一种7位氟取代Isaindigotone衍生物及其制备方法和在制备抗癌药物中的应用与流程
本发明属于药物化学领域,更具体地,涉及一种7位氟取代Isaindigotone衍生物及其制备方法和在制备抗癌药物中的应用。
背景技术:
恶性肿瘤是危害人类健康的一大类疾病,据世界卫生组织(WHO)统计,全世界每年有800万人死于癌症,而中国每年有接近200万人死于癌症。尽管肿瘤的药物治疗取得了长足的进展,并成为当前临床治疗不可或缺的主要措施。但高毒副作用、耐药等问题仍然是临床肿瘤药物治疗面临的主要障碍。国际医学界将新的作用靶点的创新抗肿瘤药物研发和靶向治疗看作是改变肿瘤治疗现状的新的希望,也是新世纪抗肿瘤药物研究的主导方向。
癌基因c-myc是一个促进细胞增殖,调节糖酵解、脂质代谢等有关基因的转录因子,其N-端是转录激活区域,C-端是DNA结合区域,也参与调节谷氨酸盐代谢、线粒体的生物合成、细胞周期、HBP途径等。c-myc的表达产物c-Myc蛋白作为转录调控因子,可以激活(或抑制)大量下游基因的表达,这些下游基因大约占人类基因组基因总数的15%,从而广泛地参与了细胞内多项生理事件,因此c-myc的异常表达或c-Myc蛋白的调控失调,是大部分肿瘤(20%的各类人类肿瘤)的特征性标志。研究发现,c-myc在多种恶性肿瘤中都有异常的表达,比如宫颈癌、乳腺癌、结肠癌、小细胞肺癌、恶性胶质瘤、黑色素瘤、骨肉瘤、骨髓行白血病等。
c-myc的P1启动子中的富G序列Pu27为转录激活因子与DNA相互识别的结合区。这一区域DNA结构存在两种能够相互转换的形式。当其保持无序的单链状态时,能与各种结合因子如单链结合蛋白CNBP和hnRNP K结合,从而激活c-myc基因的转录活性使RNA多聚酶催化反应顺利进行。当这一序列形成特殊二级结构G-四链体后,各种因子的结合被抑制,G-四链体就作为负调控因子使得c-myc的转录水平下调。而功能蛋白NM23-H2具有拆散c-myc G-四链体的能力,调控着这两种DNA结构的平衡关系。
NM23-H2最早被证明是一种二磷酸核苷的磷酸激酶(nucleoside diphosphate kinase,NDPK),在维持磷酸化及非磷酸化核酸的平衡方面起着重要作用。NM23-H2/NDP kinase B,来自于一个蛋白家族,这个蛋白家族催化α-磷酸盐在核苷三磷酸和核苷二磷酸之间的转移。对于人类,8个不同的NDP激酶基因分别被命名为nm23-H1到nm23-H8。在人类细胞中,研究最多且亚型也最多的是NM23-H1和NM23-H2,他们分别由基因nm23-H1和nm23-H2编码。研究表明,在多株肿瘤细胞中,NM23-H2蛋白都有异常高表达,且过表达NM23-H2蛋白增加了肿瘤细胞的生长,同时提高原癌基因c-myc的表达,因此靶向NM23-H2蛋白的转录调控抑制剂的研究可能成为新型的肿瘤分子靶向治疗策略。
Isaindigotone是由中药板蓝根中提取得到的喹唑啉酮衍生物,其母体是由吡咯喹唑啉酮与共轭的苯亚甲基连接而成,属于非稠环芳香体系的小分子,目前研究报道其生理活性有抗菌,抗病毒和抗肿瘤。专利CN101250189A已公开了一种双脂肪氨基取代喹唑酮衍生物及其制备方法与作为抗癌药物的应用,专利CN101857595A已公开了一种喹唑酮衍生物及其制备方法和作为抗癌药物的用途,但目前具备靶向NM23-H2蛋白的上述Isaindigotone衍生物尤其少,因而急需开发一类新型的具备靶向NM23-H2蛋白的Isaindigotone衍生物。
技术实现要素:
本发明的目的是针对现有技术中的不足,提供一种7位氟取代Isaindigotone衍生物。
本发明还提供上述衍生物的制备方法以及在制备抗癌药物中的应用。
本发明通过以下的技术方案实现上述技术目的:
本发明提供了一种7位氟取代Isaindigotone衍生物,所述衍生物结构式如式(I)或式(II)所示:
其中:X为C或N;
R1为氢、胺基、取代胺基、五元或六元杂环基;
R2为氢、羟基、胺基、C1-8烷基、C1-8烷氧基或卤基;R3、R5和R6的定义与R2相同;
R4为氢、羟基、胺基、苯基、C1-8环烷基、C1-8烷基、C1-8烷氧基、C1-6烷基酰氧基、C1-6炔基、C1-6烯基、卤基、五元或六元杂环基;
所述R4中任意一个或多个氢被R’取代,R’独立地选自:
(1)C1-5烷基;
(2)卤基;
(3)苯基;
(4)醛基;
(5)炔基;
(6)五元或六元杂环基。
本发明在Isaindigotone结构基础上,在7位采用氟取代得到新型的7位氟取代Isaindigotone衍生物,该类衍生物具备靶向NM23-H2蛋白的抗肿瘤作用。
进一步地,本发明所提供的该类7位氟取代Isaindigotone衍生物能够与NM23‐H2蛋白结合,并阻断NM23‐H2蛋白与c‐myc G‐四链体DNA相互作用,进而导致原癌基因c‐myc转录和翻译的下调,抑制多株肿瘤细胞的增殖,具有广泛的抗肿瘤效果。
优选地,R1为氢、NH2、NH(CH2)nR7或含氮氧的五元或六元杂环基;R7为氢、胺基、N,N‐二甲氨基、N,N‐二乙氨基、吗啉基、四氢吡咯基、哌啶基,n为0~6中任意一个整数。
优选地,R2为氢、C1‐5烷基、C1‐5烷氧基、羟基、胺基或卤基。优选地,R4为氢、羟基、胺基、苯基、C1-5烷基、C1-5烷氧基、C1-3烷基酰氧基、C1-3炔基、C1-3烯基、卤基、五元或六元杂环基;
所述R4中任意一个或多个氢被R’取代,R’独立地选自:
(1)C1-5烷基;
(2)卤基;
(3)苯基;
(4)醛基;
(5)炔基;
(6)五元或六元含氮或含氮氧杂环基。
优选地,R4为氢、N,N-二甲基氨基、N,N-二乙基氨基、苄氧基、甲氧基、卤基、吗啉基、三氟甲基、异丙基、哌啶基、吡啶乙烯基、羟基、乙酰氧基、丙氧基、苯氧基、异丙基氧基、丙炔氧基或醛基。
本发明另外提供所述7位氟取代Isaindigotone衍生物,所述衍生物结构式如式(III)所示或式(IV)所示:
当结构式如式(III)所示时,X为N;
当结构式如式(IV)所示时,X为C;
当结构式如式(III)所示时,R1为NH(CH2)nR8,R8为氢、胺基、N,N-二甲氨基、N,N-二乙氨基、五元或六元含氮或含氮氧杂环基,n为0~6中任意一个整数;R4为氢、羟基、苯基、C1-5烷基、C1-5烷氧基或C1-3烯基;
所述R4中任意一个或多个氢被R”取代,R”独立地选自:
(1)C1-5烷基;
(2)苯基;
(3)五元或六元含氮或含氮氧杂环基;
当结构式如式(IV)所示时,R1为R2为羟基,R3为氢,R5为氢、R6为氢;R4为苄氧基。
优选地,当结构式如式(III)所示时,R1为R4为苄氧基、异丙氧基、苯氧基、丙氧基、吡啶乙烯基或异丙基苯基;R2为羟基或氢,R3为羟基或氢,R5为氢、R6为氢。
本发明另外提供所述的7位氟取代Isaindigotone衍生物的制备方法,当所述衍生物结构式如式(I)所示时,且当X为N时,包括如下步骤:
S1.2-氨基-4,5-二氟苯甲酸与2-吡咯烷酮反应得到化合物
S2.化合物与胺类化合物NH-R1反应得到中间体
S3.将S2中所得化合物与苯甲醛类化合物反应,得到目标衍生物。
本发明实施例9~54所述衍生物具体合成路线如下:
本发明另外提供所述的7位氟取代Isaindigotone衍生物的制备方法,当所述衍生物结构式如式(II)所示时,且当X为N时,包括如下步骤:
S1.2-氨基-4,5-二氟苯甲酸与环戊酮反应得到化合物
S2.化合物在钯碳条件下通过还原反应得到中间体
S3.化合物与胺类化合物NH-R1反应得到中间体
S4.将S3中所得化合物与苯甲醛类化合物反应,得到目标衍生物。
本发明实施例55中衍生物合成路线如下:
本发明所提供的实施例9~54中,化合物的具体合成步骤如下:
2-氨基-4,5-二氟苯甲酸与2-吡咯烷酮反应得到化合物化合物m1与不同的胺反应得到中间体
中间体m2、m3、m4、m5、m6、m7、m8再与含R2、R3、R4、R5、R6取代基的苯甲醛溶于DMF后,在三甲基氯硅烷的作用下发生取代反应,得到最终化合物。
中间体m2、m3、m4、m5、m6、m7、m8的制备方法,使用胺链既为反应物又为反应溶剂,中间体m1与胺链反应的摩尔比为1:5~10,反应温度为100℃,反应时间为12~48小时。
进一步地,终产物的制备方法,所述S3步骤中中间体m2、m3、m4、m5、m6、m7、m8再与含取代基的苯甲醛反应的摩尔比为1:(1.05~3.0),反应温度为50~120℃,反应时间为12~48小时。
本发明还提供了所述的衍生物在抑制肿瘤细胞增殖和抗肿瘤的药物中的应用,所述应用领域包含卵巢癌、宫颈癌、乳腺癌、肺腺癌、结肠癌、肝癌、白血病、小细胞肺癌、皮肤癌、上皮细胞癌、前列腺癌、非小细胞肺癌、鼻咽癌、恶性胶质瘤、淋巴瘤及黑色素瘤中的一种或多种。
进一步地,本发明所述药物还包括和其药学上可接受的盐、载体、立体异构体或其前药分子。
进一步地,本发明所述药物可以为注射剂、片剂、丸剂、胶囊剂、悬浮剂或乳剂。
本发明同时提供一种NM23-H2蛋白转录调控阻断剂,所述NM23-H2蛋白转录调控阻断剂含有所述7位氟取代Isaindigotone衍生物,其能够与NM23-H2蛋白结合,并阻断NM23-H2蛋白与c-myc G-四链体DNA相互作用,进而导致原癌基因c-myc转录和翻译的下调,抑制多株肿瘤细胞的增殖,具有广泛的抗肿瘤效果。
将本发明所提供的衍生物应用于抑制癌细胞实验,发现其具备显著的抑制效果,尤其是化合物3、11、16、21、22、24、25、26、40、43、44、46的抑制癌细胞株效果非常显著。
与现有技术相比,本发明具有如下优点和有益效果:
本发明涉及具有通式(I)或(II)结构特征的氟取代Isaindigotone衍生物可以有效抑制多种肿瘤细胞的生长,并与转录因子NM23-H2蛋白有较强的选择性,结合能力强,能够显著抑制原癌基因c-myc的转录和表达,而对正常细胞毒性较小,在制备抗肿瘤药物上有着广阔的应用空间。
附图说明
图1为本发明提供的7位氟取代Isaindigotone衍生物对NM23-H2蛋白活性抑制的影响图。
图2为本发明提供的7位氟取代Isaindigotone衍生物对NM23-H2和c-myc转录水平的影响图。
图3为本发明提供的7位氟取代Isaindigotone衍生物对NM23-H2和C-MYC翻译水平的影响图。
图4为本发明提供的7位氟取代Isaindigotone衍生物对小鼠移植瘤生长抑制的影响图。
具体实施方式
下面结合具体实施例和附图进一步详细说明本发明。除非特别说明,本发明采用的试剂、设备和方法为本技术领域常规市购的试剂、设备和常规使用的方法。
实施例1:化合物m1的合成
将2-氨基-4,5-二氟苯甲酸(5g,29mmol),2-吡咯烷酮(5mL)至于100mL单颈烧瓶中,于冰浴搅拌下逐滴加入20mL三氯氧磷(POCl3),使其30min滴完,103℃加热回流24h,将反应体系逐滴加入200mL冰水中,用浓NaOH溶液调节pH至弱碱性,抽滤,得到粗产物,硅胶柱层析纯化(洗脱剂:V(石油醚):V(乙酸乙酯)=1:1)得到化合物m1,5.4g,产率85%。
1H NMR(400MHz,CDCl3)δ8.20–7.85(m,1H),7.60–7.34(m,1H),4.42–4.06(m,2H),3.19(td,J=7.9,2.7Hz,2H),2.43–2.16(m,2H).LC-MS m/z:223[M+H]+.
