一种烟碱与吲哚类杂化化合物、其合成方法及其用途
1.本发明属于农药合成领域,具体涉及烟碱与吲哚类杂化化合物、其合成方法及其抑制植物病原菌的用途应用。
背景技术:
2.我国农业生产形势越发严重,保证农业作物的生产是最有效的解决手段。而伴随着各类作物的种植,病虫害、杂草和其他有害生物则会不可避免地造成粮食减产及经济作物效益下降。农药是保证农作物高产丰收的重要农业生产资料,一直是农药发展的重点。大量农药的使用,在带来巨大的经济效益的同时也带来了其他问题,伴随着对人类和环境的风险,急性毒性危害,造成人畜中毒和植物药害;破坏生态平衡;环境污染和慢性毒性,环境中的农药通过生物富集,最终进入人体对人产生潜在的慢性毒性;防治对象产生抗药性,促使农药使用量加大,进一步提高抗药性,形成恶性循环在高度重视生态安全和食品安全的当今社会,这些问题引起了广泛的关注,也给农药带来了极其负面的影响
3.吲哚类化合物是在自然界中存在最广泛的杂环种类,许多含有吲哚结构的化合物已被证实具有显著的生物活性。以天然产物吲哚类化合物开发新农药具有低毒,环保特性,但由于吲哚类生物碱从天然产物中的提取量较少,大家难以从自然界中获得足够的量和更广泛的种类以满足进一步研究的需要,因此对吲哚生物碱及其类似物的化学合成研究就显得特别重要。多样性导向合成(diversity-oriented synthesis)是近年来迅速发展的一个药物合成研究领域,它是以一种“高通量”的方式产生“类天然产物”(natural product-like)的化合物。其方法是从简单的起始原料出发,通过选择合适的构建模块、条件控制因素和分支反应路径,灵活构建结构复杂多样的化合物集合体,再对它们进行生物学筛选。多样性合成策略以其简便、快速、高效、广泛的特点对于寻找活性药物先导物的发现和优化有着重要意义。
技术实现要素:
4.为了克服现有技术的不足,本发明的目的是提供一种烟碱与吲哚类杂化化合物,该烟碱与吲哚类杂化化合物对油菜菌核病菌、番茄早疫病菌、棉花黄萎病菌、黄瓜枯萎病菌、核桃腐霉和玉米弯孢具有良好的抑制活性。
5.为了实现上述发明目的,本发明采用了以下的技术方案:
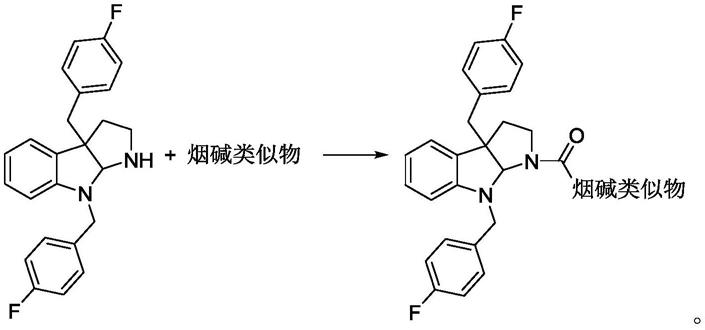
6.具有通式(i)的烟碱与吲哚类杂化的化合物:
[0007][0008]
具有式(i)的烟碱与吲哚类杂化的化合物的合成路线如下:
[0009][0010]
所述的烟碱类似物为以下基团之任意一种:
[0011][0012]
上述基团所对应的烟酸类似物为3-氯异烟酸,5-氯吡嗪-2-羧酸,2-氯-6-羧酸吡啶,2-甲酸吡嗪,对甲氧基苯甲酸,1-萘甲酸,2-氟烟酸,2-甲基烟酸,2-吡啶甲酸,2-氯异烟酸,5-甲基吡嗪-2-羧酸,异烟酸,2-氯烟酸,6-氯烟酸,5,6-二氯烟酸,5-甲基烟酸,2-羟基烟酸,烟酸,2-氨基烟酸,邻醛基苯甲酸。
[0013]
具体而言,具有式(i)的烟碱与吲哚类杂化化合物的合成工艺,步骤如下:
[0014]
步骤一、制酰氯:以烟碱类似物为原料,加入无水四氢呋喃溶解,与二氯亚砜在油浴加热情况下反应制备相应的酰氯底物。
[0015]
步骤二、步骤一得到的酰氯底物与吲哚类化合物(底物a)发生酰胺化反应,tlc跟踪检测,待反应完全,向反应液中滴加水淬灭反应,减压浓缩除去四氢呋喃,合并有机相,减压浓缩,粗产物用柱层析分离,得到烟碱与吲哚类杂化的化合物。
[0016]
具体来说,是将酰氯底物的无水四氢呋喃溶液加入添加了et3n的底物a的无水四氢呋喃溶液,发生酰胺化反应。
[0017]
上述烟碱与吲哚类杂化的化合物抑制植物病原菌的用途。所述植物病原菌为油菜菌核病菌、番茄早疫病菌、棉花黄萎病菌、黄瓜枯萎病菌、核桃腐霉或玉米弯孢。
[0018]
有益效果:本发明提供的烟碱与吲哚类杂化化合物具有结构简单、原料易得,反应条件温和,工艺简单的特点,具有抑制植物病原菌的活性。
[0019]
活性测试结果表明,化合物t-2对油菜菌核病菌的抑菌作用显著,mic值均为1.95μg/ml;化合物t-2对番茄早疫病菌表现出出色的抑制作用,mic值均为3.9μg/ml;化合物t-2对棉花黄萎病菌有同样显著的抑菌活性,它们的最小抑菌浓度为1.95μg/ml。化合物t-15对核桃腐霉的抑制作用出色,mic值均为3.9μg/ml,具有优良的应用前景。
具体实施方式
[0020]
下面结合实施例,对本发明作详细说明。
[0021]
实施例1的合成
[0022]
