋盖小瓶中装入醋酸钯(3.4mg,0.015mmol),配体l3(5.9mg,0.045mmol),二氧化锰(13.1mg,0.15mmol),苯醌(10.9mg,0.1mmol),磷酸二苄酯(48.0mg,0.2mmol),三氟乙醇(0.5ml)和二甲基亚砜(0.5ml)。然后依次向溶液中加入(z)-1-苯乙烯基-2-萘甲醛(25.9mg,0.1mmol)和丙烯脱氢枞酰胺(135.3mg,0.4mmol)。将小瓶在氧气下密封并加热至40℃并搅拌48小时。冷却后,将混合物直接应用于快速柱色谱(pe/ea),反应液经直接柱层析分离后得到白色液体(47.7mg,收率80%,ee值86%,m.p.=92.5℃)。1hnmr(500mhz,cdcl3)δ10.09(s,1h),8.08(d,j=8.5hz,1h),7.99(d,j=8.5hz,1h),7.94
–
7.91(m,3h),7.62(t,j=7.5hz,1h),7.47
–
7.44(m,2h),7.12(d,j=8.0hz,1h),7.08
–
7.05(m,1h),7.01
–
6.95(m,3h),6.85(s,1h),6.70(d,j=8.0hz,2h),5.14(t,j=6.5hz,1h),5.05(d,j=15.0hz,1h),3.21
–
3.09(m,2h),2.89
–
2.74(m,3h),2.21(d,j=12.5hz,1h),1.84
–
1.59(m,6h),1.26
–
1.16(m,8h),1.16(s,3h),0.88(s,3h);
13
cnmr(125mhz,cdcl3)δ190.94,164.62,146.07,145.41,144.65,140.90,140.62,135.49,133.71,133.68,131.22,129.93,129.49,128.61,128.43,128.07,127.76,127.54,127.51,126.78,125.83,125.56,123.04,122.90,122.83,121.62,48.81,43.94,37.08,36.45,36.33,34.85,32.38,28.89,28.68,24.18,22.93,17.79,17.74,17.41;hrms(esi):m/zforc
42h45
no2na[m+na]
+
:618.3343,found:618.3315;ftir(kbr,cm-1
):3287.85,2357.01,1656.07,1636.45,1457.01;opt.rot.[α]
20
d=-307.6(c=0.054,chcl3);hplcdaicelchiralpakic-hcolumn,n-hexane/i-proh(90/10),1.0ml/min,254nm,28.443min(majorenantiomer),48.025min(minorenantiomer).
[0084]
实施例17:(2e,4z)-4-(2-甲酰基萘基-1-基)-5-苯基-2,4-戊二烯酸β-谷甾酯的制备
[0085][0086]
向螺旋盖小瓶中装入醋酸钯(3.4mg,0.015mmol),配体l3(5.9mg,0.045mmol),二氧化锰(13.1mg,0.15mmol),苯醌(10.9mg,0.1mmol),磷酸二苄酯(48.0mg,0.2mmol),三氟
乙醇(0.5ml)和二甲基亚砜(0.5ml)。然后依次向溶液中加入(z)-1-苯乙烯基-2-萘甲醛(25.9mg,0.1mmol)和丙烯酸β-谷甾酯(187.6mg,0.4mmol)。将小瓶在氧气下密封并加热至40℃并搅拌48小时。冷却后,将混合物直接应用于快速柱色谱(pe/ea),反应液经直接柱层析分离后得到白色液体(54.7mg,收率75%,ee值98%,m.p.=76.8℃)。1hnmr(500mhz,cdcl3)δ10.11(s,1h),8.08(d,j=8.5hz,1h),8.00(d,j=8.5hz,1h),7.96(s,1h),7.93(dd,j=10.5,7.0hz,2h),7.65
–
7.62(m,1h),7.50
–
7.46(m,2h),7.09(d,j=14.5hz,1h),7.01(d,j=15.5hz,2h),6.74(d,j=7.5hz,2h),5.36(d,j=5.0hz,1h),5.12(d,j=15.5hz,1h),4.66
–
4.59(m,1h),2.33
–
2.24(m,2h),2.01
–
1.94(m,2h),1.86
–
1.80(m,3h),1.48
–
0.79(m,36h),0.67(d,j=9.0hz,3h);
13
cnmr(125mhz,cdcl3)δ190.70,165.08,148.15,141.42,140.36,138.51,135.56,133.52,131.35,129.84,129.48,128.77,128.43,128.20,128.06,127.64,127.59,126.73,125.25,121.70,121.61,120.98,73.22,55.65,54.99,48.98,44.81,41.27,38.68,35.93,35.54,35.12,32.91,30.82,28.12,27.21,26.71,25.04,23.26,22.04,19.98,18.80,18.24,18.01,17.75,17.23,14.35,10.96,10.82;hrms(esi):m/zforc
51h64
o3na[m+na]
+
:747.4748,found:747.4703;ftir(kbr,cm-1
):3251.40,2357.1,1653.27,1633.64,1451.40;opt.rot.[α]
20
d=-111.9(c=0.36,chcl3);hplcdaicelchiralpakic-hcolumn,n-hexane/i-proh(95/5),1.0ml/min,254nm,14.550min(majorenantiomer),16.680min(minorenantiomer).