实施例2:化合物m2的合成
取化合物m1(2.22g,10mmol),二乙胺基丙胺(3ml,24mmol),于厚壁耐压瓶中,100℃加热搅拌过夜,得到橙黄色溶液,反应体系降至室温后,有大量淡黄色沉淀析出,像反应体系中加入20ml乙醚,使固体进一步析出,抽滤,得到滤饼为乳白色固体,抽真空干燥,得到乳白色粉末2.3g,产率69%。
1H NMR(400MHz,CDCl3)δ7.72(d,J=11.7Hz,1H),6.72(s,1H),6.67(d,J=7.7Hz,1H),4.19–4.12(m,2H),3.31(dd,J=10.6,5.8Hz,2H),3.11(t,J=7.9Hz,2H),2.63–2.59(m,2H),2.55(q,J=7.1Hz,4H),2.30–2.19(m,2H),1.89–1.81(m,2H),1.06(t,J=7.1Hz,6H).
实施例3:化合物m3的合成
按照m2的合成方法,得2.1g淡黄色固体,产率66%。1H NMR(400MHz,CDCl3)δ7.67(d,J=11.7Hz,1H),6.63(d,J=7.7Hz,1H),4.12–4.05(m,2H),3.24(dd,J=11.6,6.3Hz,2H),3.04(t,J=7.9Hz,2H),2.37(t,J=6.4Hz,4H),2.19(s,6H),1.77(dt,J=12.8,6.2Hz,2H).
实施例4:化合物m4的合成
按照m2的合成方法,得1.8g乳白色固体,产率52%。1H NMR(400MHz,CDCl3)δ7.67(d,J=11.7Hz,1H),6.62(d,J=7.7Hz,1H),4.13–4.05(m,2H),3.72–3.67(m,2H),3.66–3.61(m,2H),3.25(dd,J=11.1,5.8Hz,2H),3.04(t,J=7.9Hz,4H),2.51–2.46(m,3H),2.24–2.12(m,4H),1.86–1.75(m,2H).
实施例5:化合物m5的合成
按照m2的合成方法,得1.7g乳白色固体,产率58%。1H NMR(400MHz,CDCl3)δ7.71(d,J=11.5Hz,1H),6.61(d,J=7.4Hz,1H),4.22–4.01(m,2H),3.44(dd,J=12.6,6.5Hz,2H),3.14(t,J=7.8Hz,2H),2.47(t,J=6.5Hz,4H),2.18(s,6H).
实施例6:化合物m6的合成
按照m2的合成方法,得2.4g乳白色固体,产率73%。1H NMR(400MHz,CDCl3)δ7.75(dd,J=11.6,4.6Hz,1H),6.71(d,J=7.5Hz,1H),5.32(s,1H),4.16(t,J=7.0Hz,2H),3.33–3.20(m,2H),3.12(dd,J=9.6,5.9Hz,2H),2.64(t,J=5.6Hz,2H),2.42(s,4H),2.30–2.21(m,2H),1.59(d,J=4.8Hz,4H),1.46(d,J=3.3Hz,2H).
实施例7:化合物m7的合成
按照m2的合成方法,得1.9g淡黄色固体,产率57%。1H NMR(400MHz,CDCl3)δ7.74(d,J=11.7Hz,1H),6.70(d,J=7.7Hz,1H),4.22–4.12(m,2H),3.33(dd,J=11.1,6.0Hz,2H),3.13(t,J=7.9Hz,2H),2.68(t,J=6.2Hz,2H),2.56(t,J=5.9Hz,5H),2.31–2.22(m,2H),1.90(dt,J=12.4,6.2Hz,2H),1.85–1.80(m,4H).
实施例8:化合物m8的合成
按照m2的合成方法,得1.9g淡黄色固体,产率66%。1H NMR(400MHz,CDCl3)δ7.84–7.72(m,1H),7.03(d,J=7.8Hz,1H),4.24–4.13(m,2H),3.95–3.83(m,4H),3.27–3.19(m,4H),3.14(t,J=8.0Hz,2H),2.34–2.22(m,2H).
实施例9:化合物1的合成
将化合物m2(0.3323g,1mmol)与4-二乙氨基水杨醛(0.2122g,1.1mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热过夜,TLC监测反应结束后,抽滤,粗产品用甲醇/二氯甲烷重结晶,得到淡黄色固体0.2380g,产率47%。
1H NMR(400MHz,CDCl3)δ8.68(s,1H),7.76(d,J=11.7Hz,1H),7.35(d,J=9.0Hz,1H),6.92(d,J=7.6Hz,1H),6.17(d,J=8.9Hz,1H),5.98(d,J=2.3Hz,1H),4.27–4.22(m,2H),3.34–3.25(m,2H),3.22–3.15(m,2H),3.10–3.01(m,4H),2.65–2.49(m,6H),1.89–1.75(m,2H),1.06(t,J=6.9Hz,6H),0.91(t,J=6.9Hz,6H).13C NMR(101MHz,CDCl3)δ160.56,159.14,149.47(t,J=117.9Hz),148.06,143.58(d,J=28.1Hz),140.89,130.11,128.19,127.41,121.81,112.11,109.90(d,J=21.3Hz),103.83(d,J=10.9Hz),98.59,52.85,46.86,44.38,44.30,43.77,29.71,25.52,24.82,12.48,11.62.ESI-HRMS[M]+m/z=508.3082,calcd for C29H38N5O2F,508.3102.Purity:95.92%by HPLC.
实施例10:化合物2的合成
将化合物m2(0.3323g,1mmol)与4-二乙氨基苯甲醛(0.1947g,1.1mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热过夜,TLC监测反应结束后,抽滤,粗产品用甲醇/二氯甲烷重结晶,得到淡黄色固体0.3568g,产率72%。
1H NMR(400MHz,CDCl3)δ7.74(dd,1H),7.71–7.62(m,2H),7.43(dd,1H),7.17–7.10(m,1H),6.75(d,J=7.5Hz,1H),6.69(dd,2H),6.40–6.31(m,1H),4.27–4.16(m,2H),3.51–3.34(m,6H),3.29–3.15(m,4H),2.80–2.65(m,5H),2.04–1.92(m,2H),1.27–1.17(m,7H),1.17–1.08(m,6H).13C NMR(101MHz,CDCl3)δ160.64,156.26,150.35(d,J=237.5Hz),149.05,147.97,143.08(d,J=12.9Hz),131.74,130.29,125.56,122.85,109.55(dd,J=19.4,3.3Hz),109.23(d,J=7.5Hz),105.41(d,J=3.7Hz),58.06,53.79,44.39,43.90,43.38,25.55,24.15,13.22,12.64.ESI-HRMS[M+H]+m/z=492.3060,calcd for C32H33N4O4F,492.3133.Purity:95.150%by HPLC.
实施例10:化合物3的合成
将化合物m2(0.3323g,1mmol)与4-苯甲氧基-2-羟基苯甲醛(0.25107g,1.1mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热过夜,TLC监测反应结束后,抽滤,粗产品用甲醇/二氯甲烷重结晶,得到淡黄色固体0.3744g,产率69%。
1H NMR(400MHz,DMSO)δ7.96(t,J=2.6Hz,1H),7.55(d,J=11.9Hz,1H),7.47–7.44(m,2H),7.43–7.38(m,2H),7.35(dt,J=5.3,2.1Hz,1H),7.30(s,1H),6.87–6.82(m,1H),6.76(d,J=8.0Hz,1H),6.58(s,1H),5.11(s,2H),4.10–4.05(m,2H),3.29–3.24(m,4H),3.18–3.12(m,2H),2.49–2.45(m,4H),1.75(dq,J=13.1,6.4Hz,2H),1.05–0.91(m,6H).13C NMR(101MHz,DMSO)δ159.97,159.34,157.92,155.86,148.54(d,J=21.9Hz),142.66,142.55(dd,J=12.1,8.2Hz),142.45,136.81,129.67,128.42,128.32,127.85,127.62,122.93,115.94,108.91(d,J=24.6Hz),108.14(d,J=6.6Hz),106.17,105.21,101.98,69.15,50.39,46.28,43.70,41.10,24.98,24.37,10.82.ESI-HRMS[M+H]+m/z=543.2693,calcd for C32H35N4O3F,543.2768.Purity:99.689%by HPLC.
实施例11:化合物4的合成
将化合物m2(0.3323g,1mmol)与4-甲氧基苯甲醛(0.1496g,1.1mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热48h,TLC监测反应结束后,抽滤,粗产品用甲醇/二氯甲烷重结晶,得到淡黄色固体,0.2021g,产率45%。
1H NMR(400MHz,DMSO)δ8.06(s,1H),7.65–7.57(m,J=10.4Hz,2H),7.42(t,J=7.1Hz,1H),7.14(d,J=8.0Hz,1H),7.07(t,J=7.5Hz,1H),6.99(d,J=7.9Hz,1H),4.15–4.07(m,2H),3.89(s,3H),3.36(t,J=6.6Hz,2H),3.23–3.18(m,2H),3.12(qd,J=12.2,6.0Hz,6H),2.06–1.96(m,2H),1.23(t,J=7.2Hz,6H).13C NMR(101MHz,CDCl3)δ160.56,158.01,153.60(d,J=337.6Hz),149.50,148.85,143.30(d,J=14.0Hz),132.02,130.12,128.99,124.76,124.67,120.33,110.85,109.60(d,J=20.8Hz),109.34(d,J=7.7Hz),105.78,105.74,55.52,52.76,46.89,43.82,43.64,25.71,25.28,11.70.ESI-HRMS[M]+m/z=451.2431,calcd for C26H31N4O2F,451.2512.Purity:98.179%by HPLC.
实施例12:化合物5的合成
将化合物m2(0.3323g,1mmol)与3,4-二甲氧基苯甲醛(0.1793g,1.08mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热48h,TLC监测反应结束后,抽滤,粗产品用甲醇/二氯甲烷重结晶,得到淡黄色固体,0.2643g,产率55%。
1H NMR(400MHz,CDCl3)δ7.78(d,J=11.7Hz,1H),7.74(t,J=2.7Hz,1H),7.11(d,J=1.9Hz,1H),7.04(d,J=1.9Hz,1H),6.97(s,1H),6.80(s,1H),6.65(s,1H),4.31–4.25(m,2H),3.96(d,J=1.6Hz,6H),3.51–3.34(m,4H),2.72–2.58(m,6H),1.97–1.89(m,2H),1.12(dd,J=12.6,6.8Hz,6H).13C NMR(101MHz,CDCl3)δ160.57(d,J=3.6Hz),155.51(d,J=2.1Hz),150.28(t,J=123.1Hz),149.74,148.95,148.78,143.26(d,J=12.9Hz),129.69,129.59,128.75,123.07,112.77,111.23,109.76(d,J=21.4Hz),109.41(d,J=7.5Hz),105.55(d,J=3.7Hz),58.31,55.98,55.91,45.41,43.92,42.81,25.77,25.52,11.49.ESI-HRMS[M+H]+m/z=481.2537,calcd for C27H33N4O3F,481.2602.Purity:98.461%by HPLC.
实施例13:化合物6的合成
将化合物m2(0.3323g,1mmol)与3,4,5-三甲氧基苯甲醛(0.2058g,1.05mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热48h,TLC监测反应结束后,抽滤,粗产品用甲醇/二氯甲烷重结晶,得到淡黄色固体,0.4539g,产率89%。
1H NMR(400MHz,CDCl3)δ7.76(d,J=11.6Hz,1H),7.69(t,J=2.6Hz,1H),6.79(s,2H),6.76(d,J=7.7Hz,1H),4.28–4.23(m,2H),3.91(d,J=1.8Hz,9H),3.35(t,J=5.8Hz,2H),3.31–3.24(m,2H),2.65(t,J=6.0Hz,2H),2.59(q,J=7.1Hz,4H),1.89(dt,J=12.0,6.0Hz,2H),1.08(t,J=7.1Hz,6H).13C NMR(101MHz,CDCl3)δ160.54,155.17,153.30,150.72(d,J=241.9Hz),148.72,143.39(d,J=14.0Hz),138.91,131.22,130.96,129.79,109.69(d,J=20.9Hz),109.28(d,J=7.6Hz),107.12,105.36,61.00,56.17,52.56,46.79,43.90,43.47,25.47,25.00,11.45.ESI-HRMS[M]+m/z=511.2642,calcd for C28H35N4O4F,511.2718.Purity:97.855%by HPLC.
实施例14:化合物7的合成
将化合物m2(0.3323g,1mmol)与对氟苯甲醛(0.1364,1.1mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热48h,TLC监测反应结束后,抽滤,粗产品用硅胶柱层析纯化(洗脱剂:V(乙酸乙酯):V(甲醇):V(氨水)=10:1:0.1),得到淡黄色固体,0.2674g,产率61%。
1H NMR(400MHz,CDCl3)δ7.78–7.71(m,2H),7.52(dd,J=8.5,5.5Hz,2H),7.13(dd,J=15.8,7.2Hz,2H),6.76(d,J=7.7Hz,1H),6.71(s,1H),4.23(t,2H),3.34(d,J=4.6Hz,2H),3.22(dd,J=9.6,4.3Hz,2H),2.65–2.59(m,2H),2.55(q,J=7.1Hz,4H),1.86(dt,J=11.8,5.9Hz,2H),1.06(t,J=7.1Hz,6H).13C NMR(101MHz,CDCl3)δ163.83,161.38,157.75(d,J=544.8Hz),152.06,149.58,148.69,143.44(d,J=17.4Hz),131.94,131.91,131.62,131.60,131.47,131.39,131.35,128.45,116.04,115.82,109.69(d,J=20.9Hz),109.28(d,J=7.7Hz),105.46,52.76,46.86,43.85,43.66,25.49,25.14,11.63.ESI-HRMS[M+Na]+m/z=461.2231,calcd for C25H28N4OF2,461.2353.Purity:97.564%by HPLC.
实施例15:化合物8的合成
将化合物m2(0.3323g,1mmol)与2-甲氧基苯甲醛(0.1496g,1.1mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热48h,TLC监测反应结束后,抽滤,粗产品用硅胶柱层析纯化(洗脱剂:V(乙酸乙酯):V(甲醇):V(氨水)=10:1:0.1),得到淡黄色固体,0.3061g,产率68%。
1H NMR(400MHz,CDCl3)δ8.11(t,J=2.5Hz,1H),7.75(dd,J=11.7,3.2Hz,1H),7.47(d,J=7.6Hz,1H),7.34(dd,J=11.3,4.4Hz,1H),7.00(dd,J=9.5,5.4Hz,1H),6.95(d,J=8.3Hz,1H),6.81(dd,J=7.6,2.4Hz,1H),6.66(s,1H),4.20(dd,J=12.4,5.2Hz,2H),3.91(s,3H),3.38–3.31(m,2H),3.21(td,J=7.2,2.7Hz,2H),2.66–2.60(m,2H),2.60–2.52(m,4H),1.92–1.82(m,2H),1.07(td,J=7.0,3.6Hz,6H).13C NMR(101MHz,CDCl3)δ160.59,158.03,153.63(d,J=338.9Hz),149.52,148.86,143.33(d,J=14.1Hz),132.03,130.16,129.01,124.69,120.35,110.84,109.62(d,J=20.8Hz),109.34(d,J=7.6Hz),105.73,55.53,52.78,43.85,43.66,25.73,25.21,11.69.ESI-HRMS[M]+m/z=451.2431,calcd for C26H31N4O2F,451.2515.Purity:97.766%by HPLC.
实施例16:化合物9的合成
将化合物m2(0.3323g,1mmol)与4-(4-吗啉)苯甲醛(0.2101g,1.1mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热48h,TLC监测反应结束后,抽滤,粗产品用硅胶柱层析纯化(洗脱剂:V(乙酸乙酯):V(甲醇):V(氨水)=10:1:0.1),得到淡黄色固体,0.3840g,产率76%。
1H NMR(400MHz,CDCl3)δ7.75(d,J=11.7Hz,1H),7.69(s,1H),7.49(d,J=8.5Hz,2H),6.93(d,J=8.2Hz,2H),6.76(d,J=7.7Hz,1H),4.23(t,J=6.9Hz,2H),3.89–3.84(m,4H),3.39–3.32(m,2H),3.28–3.21(m,6H),2.69–2.52(m,6H),1.93–1.83(m,2H),1.08(t,J=6.9Hz,6H).13C NMR(101MHz,CDCl3)δ160.55,155.71,151.08,150.75(d,J=121.7Hz),148.91,143.34(d,J=14.0Hz),131.16,129.47,128.40,126.89,114.74,109.63(d,J=20.7Hz),109.15(d,J=7.6Hz),105.36(d,J=4.1Hz),66.69,52.79,48.15,46.86,43.87,43.68,25.58,25.18,11.68.ESI-HRMS[M]+m/z=506.2853,calcd for C29H36N5O2F,506.2919.Purity:99.142%by HPLC.
实施例17:化合物10的合成
将化合物m2(0.2658g,0.80mmol)与对三氟甲基苯甲醛(0.123μL,0.90mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热48h,TLC监测反应结束后,抽滤,粗产品用硅胶柱层析纯化(洗脱剂:V(乙酸乙酯):V(甲醇):V(氨水)=10:1:0.1),得到淡黄色固体,0.3201g,产率82%。
1H NMR(400MHz,CDCl3)δ7.78(d,J=2.7Hz,1H),7.76(d,J=11.7Hz,1H),7.69(d,J=8.3Hz,2H),7.64(d,J=8.3Hz,2H),6.86(s,1H),6.78(d,J=7.7Hz,1H),4.27(t,J=7.1Hz,2H),3.36(dd,J=10.7,5.4Hz,2H),3.29(dd,J=9.7,4.2Hz,2H),2.64(t,J=5.5Hz,2H),2.58(dd,J=13.8,6.8Hz,4H),1.92–1.84(m,2H),1.08(t,J=7.1Hz,6H).13C NMR(101MHz,CDCl3)δ160.27(d,J=3.3Hz),154.63,152.07,149.64,148.52,143.36(d,J=13.9Hz),138.92,134.73,131.12–129.84(m),129.64,127.82,125.71(d,J=3.7Hz),123.92(d,J=272.1Hz),109.74(d,J=20.9Hz),105.58(d,J=4.0Hz),66.87,58.12,53.77,43.87,43.48,25.63,23.91.ESI-HRMS[M]+m/z=489.2199,calcd for C26H28N4OF4,489.2283.Purity:99.365%by HPLC.
实施例18:化合物11的合成
将化合物m2(0.2658g,0.80mmol)与4-异丙基苯甲醛(0.133μL,0.90mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热48h,TLC监测反应结束后,抽滤,粗产品用硅胶柱层析纯化(洗脱剂:V(乙酸乙酯):V(甲醇):V(氨水)=10:1:0.1),得到淡黄色固体,0.2738g,产率74%。
1H NMR(400MHz,CDCl3)δ7.79–7.72(m,2H),7.50(d,J=7.5Hz,2H),7.30(d,J=7.7Hz,2H),6.78(d,J=7.5Hz,1H),6.72(s,1H),4.24(t,J=6.7Hz,2H),3.40–3.32(m,2H),3.31–3.22(m,2H),2.67–2.54(m,6H),1.94–1.82(m,2H),1.28(d,J=6.7Hz,6H),1.08(t,J=6.8Hz,6H).13C NMR(101MHz,CDCl3)δ160.49(d,J=3.5Hz),155.32,150.69(d,J=87.8Hz),149.83,148.77,143.36(d,J=14.0Hz),133.31,131.00,129.80,129.62,126.88,109.61(d,J=20.8Hz),109.26(d,J=7.7Hz),105.47(d,J=4.2Hz),52.80,46.88,43.86,43.68,34.00,25.59,25.25,23.78,11.71.ESI-HRMS[M]+m/z=463.2795,calcd for C28H35N4OF,463.2881.Purity:99.113%by HPLC.
实施例19:化合物12的合成
将化合物m2(0.3323g,1mmol)与4-哌啶-1-基-苯甲醛(0.2081g,1.1mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热48h,TLC监测反应结束后,抽滤,粗产品用硅胶柱层析纯化(洗脱剂:V(乙酸乙酯):V(甲醇):V(氨水)=10:1:0.1),得到淡黄色固体,0.4281g,产率85%。
1H NMR(400MHz,CDCl3)δ7.72(s,1H),7.68(s,1H),7.45(d,J=8.4Hz,2H),6.92(d,J=8.4Hz,2H),6.76(d,J=7.7Hz,1H),6.59(s,1H),4.22(t,J=7.0Hz,2H),3.37–3.18(m,8H),2.65–2.60(m,2H),2.56(dd,J=13.9,6.9Hz,4H),1.91–1.82(m,2H),1.70(s,4H),1.63(d,J=4.2Hz,2H),1.06(t,J=7.0Hz,6H).13C NMR(101MHz,CDCl3)δ160.68(d,J=3.3Hz),155.94,151.76,150.92(t,J=121.6Hz),148.99,143.32(d,J=13.9Hz),131.27,129.92,127.37,125.58,115.03,109.65(d,J=20.8Hz),109.14(d,J=7.6Hz),105.37(d,J=4.0Hz),52.74,49.23,46.88,43.91,43.63,25.62,25.53,25.20,24.35,11.65.ESI-HRMS[M+H]+m/z=504.3060,calcd for C30H38N5OF,504.3139.Purity:99.019%by HPLC.