[0087]
应用实施例1.轴手性羧酸(cca)制备的通用方法:
[0088][0089]
在干燥的反应瓶中加入(2e,4z)-4-(2-甲酰基萘基-1-基)-5-(4-氟苯基)-2,4-戊二烯酸正丁酯(0.2mmol),t-buoh/thf/h2o(3ml,2:1:3)的混合溶剂,冷却到0℃,加入nah2po4(4.8mmol,24.0equiv)、2-甲基-2-丁烯(2.6mmol,13.0equiv),naclo2(0.74mmol,3.7equiv),升温到室温反应至反应完全。将有机溶液真空浓缩,得到橙色油状物,将其溶解在10mletoac中。有机溶液依次用hcl(2m,10ml
×
2)、水(10ml)和饱和食盐水(10ml)洗涤。然后将有机层用无水na2so4干燥并真空浓缩,得到粗羧酸产物。通过柱色谱法(pe/ea)纯化得到相应的羧酸产物(38.9mg,收率93%,ee值92%,黄色液体)。1hnmr(500mhz,cdcl3)δ8.18(d,j=8.7hz,1h),7.98(d,j=8.7hz,1h),7.95
–
7.81(m,3h),7.57(t,j=7.5hz,1h),7.42(t,j=7.7hz,1h),7.22(s,1h),6.71(dd,j=8.8,5.6hz,2h),6.65(t,j=8.7hz,2h),5.07(d,j=15.5hz,1h),4.06(t,j=6.7hz,2h),1.55(p,j=6.8hz,2h),1.32(dd,j=15.1,7.6hz,2h),0.87(t,j=7.4hz,3h);
13
cnmr(125mhz,cdcl3)δ170.33,166.37,161.28(d,j
cf
=248.6hz),148.33,137.15,137.04,134.76,134.64,134.63,130.70(d,j
cf
=3.7hz),130.06,130.00,129.68,127.71(d,j
cf
=7.5hz),127.27,126.59,125.75,125.44,118.27,114.42(d,j
cf
=21.3hz),63.33,29.60,18.08,12.65;ftir(kbr,cm-1
)3448.85,2960.44,2831.32,2716.53,2359.81,2343.55,1597.12,1364.17,1068.5,776.01,556.91,454.38;opt.rot.[α]
20
d=-60.2(c=0.54,chcl3);hplcdaicelchiralpakia
column,n-hexane/i-proh(60/40),1ml/min,254nm,7.390min(majorenantiomer),17.881min(minorenantiomer).
[0090][0091]
在干燥的反应瓶中加入(2e,4z)-4-(2-甲酰基萘基-1-基)-5-(2-甲基苯基)-2,4-戊二烯酸正丁酯(0.2mmol),t-buoh/thf/h2o(3ml,2:1:3)的混合溶剂,冷却到0℃,加入nah2po4(4.8mmol,24.0equiv)、2-甲基-2-丁烯(2.6mmol,13.0equiv),naclo2(0.74mmol,3.7equiv),升温到室温反应至反应完全。将有机溶液真空浓缩,得到橙色油状物,将其溶解在10mletoac中。有机溶液依次用hcl(2m,10ml
×
2)、水(10ml)和饱和食盐水(10ml)洗涤。然后将有机层用无水na2so4干燥并真空浓缩,得到粗羧酸产物。通过柱色谱法(pe/ea)纯化得到相应的羧酸产物(37.3mg,收率90%,ee值96%,黄色液体)。1hnmr(500mhz,cdcl3)δ8.07(d,j=8.7hz,1h),7.94(d,j=15.5hz,1h),7.92
–
7.85(m,3h),7.55(t,j=7.2hz,1h),7.46(d,j=7.4hz,2h),7.01(d,j=7.5hz,1h),6.91(t,j=7.3hz,1h),6.55(t,j=7.6hz,1h),6.37(d,j=7.8hz,1h),5.12(d,j=15.5hz,1h),4.08(t,j=6.6hz,2h),2.39(s,3h),1.57(dt,j=14.7,6.9hz,2h),1.33(dd,j=15.1,7.5hz,2h),0.88(t,j=7.4hz,3h);
13
cnmr(125mhz,cdcl3)δ170.70,166.45,148.29,137.12,137.04,136.07,135.51,134.46,133.36,130.35,128.99,127.39,127.33,127.24,127.01,126.54,126.31,126.09,125.78,125.47,124.36,118.32,63.32,29.64,18.93,18.10,12.68;ftir(kbr,cm-1
)2956.02,2925.70,2852.24,1700.81,1596.48,1366.13,1285.36,1163.94,744.50;opt.rot.[α]
20
d=-56.2(c=0.47,chcl3);hplcdaicelchiralpakad-hcolumn,n-hexane/i-proh(60/40),1ml/min,254nm,4.164min(majorenantiomer),5.654min(minorenantiomer).