实施例20:化合物13的合成
将化合物m2(0.3323g,1mmol)与(E)-4-(2-(pyridin-2-yl)vinyl)benzaldehyde(0.2301g,1.1mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热48h,TLC监测反应结束后,抽滤,粗产品用硅胶柱层析纯化(洗脱剂:V(乙酸乙酯):V(甲醇):V(氨水)=10:1:0.1),得到淡黄色固体,0.3298g,产率63%。
1H NMR(400MHz,MeOD)δ8.41(d,J=4.7Hz,1H),7.72–7.63(m,2H),7.57(t,J=8.6Hz,3H),7.54–7.46(m,4H),7.16(dd,J=17.0,11.0Hz,2H),6.76(d,J=7.9Hz,1H),4.12(t,J=7.0Hz,2H),3.26(t,J=6.9Hz,2H),2.52(dt,J=21.8,7.1Hz,6H),1.84–1.75(m,2H),1.26–1.16(m,2H),0.98(t,J=7.1Hz,6H).13C NMR(101MHz,MeOD)δ160.97,155.66,155.58,150.81(d,J=244.0Hz),148.89,148.83,143.79–143.56(m),137.25,137.03,135.80,132.38,132.25,129.92,129.22,128.42,127.15,126.28,122.24,121.94,108.85(d,J=21.3Hz),108.63(d,J=5.0Hz),105.09(d,J=4.3Hz),99.99,51.06,46.70,43.93,41.74,25.30,25.21,10.30.ESI-HRMS[M+H]+m/z=524.2747,calcd for C32H34N5OF,524.2821.Purity:99.666%by HPLC.
实施例21:化合物14的合成
将化合物m2(0.3323g,1mmol)与4-苯甲氧基苯甲醛(0.2333g,1.1mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热48h,TLC监测反应结束后,抽滤,粗产品用硅胶柱层析纯化(洗脱剂:V(乙酸乙酯):V(甲醇):V(氨水)=10:1:0.1),得到淡黄色固体,0.4470g,产率85%。
1H NMR(400MHz,CDCl3)δ7.70(d,J=11.7Hz,1H),7.67–7.64(m,1H),7.44(d,J=8.8Hz,2H),7.41(t,J=7.2Hz,2H),7.37(t,J=7.3Hz,2H),7.31(dd,J=8.2,5.8Hz,1H),6.98(d,J=8.7Hz,2H),6.73(d,J=7.7Hz,1H),6.54(s,1H),5.07(s,2H),4.22–4.09(m,2H),3.31(dd,J=10.5,5.6Hz,2H),3.12(t,J=5.9Hz,2H),2.61–2.56(m,2H),2.53(q,J=7.1Hz,4H),1.83(dd,J=11.7,5.8Hz,2H),1.04(t,J=7.1Hz,6H).13C NMR(101MHz,CDCl3)δ160.49,159.18,155.43,150.64(d,J=243.8Hz),148.81,143.35(d,J=14.0Hz),136.56,131.30,129.53,129.21,128.74,128.64,128.11,127.43,115.18,109.62(d,J=20.8Hz),109.22(d,J=7.7Hz),105.43(d,J=4.0Hz),70.07,52.76,46.88,43.85,43.64,29.69,25.51,25.23,11.68.ESI-HRMS[M]+m/z=527.2744,calcd for C32H35N4O2F,527.2823.Purity:97.562%by HPLC.
实施例22:化合物15的合成
将化合物m2(0.3323g,1mmol)与4-苯甲氧基-3-羟基苯甲醛(0.2336g,1.1mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热48h,TLC监测反应结束后,抽滤,粗产品用硅胶柱层析纯化(洗脱剂:V(乙酸乙酯):V(甲醇):V(氨水)=10:1:0.1),得到淡黄色固体,0.3418g,产率63%。
1H NMR(400MHz,CDCl3)δ7.74(d,J=11.7Hz,1H),7.66(s,1H),7.46–7.34(m,5H),7.17(d,J=1.0Hz,1H),7.04(d,J=8.4Hz,1H),6.96(d,J=8.4Hz,1H),6.76(d,J=7.7Hz,1H),6.57(s,1H),5.14(s,2H),4.20(t,J=7.2Hz,2H),3.34(s,2H),3.21(t,J=6.0Hz,2H),2.63(t,J=5.9Hz,2H),2.58(dd,J=14.2,7.1Hz,4H),1.92–1.82(m,2H),1.07(t,J=7.1Hz,6H).13C NMR(101MHz,CDCl3)δ160.59(d,J=3.3Hz),155.48,150.69(d,J=244.0Hz),148.80,146.51,145.99,143.36(d,J=14.0Hz),135.91,130.06,129.65,129.53,128.80,128.57,127.84,123.00,115.55,112.17,109.69(d,J=20.6Hz),109.27(d,J=7.6Hz),105.43(d,J=3.9Hz),71.15,52.59,46.83,43.92,43.49,25.52,25.10,11.51.ESI-HRMS[M]+m/z=543.2693,calcd for C32H35N4O3F,543.2771.Purity:99.069%by HPLC.
实施例23:化合物16的合成
将化合物m3(0.3042g,1mmol)与4-苯甲氧基-2-羟基苯甲醛(0.2336g,1.1mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热48h,TLC监测反应结束后,抽滤,粗产品用硅胶柱层析纯化(洗脱剂:V(乙酸乙酯):V(甲醇):V(氨水)=10:1:0.1),得到淡黄色固体,0.3805g,产率74%。
1H NMR(400MHz,DMSO)δ7.97(s,1H),7.56(d,J=11.7Hz,1H),7.50–7.29(m,6H),6.86(d,J=7.7Hz,1H),6.64(d,J=10.9Hz,1H),6.58(d,J=8.5Hz,1H),5.10(s,2H),4.12–4.03(m,2H),3.34(d,J=5.9Hz,2H),3.14(m,4H),2.75(s,6H),2.06–1.95(m,2H).13C NMR(101MHz,DMSO)δ160.47,159.85(d,J=3.0Hz),158.49,153.87(d,J=517.8Hz),148.89,142.75(d,J=13.6Hz),137.30,130.17,128.95,128.75,128.38,128.16,123.42,116.40,109.60(d,J=20.1Hz),108.89(d,J=7.2Hz),106.68,105.88(d,J=3.6Hz),102.50,69.63,54.97,44.25,42.51,25.47,23.55.ESI-HRMS[M+H]+m/z=515.2380,calcd for C30H31N4O3F,515.2456.Purity:98.868%by HPLC.
实施例24:化合物17的合成
将化合物m3(0.3042g,1mmol)与3,4-二羟基苯甲醛(0.1519g,1.1mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热48h,TLC监测反应结束后,抽滤,粗产品用硅胶柱层析纯化(洗脱剂:V(乙酸乙酯):V(甲醇):V(氨水)=10:1:0.1),得到淡黄色固体,0.2844g,产率67%。
1H NMR(400MHz,DMSO)δ7.58(d,J=11.8Hz,1H),7.49(s,1H),7.09(s,1H),6.94(d,J=8.0Hz,1H),6.84(dd,J=11.3,8.2Hz,2H),6.61(s,1H),4.16–4.08(m,2H),3.14(dd,J=14.9,7.7Hz,4H),2.74(s,6H),2.06–1.95(m,2H),1.42–1.21(m,2H).13C NMR(101MHz,CDCl3)δ160.57(d,J=3.4Hz),155.51(d,J=1.9Hz),150.28(d,J=121.8Hz),149.74,148.95,148.78,143.26(d,J=13.8Hz),129.69,129.59,128.75,123.07,112.77,111.23,109.76(d,J=20.8Hz),109.41(d,J=7.6Hz),105.55(d,J=3.8Hz),58.31,45.41,43.92,42.81,25.77,25.52.ESI-HRMS[M+H]+m/z=425.1911,calcd for C23H25N4O3F,425.1983.Purity:97.171%by HPLC.
实施例25:化合物18的合成
将化合物m3(0.3042g,1mmol)与丁香醛(0.1519g,1.1mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热48h,TLC监测反应结束后,抽滤,粗产品用硅胶柱层析纯化(洗脱剂:V(乙酸乙酯):V(甲醇):V(氨水)=10:1:0.1),得到淡黄色固体,0.2844g,产率67%。
1H NMR(400MHz,MeOD)δ7.63(dd,J=19.7,12.3Hz,2H),6.86(d,J=14.2Hz,3H),4.26–4.15(m,2H),3.92(d,J=5.2Hz,6H),3.45(dd,J=12.3,6.0Hz,2H),3.31–3.19(m,4H),2.96(s,6H),2.24–2.12(m,2H).13C NMR(101MHz,MeOD)δ160.86,156.21,154.00,150.57(d,J=242.7Hz),148.73,148.27,142.96(d,J=14.5Hz),137.71,130.51,129.01,126.68,109.23(d,J=7.9Hz),109.10(d,J=5.4Hz),105.28(d,J=2.9Hz),55.89,55.80,44.00,42.40,39.56,24.99,23.70,16.87.ESI-HRMS[M+H]+m/z=469.2173,calcd for C25H29N4O4F,469.2242.Purity:99.708%by HPLC.
实施例26:化合物19的合成
将化合物m3(0.3042g,1mmol)与3,4-二甲氧基苯甲醛(0.1793g,1.08mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热48h,TLC监测反应结束后,抽滤,粗产品用硅胶柱层析纯化(洗脱剂:V(乙酸乙酯):V(甲醇):V(氨水)=10:1:0.1),得到淡黄色固体,0.3484g,产率77%。
1H NMR(400MHz,CDCl3)δ7.76(d,J=11.7Hz,1H),7.70(t,J=2.5Hz,1H),7.17(dd,J=8.4,1.5Hz,1H),7.08(d,J=1.5Hz,1H),6.94(d,J=8.4Hz,1H),6.79(d,J=7.7Hz,1H),5.96(s,1H),4.28–4.21(m,2H),3.93(d,J=2.9Hz,6H),3.35(dd,J=11.5,6.1Hz,2H),3.29–3.22(m,2H),2.49(t,J=6.3Hz,2H),2.30(s,6H),1.88(p,J=6.3Hz,2H).13C NMR(101MHz,CDCl3)δ160.57(d,J=3.4Hz),155.51(d,J=1.9Hz),150.28(t,J=121.8Hz),149.74,148.95,148.78,143.26(d,J=13.8Hz),129.69,129.59,128.75,123.07,112.77,111.23,109.76(d,J=20.8Hz),109.41(d,J=7.6Hz),105.55(d,J=3.8Hz),58.31,55.98,55.91,45.41,43.92,42.81,25.77,25.52.ESI-HRMS[M+H]+m/z=453.2224,calcd for C25H29N4O3F,453.2292.Purity:99.609%by HPLC.
实施例27:化合物20的合成
将化合物m3(0.3042g,1mmol)与3,4,5-三甲氧基苯甲醛(0.2158g,1.1mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热48h,TLC监测反应结束后,抽滤,粗产品用硅胶柱层析纯化(洗脱剂:V(乙酸乙酯):V(甲醇):V(氨水)=10:1:0.1),得到淡黄色固体,0.3281g,产率68%。
1H NMR(400MHz,DMSO)δ7.71(s,1H),7.60(d,J=11.9Hz,1H),6.94–6.83(m,3H),4.14(t,J=6.9Hz,2H),3.86(s,6H),3.75(s,2H),3.38–3.32(m,4H),3.16(s,2H),2.75(s,6H),2.11–1.99(m,2H).13C NMR(101MHz,DMSO)δ159.63(d,J=3.1Hz),156.03,153.62,149.90(t,J=144.2Hz),149.25,148.25,142.76(d,J=14.1Hz),132.19,131.32,129.85,109.82(d,J=20.5Hz),109.31(d,J=7.1Hz),105.77(d,J=2.4Hz),61.04,60.68,56.76,55.27,44.56,42.69,25.41,23.55.ESI-HRMS[M+H]+m/z=483.2329,calcd for C26H31N4O4F,483.2399.Purity:99.741%by HPLC.
实施例28:化合物21的合成
将化合物m3(0.3042g,1mmol)与(E)-4-(2-(pyridin-2-yl)vinyl)benzaldehyde(0.2301g,1.1mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热48h,TLC监测反应结束后,抽滤,粗产品用硅胶柱层析纯化(洗脱剂:V(乙酸乙酯):V(甲醇):V(氨水)=10:1:0.1),得到淡黄色固体,0.2726g,产率55%。
1H NMR(400MHz,MeOD)δ8.61(d,J=5.2Hz,1H),8.20(t,J=7.8Hz,1H),8.01(d,J=8.0Hz,1H),7.75(d,J=8.9Hz,3H),7.66–7.59(m,4H),7.36(d,J=16.4Hz,2H),6.94(d,J=7.3Hz,1H),4.25(t,J=6.8Hz,2H),3.49(t,J=6.4Hz,2H),3.39–3.34(m,4H),2.97(s,6H),2.20(dd,J=14.4,6.9Hz,2H),1.37–1.29(m,2H)..13C NMR(101MHz,MeOD)δ160.55,152.80,150.76(d,J=244.6Hz),149.55,148.97,148.14,144.40,142.00,136.92,136.06,134.29(dd,J=15.2,8.5Hz),133.05,130.07,129.23,127.59–127.29(m),123.55,123.24,123.01,109.31(d,J=8.1Hz),109.08(d,J=11.7Hz),105.22(d,J=2.7Hz),55.85,44.11,42.38,39.59,25.27,23.68.ESI-HRMS[M+H]+m/z=496.2482,calcd for C30H30N5OF,496.2498.Purity:96.195%by HPLC.
实施例29:化合物22的合成
将化合物m3(0.3042g,1mmol)与4-苯甲氧基-3-羟基苯甲醛(0.2336g,1.1mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热24h,TLC监测反应结束后,抽滤,粗产品用硅胶柱层析纯化(洗脱剂:V(乙酸乙酯):V(甲醇):V(氨水)=10:1:0.1),得到淡黄色固体,0.2985g,产率58%。
1H NMR(400MHz,DMSO)δ7.98(t,J=2.6Hz,1H),7.58(d,J=11.9Hz,1H),7.48–7.44(m,2H),7.43–7.38(m,2H),7.35(dt,J=5.1,2.0Hz,1H),6.87(d,J=8.0Hz,1H),6.64(s,1H),6.60(dd,J=8.6,2.5Hz,2H),5.11(s,2H),4.15–4.05(m,2H),3.30–3.22(m,2H),3.19–3.09(m,4H),2.75(s,6H),2.08–1.93(m,2H).13C NMR(101MHz,DMSO)δ160.47,159.85(d,J=3.0Hz),158.49,153.87(d,J=517.8Hz),148.89,142.75(d,J=13.6Hz),137.30,130.17,128.95,128.75,128.38,128.16,123.42,116.40,109.62(d,J=20.2Hz),108.69(d,J=7.3Hz),106.68,105.88(d,J=3.4Hz),102.50,69.63,54.97,44.25,42.51,25.47,23.55.ESI-HRMS[M+H]+m/z=515.2380,calcd for C30H31N4O3F,515.2458.Purity:99.383%by HPLC.
实施例30:化合物23的合成
将化合物m3(0.2432g,0.8mmol)与4-苯甲氧基-3-羟基苯甲醛(0.12mL,0.85mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热24h,TLC监测反应结束后,抽滤,粗产品用乙醇重结晶,得到黄色固体,0.2198g,产率61%。
1H NMR(400MHz,MeOD)δ7.74(d,J=11.4Hz,1H),7.65(d,J=8.6Hz,2H),7.56(d,J=8.5Hz,2H),7.30(d,J=7.1Hz,1H),6.99(s,1H),3.91(t,J=6.5Hz,2H),3.52(t,J=6.9Hz,2H),3.45–3.38(m,4H),2.97(s,6H),2.94(s,3H),2.13–2.03(m,2H).13C NMR(101MHz,MeOD)δ171.93,161.14,157.99,157.44,150.81(d,J=247.2Hz),144.38(d,J=16.3Hz),139.62,133.36,132.31,125.81,122.51,116.11,110.50(d,J=22.4Hz),106.79(d,J=7.9Hz),98.19,55.59,46.81,42.43,39.78,34.14,25.23,23.45.ESI-HRMS[M+H]+m/z=541.2067,calcd for C25H27N4O3F,451.2124.Purity:97.017%by HPLC.
实施例31:化合物24的合成
将化合物m3(0.3042g,1.0mmol)与4-苯甲氧基-3-羟基苯甲醛(0.1806g,1.1mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热24h,TLC监测反应结束后,抽滤,粗产品用乙醇重结晶,得到淡黄色固体,0.3830g,产率85%。
1H NMR(400MHz,MeOD)δ8.37(s,1H),7.65–7.53(m,3H),7.18(d,J=7.0Hz,1H),6.93(d,J=8.2Hz,2H),4.46(t,J=6.9Hz,2H),3.96(t,J=6.3Hz,2H),3.50–3.34(m,6H),2.99(s,6H),2.25–2.12(m,2H),1.89–1.77(m,2H),1.08(t,J=7.4Hz,3H).13C NMR(101MHz,MeOD)δ162.10,157.79(d,J=5.4Hz),157.62(d,J=2.7Hz),151.57–146.00(m),149.55,144.34(d,J=4.0Hz),138.74,133.02,126.85,123.84,115.15,110.35(d,J=17.3Hz),106.92(d,J=6.9Hz),99.98,69.80,55.59,42.45,39.81,34.16,25.23,23.46,22.11,9.20.ESI-HRMS[M+H]+m/z=541.2431,calcd for C26H31N4O2F,451.2505.Purity:98.997%by HPLC.
实施例32:化合物25的合成
将化合物m3(0.2432g,0.8mmol)与4-苯甲氧基-3-羟基苯甲醛(0.148mL,0.85mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热24h,TLC监测反应结束后,抽滤,粗产品用乙醇重结晶,得到黄色固体,0.2791g,产率72%。
1H NMR(400MHz,CD3OD_SPE)δ8.36(s,1H),7.49(t,J=9.7Hz,3H),7.43(t,J=7.9Hz,2H),7.23(t,J=7.4Hz,1H),7.11–7.05(m,3H),6.91(d,J=8.6Hz,2H),4.45(t,J=6.9Hz,2H),3.42–3.31(m,6H),2.96(s,6H),2.22–2.08(m,2H).13C NMR(101MHz,CD3OD_SPE)δ160.56,157.55,157.23(d,J=6.1Hz),155.25(d,J=4.2Hz),150.56(d,J=245.1Hz),146.91,144.17,144.02,137.85,132.97,129.92,128.46,125.11,124.61,119.98,117.56,110.15(d,J=20.9Hz),106.57(d,J=5.7Hz),97.74(d,J=3.7Hz),55.36,44.69,42.26,39.61,25.23,23.25.ESI-HRMS[M+H]+m/z=485.2275,calcd for C29H29N4O2F,485.2344.Purity:99.804%by HPLC.
实施例33:化合物26的合成
将化合物m3(0.2432g,0.8mmol)与4-苯甲氧基-3-羟基苯甲醛(0.139mL,0.85mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热24h,TLC监测反应结束后,抽滤,粗产品用乙醇重结晶,得到黄色固体,0.2920g,产率81%。
1H NMR(400MHz,MeOD)δ8.39(s,1H),7.70–7.59(m,3H),7.24(d,J=7.1Hz,1H),7.01(d,J=8.4Hz,2H),4.72(dt,J=12.1,6.0Hz,1H),4.44(t,J=7.0Hz,2H),3.47(t,J=6.9Hz,2H),3.44–3.34(m,4H),2.98(s,6H),2.26–2.15(m,2H),1.38(d,J=6.0Hz,6H).13C NMR(101MHz,MeOD)δ161.07,157.84,157.41,150.78(d,J=247.4Hz),144.30(d,J=14.0Hz),139.00,138.28,133.06,126.60,123.45,116.13,110.41(d,J=22.3Hz),106.83(d,J=8.7Hz),98.37,70.41,55.57,46.81,42.41,39.78,25.22,23.43,20.85.ESI-HRMS[M+H]+m/z=451.2431,calcd for C26H31N4O2F,451.2497.Purity:99.876%by HPLC.
实施例34:化合物27的合成
将化合物m3(0.3042g,1mmol)与4-(2-Propynyloxy)benzenecarbaldehyde(0.1762g,1.1mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热48h,TLC监测反应结束后,抽滤,粗产品用硅胶柱层析纯化(洗脱剂:V(乙酸乙酯):V(甲醇):V(氨水)=10:1:0.1),得到乳白色固体,0.3260g,产率73%。
1H NMR(400MHz,DMSO)δ7.57(dd,J=18.0,10.8Hz,4H),7.07(dd,J=16.8,8.2Hz,2H),6.76(d,J=7.8Hz,1H),6.40(s,1H),4.79(d,J=10.0Hz,2H),4.13–4.04(m,2H),3.30–3.23(m,2H),3.22–3.16(m,2H),2.49(s,1H),2.36(t,J=6.5Hz,2H),2.18(s,6H),1.81–1.72(m,2H).13C NMR(101MHz,DMSO)δ159.81(d,J=3.2Hz),158.62(d,J=40.9Hz),155.88,150.35(d,J=242.1Hz),149.04,143.33(d,J=13.8Hz),131.66,129.54,128.39,121.99,115.66,109.49(d,J=20.4Hz),108.99(d,J=7.2Hz),105.99(d,J=3.9Hz),70.55,63.90,57.65,52.55,45.61,44.20,41.74,26.51,25.49.ESI-HRMS[M+K]+m/z=485.2118,calcd for C26H27N4O2FK,485.2343.Purity:98.152%by HPLC.
实施例35:化合物28的合成
将化合物m4(0.3462g,1mmol)与4-二乙氨基苯甲醛(0.1947g,1.1mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热过夜,TLC监测反应结束后,抽滤,粗产品用甲醇/二氯甲烷重结晶,得到淡黄色固体0.2427g,产率48%。
1H NMR(400MHz,CDCl3)δ7.78–7.69(m,1H),7.65(d,J=8.2Hz,1H),7.43(t,J=8.1Hz,2H),6.76(t,J=7.2Hz,1H),6.68(t,J=7.7Hz,2H),6.37(s,1H),4.26–4.12(m,2H),3.78(d,J=3.8Hz,4H),3.46–3.31(m,6H),3.25–3.10(m,2H),2.61–2.44(m,J=14.3,8.6Hz,6H),1.89(dd,J=5.2Hz,2H),1.20(dd,J=11.6,6.6Hz,6H).13C NMR(101MHz,CDCl3)δ160.64,156.26,150.37(d,J=241.0Hz),149.05,147.97,143.09(d,J=13.7Hz),131.74,130.29,125.56,122.85,109.65(dd,J=20.4,3.3Hz),109.21(d,J=7.6Hz),105.41(d,J=3.8Hz),66.91,58.06,53.79,44.39,43.90,43.38,25.55,24.15,12.64.ESI-HRMS[M+H]+m/z=506.2853,calcd for C29H30N5O2F,506.2919.Purity:97.881%by HPLC.