[0092]
应用实施例2.cca在co
iii
催化1,4-吲哚和马来酰亚胺的对映选择性加成中的应用
[0093][0094]
向烘干的25mlschlenk管中加入n-5-甲基-嘧啶基吲哚10(0.20mmol,1.0equiv)、马来酰亚胺11(0.4mmol,2equiv)、cca(0.02mmol,10mol%)、[cp*co(mecn)3][sbf6]2(0.01mmol),活化的ms13x(40mg)。冷却至0℃,向混合物中加入t-buok的tfe溶液(0.1m,240μl,0.024mmol,12mol%)、tfe(560μl)和dcm(200μl),并在25℃下搅拌72小时。反应结束后将反应混合物通过硅胶柱色谱法(石油醚/乙酸乙酯=10/1至2/1)纯化,得到白色固体(45.5mg,收率71%,er值74:26)。1hnmr(500mhz,cdcl3)δ8.55(d,j=7.8hz,1h),8.43(s,2h),7.56(d,j=7.1hz,1h),7.31(t,j=7.0hz,1h),7.23(q,j=9.1,6.8hz,1h),6.67(s,1h),4.77(s,1h),3.17
–
3.05(m,4h),2.97
–
2.84(m,1h),2.30(s,3h);
13
cnmr
(125mhz,cdcl3)δ175.99,175.44,156.71,154.84,136.26,132.53,127.52,125.21,122.96,121.29,119.30,114.48,109.57,41.18,35.50,24.05,14.02;hrms(esi)forc
18h16
o2n4h[m+h]
+
:321.1346,found:321.1137;ftir2925.20,2828.58,1699.93,1591.69,1365.93,775.34;hplcdaicelchiralpakiacolumn,n-hexane/i-proh(80/20),1.0ml/min,daicelchiralpakiacolumn,n-hexane/i-proh(80/20),1.0ml/min,254nm,17.130min(majorenantiomer),37.385min(minorenantiomer).
[0095][0096]
向烘干的25mlschlenk管中加入n-5-甲基-嘧啶基吲哚10(0.20mmol,1.0equiv)、马来酰亚胺11(0.4mmol,2equiv)、cca(0.02mmol,10mol%)、[cp*co(mecn)3][sbf6]2(0.01mmol),活化的ms13x(40mg)。冷却至0℃,向混合物中加入t-buok的tfe溶液(0.1m,240μl,0.024mmol,12mol%)、tfe(560μl)和dcm(200μl),并在25℃下搅拌72小时。反应结束后将反应混合物通过硅胶柱色谱法(石油醚/乙酸乙酯=10/1至2/1)纯化,得到白色固体(26.3mg,收率41%,er值90:10)。1hnmr(500mhz,cdcl3)δ8.55(d,j=7.8hz,1h),8.43(s,2h),7.56(d,j=7.1hz,1h),7.31(t,j=7.0hz,1h),7.23(q,j=9.1,6.8hz,1h),6.67(s,1h),4.77(s,1h),3.17
–
3.05(m,4h),2.97
–
2.84(m,1h),2.30(s,3h);
13
cnmr(125mhz,cdcl3)δ175.99,175.44,156.71,154.84,136.26,132.53,127.52,125.21,122.96,121.29,119.30,114.48,109.57,41.18,35.50,24.05,14.02;hrms(esi)forc
18h16
o2n4h[m+h]
+
:321.1346,found:321.1137;ftir2925.20,2828.58,1699.93,1591.69,1365.93,775.34;hplcdaicelchiralpakiacolumn,n-hexane/i-proh(80/20),1.0ml/min,daicelchiralpakiacolumn,n-hexane/i-proh(80/20),1.0ml/min,254nm,17.130min(majorenantiomer),37.385min(minorenantiomer)。
相关技术
网友询问留言
已有0条留言
- 还没有人留言评论。精彩留言会获得点赞!
1