实施例36:化合物29的合成
将化合物m4(0.3462g,1mmol)与4-苯甲氧基-2-羟基苯甲醛(0.2336g,1.1mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热过夜,TLC监测反应结束后,抽滤,粗产品用硅胶柱层析纯化(洗脱剂:V(乙酸乙酯):V(甲醇):V(氨水)=10:1:0.1),得到淡黄色固体0.2785g,产率50%。
1H NMR(400MHz,MeOD)δ8.41(s,1H),7.62(dd,J=8.3Hz,1H),7.46(d,J=8.8Hz,1H),7.37–7.17(m,7H),6.98(t,J=6.2Hz,1H),6.56(d,J=8.5Hz,1H),6.50(s,1H),5.04(s,1H),4.00–3.90(m,4H),3.90–3.77(m,4H),3.43(dd,J=14.7,8.1Hz,7H),3.30–3.23(m,5H),3.17–2.97(m,5H),2.22–2.06(m,4H).13C NMR(101MHz,MeOD)δ163.56,δ157.79(d,J=57.8Hz),157.50,144.36(d,J=14.0Hz),136.65,134.02,131.08,128.19,127.70,127.10,121.91,115.14,110.54(d,J=22.1Hz),108.92(d,J=99.2Hz),107.67,106.77,102.32,101.89,98.16,98.12,70.02,63.57,54.97,52.02,46.56,39.85,25.62,22.50.ESI-HRMS[M+H]+m/z=557.2486,calcd for C32H33N4O4F,557.2559.Purity:98.517%by HPLC.
实施例37:化合物30的合成
将化合物m4(0.3462g,1mmol)与4-甲氧基苯甲醛(0.1496g,1.1mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热过夜,TLC监测反应结束后,抽滤,粗产品用硅胶柱层析纯化(洗脱剂:V(乙酸乙酯):V(甲醇):V(氨水)=10:1:0.1),得到淡黄色固体0.3484g,产率75%。
1H NMR(400MHz,DMSO)δ7.80(s,1H),7.62(d,J=8.4Hz,1H),7.60(d,J=5.4Hz,2H),7.07(d,J=8.8Hz,2H),6.94(d,J=7.6Hz,1H),6.75(s,1H),4.19–4.10(m,2H),3.96(dd,J=12.4,2.6Hz,3H),3.83(s,4H),3.43(d,J=12.0Hz,3H),3.34(t,J=6.3Hz,2H),3.27–3.16(m,4H),3.07(dd,J=21.0,9.1Hz,3H),2.53(d,J=5.6Hz,2H),2.13–2.01(m,2H).13C NMR(101MHz,CDCl3)δ160.56,158.01,153.60(d,J=337.6Hz),149.50,148.85,143.30(d,J=14.0Hz),132.02,130.12,128.99,124.76,124.67,120.33,110.85,109.60(d,J=20.8Hz),109.34(d,J=7.7Hz),105.78,105.74,55.52,52.76,46.89,43.82,43.64,25.71,25.28,11.70.ESI-HRMS[M+1]+m/z=465.2296,calcd for C26H29N4O3F,464.2224.Purity:95.778%by HPLC.
实施例38:化合物31的合成
将化合物m4(0.3462g,1mmol)与3,4,5-三甲氧基苯甲醛(0.2158g,1.1mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热过夜,TLC监测反应结束后,抽滤,粗产品用硅胶柱层析纯化(洗脱剂:V(乙酸乙酯):V(甲醇):V(氨水)=10:1:0.1),得到淡黄色固体0.4092g,产率78%。
1H NMR(400MHz,CDCl3)δ7.69(d,J=11.7Hz,1H),7.61(t,J=2.5Hz,1H),6.71(s,2H),6.42(s,1H),4.22–4.13(m,2H),3.84(s,9H),3.71(t,J=4.5Hz,4H),3.28(dd,J=10.8,5.7Hz,2H),3.22–3.15(m,2H),2.54–2.48(m,2H),2.45(s,2H),1.83(dt,J=11.9,6.0Hz,2H),1.73(s,2H).13C NMR(101MHz,CDCl3)δ160.51(d,J=3.3Hz),155.26(d,J=1.5Hz),153.29,150.70(d,J=244.0Hz),148.70,143.29(d,J=14.0Hz),138.86,131.18,130.90,129.84,109.75(d,J=20.8Hz),109.40(d,J=7.7Hz),105.44(d,J=4.0Hz),66.83,61.02,58.07,56.15,53.73,43.94,43.39,25.46,23.90.ESI-HRMS[M+1]+m/z=525.2512,calcd for C28H33N4O5F,524.2435.Purity:98.739%by HPLC.
实施例39:化合物32的合成
将化合物m4(0.3462g,1mmol)与对氟苯甲醛(0.1364,1.1mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热过夜,TLC监测反应结束后,抽滤,粗产品用硅胶柱层析纯化(洗脱剂:V(乙酸乙酯):V(甲醇):V(氨水)=10:1:0.1),得到淡黄色固体0.3484g,产率77%。
1H NMR(400MHz,CDCl3)δ7.78(d,J=11.7Hz,1H),7.73(t,J=2.5Hz,1H),7.53(dd,J=8.7,5.4Hz,2H),7.13(t,J=8.6Hz,2H),6.80(d,J=7.7Hz,1H),6.46(s,1H),4.28–4.22(m,2H),3.85–3.75(m,4H),3.42–3.33(m,2H),3.24(t,J=5.9Hz,2H),2.68–2.47(m,6H),1.97–1.87(m,2H).13C NMR(101MHz,CDCl3)δ163.93,161.44,δ160.42(d,J=3.2Hz),155.13,150.74(d,J=243.8Hz),148.68,143.30(d,J=13.9Hz),131.50(t,J=9.7Hz),128.45,116.06,115.85,109.77(d,J=20.8Hz),109.51(d,J=7.7Hz),105.58(d,J=4.0Hz),66.90,58.09,53.79,43.86,43.43,25.48,24.05.ESI-HRMS[M+1]+m/z=453.2095,calcd for C25H26N4O2F,452.2024.Purity:96.492%by HPLC.
实施例40:化合物33的合成
将化合物m4(0.3462g,1mmol)与4-(4-吗啉)苯甲醛(0.2101g,1.1mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热过夜,TLC监测反应结束后,抽滤,粗产品用硅胶柱层析纯化(洗脱剂:V(乙酸乙酯):V(甲醇):V(氨水)=10:1:0.1),得到淡黄色固体0.3325g,产率64%。
1H NMR(400MHz,CDCl3)δ7.77(d,J=11.7Hz,1H),7.70(t,J=2.4Hz,1H),7.49(d,J=8.8Hz,2H),6.94(d,J=8.9Hz,2H),6.79(d,J=7.7Hz,1H),6.44(s,1H),4.28–4.21(m,2H),3.91–3.84(m,4H),3.84–3.74(m,4H),3.36(dd,J=9.9,4.9Hz,2H),3.25(dd,J=9.6,4.8Hz,6H),2.68–2.42(m,6H),1.92(s,2H).13C NMR(101MHz,CDCl3)δ160.60(d,J=3.3Hz),155.84,151.79,150.77(t,J=121.6Hz),148.91,143.28,143.21(d,J=13.9Hz),131.21,128.33,114.80,109.77(d,J=20.8Hz),109.40(d,J=7.6Hz),105.52(d,J=3.8Hz),66.89,66.71,58.04,53.80,48.19,43.92,43.36,25.60,24.15.ESI-HRMS[M+1]+m/z=520.2718,calcd for C29H34N5O3F,519.2646.Purity:98.118%by HPLC.
实施例41:化合物34的合成
将化合物m4(0.3462g,1mmol)与对三氟甲基苯甲醛(0.137μL,1.1mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热过夜,TLC监测反应结束后,抽滤,粗产品用硅胶柱层析纯化(洗脱剂:V(乙酸乙酯):V(甲醇):V(氨水)=10:1:0.1),得到淡黄色固体0.3568g,产率71%。
1H NMR(400MHz,CDCl3)δ7.77–7.71(m,2H),7.67(d,J=8.2Hz,2H),7.61(d,J=8.2Hz,2H),6.77(d,J=7.6Hz,1H),6.64(s,1H),4.24(t,J=7.0Hz,2H),3.84–3.74(m,4H),3.41–3.32(m,2H),3.23(t,J=5.5Hz,2H),2.57(dd,J=17.1,11.5Hz,6H),1.96–1.86(m,2H).13C NMR(101MHz,CDCl3)δ160.27(d,J=3.4Hz),154.63,150.85(d,J=244.5Hz),148.52,143.36(d,J=14.0Hz),138.91,134.73,130.72–129.83(m),129.63,127.80,125.70(d,J=3.7Hz),109.74(d,J=20.8Hz),109.54(d,J=7.7Hz),105.56(d,J=4.0Hz),66.89,58.16,53.77,43.86,43.52,25.62,23.87.ESI-HRMS[M+1]+m/z=503.2057,calcd for C26H26N4O2F4,502.1992.Purity:95.469%by HPLC.
实施例42:化合物35的合成
将化合物m4(0.3462g,1mmol)与对4-异丙基苯甲醛(0.163μL,1.1mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热过夜,TLC监测反应结束后,抽滤,粗产品用硅胶柱层析纯化(洗脱剂:V(乙酸乙酯):V(甲醇):V(氨水)=10:1:0.1),得到淡黄色固体0.2955g,产率62%。
1H NMR(400MHz,CDCl3)δ7.75(d,J=12.3Hz,2H),7.48(d,J=7.9Hz,2H),7.30(d,J=7.9Hz,1H),6.78(d,J=7.7Hz,1H),6.50(s,1H),4.22(t,J=7.1Hz,2H),3.78(t,J=7.0Hz,4H),3.36(dd,J=10.4,5.2Hz,2H),3.23(t,J=6.2Hz,2H),2.95(dt,J=13.8,6.9Hz,1H),2.62–2.48(m,6H),1.91(t,J=5.6Hz,2H),1.28(d,J=6.9Hz,6H).13C NMR(101MHz,CDCl3)δ160.51(d,J=3.4Hz),155.46(d,J=1.8Hz),150.43(t,J=121.8Hz),149.94,148.76,143.24(d,J=13.9Hz),133.27,130.97,129.85,129.72,126.95,109.73(d,J=20.8Hz),109.47(d,J=7.6Hz),105.56(d,J=3.9Hz),66.90,58.11,53.78,43.91,43.44,34.03,25.61,24.02,23.83.ESI-HRMS[M+1]+m/z=477.2653,calcd for C28H33N4O2F,476.2588.Purity:99.239%by HPLC.
实施例43:化合物36的合成
将化合物m4(0.3462g,1mmol)与4-哌啶-1-基-苯甲醛(0.2081g,1.1mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热过夜,TLC监测反应结束后,抽滤,粗产品用硅胶柱层析纯化(洗脱剂:V(乙酸乙酯):V(甲醇):V(氨水)=10:1:0.1),得到淡黄色固体0.4090g,产率79%。
1H NMR(400MHz,CDCl3)δ7.73(d,J=11.2Hz,1H),7.64(s,1H),7.42(d,J=7.5Hz,1H),6.90(d,J=7.9Hz,2H),6.76(d,J=7.0Hz,1H),6.39(s,1H),4.27–4.12(m,2H),3.78(m,4H),3.43–3.22(m,6H),3.17(s,2H),2.64–2.41(m,6H),1.89(s,2H),1.67(s,6H).13C NMR(101MHz,CDCl3)δ160.59,155.96,150.90(t,J=134.1Hz),149.22,148.93,143.12(d,J=12.1Hz),131.27,129.85,127.26,114.95,113.23,109.64(d,J=20.1Hz),109.21(d,J=6.8Hz),105.38,66.87,58.06,53.74,49.14,48.36,43.90,43.40,25.50,24.32,24.00.ESI-HRMS[M+1]+m/z=518.2909,calcd for C30H36N5O2F,517.2853.Purity:98.323%by HPLC.
实施例44:化合物37的合成
将化合物m4(0.3462g,1mmol)与4-甲酰基苯甲醛(0.1475g,1.1mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热过夜,TLC监测反应结束后,抽滤,粗产品用硅胶柱层析纯化(洗脱剂:V(乙酸乙酯):V(甲醇):V(氨水)=10:1:0.1),得到淡黄色固体0.3145g,产率68%。
1H NMR(400MHz,CDCl3)δ10.04(s,1H),7.98(s,1H),7.85(d,J=7.4Hz,1H),7.79–7.66(m,3H),7.60(t,J=7.5Hz,1H),6.77(d,J=7.5Hz,1H),6.60(s,1H),4.24(t,J=6.9Hz,2H),3.78(s,4H),3.42–3.30(m,2H),3.29–3.20(m,2H),2.66–2.42(m,6H),1.91(t,J=4.9Hz,2H).13C NMR(101MHz,CDCl3)δ191.74,160.28(d,J=3.3Hz),154.70,150.78(d,J=244.2Hz),148.54,143.33(d,J=14.0Hz),135.37,134.01,129.83,129.75,129.58,127.85,109.72(d,J=20.9Hz),109.52(d,J=7.8Hz),105.54(d,J=4.0Hz),66.91,58.14,53.78,43.89,43.50,25.65,23.93.ESI-HRMS[M+1]+m/z=463.2134,calcd for C26H27N4O3F,462.2067.Purity:97.925%by HPLC.
实施例45:化合物38的合成
将化合物m4(0.3462g,1mmol)与4-苯甲氧基苯甲醛(0.2333g,1.1mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热过夜,TLC监测反应结束后,抽滤,粗产品用硅胶柱层析纯化(洗脱剂:V(乙酸乙酯):V(甲醇):V(氨水)=10:1:0.1),得到淡黄色固体0.3189g,产率59%。
1H NMR(400MHz,CDCl3)δ7.75(d,J=9.8Hz,1H),7.71(s,1H),7.48(d,J=8.8Hz,2H),7.46–7.30(m,5H),7.03(d,J=8.5Hz,2H),6.79(d,J=7.6Hz,1H),6.42(s,1H),5.11(s,2H),4.22(t,J=6.9Hz,2H),3.78(s,4H),3.42–3.31(m,2H),3.27–3.17(m,2H),2.62–2.43(m,6H),1.89(t,J=4.8Hz,2H).13C NMR(101MHz,CDCl3)δ160.50(d,J=3.4Hz),159.25,155.57(d,J=1.8Hz),150.61(d,J=243.1Hz),148.79,143.18(d,J=14.0Hz),136.57,131.32,130.32,129.44,128.65,127.88,127.42,115.23,109.76(d,J=20.9Hz),109.48(d,J=7.9Hz),105.60(d,J=3.7Hz),70.12,53.76,50.92,43.88,43.23,29.68,25.51,24.21.ESI-HRMS[M+1]+m/z=541.2607,calcd for C32H33N4O3F,540.2537.Purity:95.400%by HPLC.
实施例46:化合物39的合成
将化合物m5(0.2903g,1mmol)与4-二乙氨基苯甲醛(0.1947g,1.1mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热过夜,TLC监测反应结束后,抽滤,粗产品用硅胶柱层析纯化(洗脱剂:V(乙酸乙酯):V(甲醇):V(氨水)=10:1:0.1),得到淡黄色固体0.2427g,产率54%。
1H NMR(400MHz,CDCl3)δ7.75(d,J=11.7Hz,1H),7.66(s,1H),7.44(d,J=8.6Hz,2H),6.79(d,J=7.7Hz,1H),6.69(d,J=8.6Hz,2H),5.13(s,1H),4.21(t,J=7.2Hz,2H),3.40(q,J=6.8Hz,4H),3.28(dd,J=10.5,5.2Hz,2H),3.23–3.15(m,2H),2.63(t,J=5.8Hz,2H),2.29(s,6H),1.20(t,J=6.9Hz,6H).13C NMR(101MHz,CDCl3)δ160.69(d,J=11.8Hz),148.96,148.55(d,J=213.8Hz),142.81(d,J=11.8Hz),131.79,130.45,125.44,122.80,111.29,109.94–109.51(m),105.88(d,J=3.9Hz),57.38,45.16,44.42,43.96,40.21,25.58,12.64.ESI-HRMS[M+1]+m/z=449.2591,calcd for C26H32N5OF,450.2650.Purity:98.779%by HPLC.
实施例47:化合物40的合成
将化合物m5(0.2903g,1mmol)与4-苯甲氧基-2-羟基苯甲醛(0.2336g,1.1mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热过夜,TLC监测反应结束后,抽滤,粗产品用硅胶柱层析纯化(洗脱剂:V(乙酸乙酯):V(甲醇):V(氨水)=10:1:0.1),得到淡黄色固体0.3154g,产率63%。
1H NMR(400MHz,CDCl3)δ8.32(s,1H),7.75(d,J=11.4Hz,1H),7.37–7.31(m,2H),7.29–7.28(m,4H),6.83(d,J=6.9Hz,1H),6.55(d,J=7.8Hz,1H),6.44(s,1H),5.20(s,1H),4.94(s,2H),4.21–4.18(m,2H),3.16(d,J=25.2Hz,4H),2.57(m,2H),2.39(s,6H).13C NMR(101MHz,DMSO)δ159.97,159.34,157.92,155.86,148.56(d,J=22.9Hz),142.66,142.65(dd,J=12.1,8.2Hz),142.45,136.81,129.67,128.42,128.32,127.85,127.62,122.93,115.94,108.91(d,J=25.6Hz),108.14(d,J=6.8Hz),106.17,105.21,101.98,69.15,50.39,46.28,43.70,41.10,24.98.ESI-HRMS[M+H]+m/z=501.2224,calcd for C29H29N4O3F,501.2001.Purity:98.675%by HPLC.
实施例48:化合物41的合成
将化合物m5(0.2903g,1mmol)与4-甲酰基苯甲醛(0.1475g,1.1mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热过夜,TLC监测反应结束后,抽滤,粗产品用硅胶柱层析纯化(洗脱剂:V(乙酸乙酯):V(甲醇):V(氨水)=10:1:0.1),得到淡黄色固体0.2886g,产率71%。
1H NMR(400MHz,CDCl3)δ10.05(s,1H),7.95(d,J=8.1Hz,2H),7.83–7.77(m,2H),7.70(d,J=8.1Hz,2H),6.83(d,J=7.7Hz,1H),4.28(t,J=7.1Hz,2H),3.37–3.26(m,4H),2.65(t,J=5.9Hz,2H),2.30(s,6H).13C NMR(101MHz,CDCl3)δ191.51,160.33,153.36(d,J=271.8Hz),149.58,148.46,143.08(d,J=13.8Hz),141.39,135.74,135.47,130.11,130.02,128.31,110.17–109.82(m),106.20(d,J=3.8Hz),99.99,57.27,45.15,43.93,40.17,25.88.ESI-HRMS[M+1]+m/z=407.1805,calcd for C23H23N4O2F,407.1882.Purity:98.152%by HPLC.
实施例49:化合物42的合成
将化合物m5(0.2903g,1mmol)与4-(2-Propynyloxy)benzenecarbaldehyde(0.1762g,1.1mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热过夜,TLC监测反应结束后,抽滤,粗产品用硅胶柱层析纯化(洗脱剂:V(乙酸乙酯):V(甲醇):V(氨水)=10:1:0.1),得到淡黄色固体0.2811g,产率65%。
1H NMR(400MHz,CDCl3)δ7.77(d,J=11.6Hz,1H),7.71(s,1H),7.51(dd,J=8.5,4.8Hz,2H),7.04(d,J=8.7Hz,1H),6.98(dd,J=8.5,5.5Hz,1H),6.81(d,J=7.7Hz,1H),5.18(s,1H),4.69(d,J=43.9Hz,2H),4.24(t,J=7.2Hz,2H),3.26(dt,J=15.5,5.7Hz,4H),2.63(t,J=5.9Hz,2H),2.29(s,6H).13C NMR(101MHz,DMSO)δ159.81(d,J=3.2Hz),158.62(d,J=40.9Hz),155.88,150.45(d,J=244.2Hz),149.04,143.35(d,J=12.9Hz),131.66,129.54,128.39,121.99,115.66,109.47(d,J=20.1Hz),108.89(d,J=7.3Hz),105.89(d,J=4.0Hz),70.55,63.90,57.65,52.55,45.61,44.20,41.74,26.51.ESI-HRMS[M+H]+m/z=433.1962,calcd for C25H25N4O2F,433.2039.Purity:99.858%by HPLC.
实施例50:化合物43的合成
将化合物m6(0.3304g,1mmol)与4-苯甲氧基-2-羟基苯甲醛(0.2336g,1.1mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热过夜,TLC监测反应结束后,抽滤,粗产品用硅胶柱层析纯化(洗脱剂:V(乙酸乙酯):V(甲醇):V(氨水)=10:1:0.1),得到淡黄色固体0.2595g,产率48%。
1H NMR(400MHz,DMSO)δ7.96(t,J=2.7Hz,1H),7.55(d,J=11.9Hz,1H),7.47–7.44(m,2H),7.43–7.38(m,2H),7.35(dt,J=5.2,2.1Hz,1H),7.30(s,1H),6.87–6.82(m,1H),6.76(d,J=8.0Hz,1H),6.58(s,1H),5.11(s,2H),4.10–4.05(m,2H),3.29–3.24(m,4H),3.18–3.12(m,2H),2.49–2.45(m,4H),1.75(dq,J=13.1,6.4Hz,2H),1.03–0.89(m,4H).13C NMR(101MHz,DMSO)δ159.97,159.34,157.92,155.86,148.54(d,J=21.9Hz),142.66,142.55(dd,J=12.2,8.2Hz),142.45,136.81,129.67,128.42,128.32,127.85,127.62,122.93,115.94,108.91(d,J=24.6Hz),108.14(d,J=6.6Hz),106.17,105.21,101.98,69.15,50.39,46.28,43.70,41.10,24.98,24.37,10.82.ESI-HRMS[M+H]+m/z=541.2537,calcd for C32H33N4O3F,541.2611.Purity:98.148%by HPLC.
实施例51:化合物44的合成
将化合物m7(0.3304g,1mmol)与4-苯甲氧基-2-羟基苯甲醛(0.2336g,1.1mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热过夜,TLC监测反应结束后,抽滤,粗产品用硅胶柱层析纯化(洗脱剂:V(乙酸乙酯):V(甲醇):V(氨水)=10:1:0.1),得到淡黄色固体0.2865g,产率53%。
1H NMR(400MHz,CDCl3)δ8.32(s,1H),7.76(d,J=11.3Hz,1H),7.46–7.33(m,2H),7.33–7.26(m,3H),6.83(d,J=6.9Hz,1H),6.55(d,J=7.8Hz,1H),6.44(s,1H),5.20(s,1H),4.94(s,2H),4.25–4.13(m,2H),3.16(d,J=26.2Hz,4H),2.57(s,2H),2.39(s,4H),1.57(s,4H),1.43(s,2H).13C NMR(101MHz,CDCl3)δ160.94,160.33,156.66,155.00(d,J=111.1Hz),151.61,143.09(d,J=13.7Hz),136.59,129.88,128.52,127.90,127.10,125.62,116.92,110.06(d,J=21.2Hz),107.60,105.40(d,J=8.3Hz),102.94,100.00,70.01,56.61,54.27,44.09,39.53,25.85,25.53,24.25.ESI-HRMS[M+1]+m/z=541.2537,calcd for C32H33N4O3F,541.2606.Purity:98.976%by HPLC.
实施例52:化合物45的合成
将化合物m8(0.2893g,1mmol)与4-苯甲氧基-2-羟基苯甲醛(0.2336g,1.1mmol)加入后壁耐压瓶,再加入2mL的DMSO,10mL的三甲基氯硅烷,100℃加热过夜,TLC监测反应结束后,抽滤,粗产品用硅胶柱层析纯化(洗脱剂:V(乙酸乙酯):V(甲醇):V(氨水)=10:1:0.1),得到淡黄色固体0.3147g,产率63%。
1H NMR(400MHz,DMSO)δ8.13(s,1H),7.61(d,J=11.5Hz,1H),7.50–7.43(m,3H),7.41(dd,J=7.1,5.6Hz,2H),7.35(dt,J=5.2,2.1Hz,1H),6.97(d,J=7.7Hz,1H),6.65(d,J=2.4Hz,0H),6.61(dd,J=8.7,2.4Hz,0H),5.12(s,2H),4.18–4.08(m,2H),3.95(d,J=12.4Hz,2H),3.81(t,J=11.7Hz,2H),3.19(d,J=5.0Hz,4H),3.06(dd,J=20.9,9.1Hz,2H).13C NMR(101MHz,MeOD)δ163.56,δ157.76(d,J=58.8Hz),157.50,144.28(d,J=14.2Hz),136.65,134.02,131.08,128.19,127.70,127.10,121.91,115.14,110.52(d,J=21.6Hz),108.94(d,J=94.2Hz),107.67,106.77,102.32,101.89,98.16,98.12,70.02,63.57,52.02,46.56,25.62.ESI-HRMS[M+H]+m/z=500.1907,calcd for C29H26N3O4F,500.1968.Purity:98.779%by HPLC.
实施例53:化合物m9的合成
将2-氨基-4,5-二氟苯甲酸(10g,29mmol),环戊酮(10mL)置于100mL单颈烧瓶中,于冰浴搅拌下逐滴加入35mL三氯氧磷(POCl3),使其30min滴完,加热回流8h,将反映物中大部分三氯氧磷蒸出后,逐滴加入到200mL冰水中,用浓NaOH溶液调节pH至碱性,有大量固体析出,抽滤得粗产物,滤液用CH2Cl2萃取多次,有机层用无水MgSO4干燥,抽滤,浓缩溶剂得到粗产物。粗产物硅胶柱层析纯化(洗脱剂:V(石油醚):V(二氯甲烷)=2:1)得到化合物m9(8g),产率58%。
1H NMR(400MHz,CDCl3)δ7.85(dd,J=11.1,8.4Hz,1H),7.74(dd,J=11.1,7.6Hz,1H),3.21(t,J=7.7Hz,2H),3.14(t,J=7.5Hz,2H),2.36–2.16(m,2H).
实施例54:化合物m10的合成
将化合物m9(1.18g,5mmol),10%Pd C0.52g,甲醇40mL,三乙胺0.5mL,至于三颈瓶中,充氢气,常温反应过夜;TLC监测反应完毕后,过滤除去Pd C,将滤液浓缩干燥,得到灰色固体,硅胶柱层析纯化后得到灰色固体m10 0.9g,产率90%。
1H NMR(400MHz,DMSO)δ8.08(s,1H),7.93(dd,J=11.2,9.1Hz,1H),7.86(dd,J=11.9,8.0Hz,1H),3.03(t,J=7.1Hz,4H),2.18–2.03(m,2H).
实施例55:化合物46的合成
取m10(300mg)和少量3-二乙胺基丙胺(搅拌下能溶解即可)于38mL耐压管中,90℃下搅拌过夜。反应完全后,TCL点板显示产物较纯。加入乙醚析出沉淀。抽滤,将滤渣烘干,TLC点板,得到产物m11(346mg)。再将化合物m11(346mg,1.1mmol)、4-苯甲氧基-2-羟基苯甲醛(275.4mg,1.2mmol)、置于38mL耐压管中,加入DMF(1mL)和TMSCl(8mL)。100℃下搅拌过夜。经TLC点板监测反应结束后,抽滤,粗产品用硅胶柱层析纯化(洗脱剂:V(乙酸乙酯):V(甲醇):V(氨水)=10:1:0.1),得到淡黄色固体(312mg),产率为54%。
1H NMR(400MHz,CDCl3)δ8.28(s,1H),7.66(s,1H),7.36(d,J=8.6Hz,2H),7.20–6.98(m,6H),6.59–6.31(m,2H),5.04(s,2H),4.78(s,2H),3.21(t,J=5.6Hz,4H),2.72–2.42(m,6H),1.94–1.69(m,2H),1.02(dd,J=15.6,8.6Hz,6H).13C NMR(101MHz,CDCl3)δ160.48,158.58,156.77,152.00,149.49(d,J=162.6Hz),146.35,145.42,143.47(d,J=68.0Hz),135.76,133.80,129.35,127.47,126.90,126.32,122.15,119.25(d,J=30.3Hz),117.86,109.44(d,J=20.3Hz),108.80(d,J=16.2Hz),105.50,101.79,68.73,49.93,45.46,30.91,28.75,26.13,25.69,11.21.ESI-HRMS[M+1]+m/z=525.2792,calcd for C33H36N3O2F,526.2844.Purity:99.196%by HPLC.
实施例56:本发明所述的7位氟取代Isaindigotone衍生物对NM23-H2蛋白活性的抑制作用
选取本发明所述的7位氟取代Isaindigotone衍生物,使用ELISA方法检测化合物阻碍NM23-H2蛋白与G-四链体结合的能力。我们以KD with ligand/KD without ligand作为评价化合物阻断NM23-H2蛋白与c-myc G-四链体DNA相互作用的指标,比值越大,说明化合物抑制NM23-H2蛋白活性,阻断NM23-H2蛋白与c-myc G-四链体DNA相互作用的能力越强。
结果如图1所示,所有化合物均表现出对NM23-H2的抑制活性,部分化合物(例如1,3,13,14,15,16,21,22,26,29,38,39,40,43,44,46等)对NM23-H2的活性表现出明显的抑制作用,当R2为羟基R4为烷氧基时,活性最好。
实施例57:本发明所述的7位氟取代Isaindigotone衍生物对肿瘤细胞生长的抑制作用
选取本发明所述的7位氟取代Isaindigotone衍生物,对多种肿瘤细胞株采用MTT法进行体外细胞毒测定。对数生长期的细胞加入不同浓度的氟取代Isaindigotone衍生物,作用48h后,加入MTT,测定其吸光度。分别计算出抑制细胞生长50%时的化合物浓度,以IC50值表示,结果如表1所示。结果表明这系列化合物在体外对肿瘤细胞株有较强的抑制作用,可用于制备抗肿瘤药物。
表1:7位氟取代Isaindigotone衍生物对肿瘤细胞株生长的抑制作用(IC50值/μM)
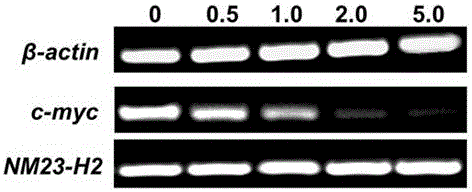
实施例58:代表化合物16对细胞内NM23-H2蛋白及c-myc基因的转录与表达的影响
RT-PCR实验方法:
细胞培养:将细胞接种到6孔板中,200 000个/孔,待24小时细胞贴壁后,加入化合物,培养3-72小时后,收集细胞,提取RNA,检测RNA浓度,后按照下面体系做逆转录反应,及PCR实验
使用的PCR产物、引物、配制体系如下:
10×Dream Taq Green buffer,2.5μL;dNTP mixture(2.5mM),0.5μL;cDNA,1μL;引物(sense),0.5μL;引物(antisense),0.5μL;Dream TaqDNA聚合酶,0.25μL;ddH20,20.25μL;total,25μL
程序:95℃变性,5min;95℃变性,30s;58℃退火,30s;72℃延伸,60s(30cycles);72℃,10min;最终降温至10℃。
1.5%琼脂糖电泳120V,0.5h,电泳结束后,凝胶成像。
结果如图2所示,7位氟取代Isaindigotone衍生物16能够不改变NM23-H2基因的转录水平,降低c-myc的转录水平。
Western blot实验方法:
细胞培养:细胞记数,接种,培养在六孔板中,长至500万个细胞后,取出,裂解,细胞被收集后,加入50μL细胞裂解液,提取上清总蛋白液。使用BCA法检测总蛋白浓度,后变性蛋白样品,取相同质量的蛋白上样,SDS-PAGE胶电泳分离蛋白条带。根据目标蛋白计算分子量,将相应位置的电泳胶条带切下,湿转法将蛋白条带转到PVDF膜上。
配置TBST缓冲液:25mM NaCl,100mM Tris,0.2%Tween-20,pH 7.4,用TBST缓冲液溶解的5%脱脂奶粉溶液(w/v)封闭PVDF膜。分别相应用一抗和二抗孵育PVDF膜,TBST缓冲液漂洗适当次数后,使用SuperECL Plus超敏发光试剂盒显色成像。
结果如图3所示,7位氟取代Isaindigotone衍生物16能够不改变NM23-H2蛋白的表达水平,降低C-MYC的表达水平。
实施例59:代表化合物16对小鼠移植瘤生长抑制的影响
该实验遵照中山大学实验动物中心规则下完成。Siha宫颈癌模型的建立:处于对数生长期的Siha细胞,用不含EDTA的胰酶消化并离心收集,用不含血清的DMEM培养基重悬至1×107cells/100μL。每只4-5周BALB/C-nu/nu裸鼠接种100μL于小鼠前肢腋下,饲养3-4周,等待肿瘤体积(约300mm3)适合后续试验时,取瘤进行二次建模。二次建模时,将瘤体取出,置于不含血清的DMEM培养基中切割成3mm3左右的小块,将瘤体移植至4-5周小鼠前肢腋下,期间用游标卡尺测量肿瘤长和宽,肿瘤体积计算公式为:长×宽2/2。待肿瘤长至给药初始体积约100mm3时(约14天),进行抗肿瘤实验给药。
测定药物的抗肿瘤效果:将小鼠随机分组,每组8只共3组,分别为溶剂组,阿霉素组(1mg/kg),化合物高浓度组(5mg/kg),低浓度组(2.5mg/kg)。每天测量小鼠体重和肿瘤体积,并腹腔注射相应浓度的药物,注射体积为100μL/10g,连续给药21天。
实验结果见图4,7位氟取代Isaindigotone衍生物16能够有效抑制小鼠体内肿瘤生长,且不影响小鼠生存。
- 还没有人留言评论。精彩留言会获得点赞!