第一步:制酰氯。制备方法如下:首先准确称量3-氯烟酸0.32g(2mmol)于50ml的圆底烧瓶中,加入10ml无水四氢呋喃,随后用1ml注射器取0.31ml(4mmol)的氯化亚砜注入反应体系中,在设定为55℃的油浴锅中回流2h,用旋转蒸发仪除去氯化亚砜和二氯甲烷,得到酰氯底物。
[0023]
第二步:称取0.29g(0.77mmol,1equiv)底物a,并用10ml无水四氢呋喃溶解,加入0.12ml(1.16mmol,1.5equiv)et3n(三乙胺),将反应体系置于冰浴中。用10ml无水四氢呋喃与第一步制好的酰氯混合均匀,缓慢的滴加至反应体系中,此时伴随白烟产生,随后在室温下反应2h,用tlc监测原料点是否消失。加入3ml饱和碳酸氢钠溶液淬灭反应,搅拌10min,用乙酸乙酯(30ml
×
3)萃取,饱和nacl溶液洗涤,干燥、浓缩、层析柱纯化(石油醚∶乙酸乙酯=5∶1),得到t-1的白色固体(0.35g,89%)。
[0024]
性状鉴定:白色固体,1h-nmr(400mhz,acetone-d6),δ8.65(s,1h),8.57(d,j=4.8hz,1h),7.30
–
6.89(m,11h),6.72(dt,j=14.8,7.5hz,1h),6.17(d,j=7.8hz,1h),5.97(d,j=10.1hz,1h),4.57(dd,j=49.8,16.5hz,2h),3.42
–
3.02(m,4h),2.40
–
2.20(m,2h).
13
c-nmr(100mhz,acetone-d6),δ164.58(c),163.00(c),162.89(c),160.58(c),160.48(c),150.66(c),149.58(ch),148.58(ch),143.54(c),135.18(c),133.76(c),131.75(ch),131.67(ch),131.29(c),128.78(ch),128.64(ch),128.58(ch),128.57(c),123.49(ch),121.83(ch),117.64(ch),114.87(ch),114.68(ch),114.66(ch),114.47(ch),105.94(ch),81.88(ch),57.25(ch2),48.53(c),47.24(ch2),43.38(ch2),38.15(ch2).ms(esi(+))calcd for c
30h24
clf2n3o[m+h]
+
:516.1;found:516.1。
[0025]
实施例2~19施例1不同之处在于所采用的烟碱试剂不同,具体如表1所示:
[0026]
表1烟碱与吲哚类杂化化合物的理化常数
[0027][0028][0029]
实施例2
[0030]
[0031]
淡黄色油状,1h-nmr(400mhz,acetone-d6),δ8.73(t,j=1.2hz,1h),8.69
–
8.66(m,1h),7.16
–
7.11(m,1h),7.08
–
7.02(m,3h),6.99
–
6.92(m,6h),6.71(td,j=7.4,1.0hz,1h),6.16(d,j=7.8hz,1h),6.02(s,1h),4.53(q,j=16.3hz,2h),3.93(ddd,j=11.5,7.1,1.2hz,1h),3.46
–
3.39(m,1h),3.21(d,j=13.4hz,1h),3.03(d,j=13.5hz,1h),2.34
–
2.25(m,2h).
13
c-nmr(100mhz,acetone-d6),δ164.69(c),162.90(c),160.49(c),150.57(c),147.71(c),145.99(c),145.02(ch),142.49(ch),141.50(c),135.27(c),133.82(c),131.75(ch),131.67(ch),131.55(ch),128.70(ch),128.62(ch),128.55(c),123.48(ch),117.66(ch),114.88(ch),114.81(ch),114.66(ch),114.44(ch),106.21(ch),82.83(ch),56.51(ch2),48.80(c),48.63(ch2),43.51(ch2),37.98(ch2).ms(esi(+))calcd for c
30h23
clf2n4o[m+h]
+
:517.1;found:517.1。
[0032]
实施例3
[0033][0034]
黄色油状,1h-nmr(400mhz,acetone-d6),δ8.00
–
7.94(m,1h),7.71(dd,j=7.6,0.8hz,1h),7.57
–
7.54(m,1h),7.15
–
7.12(m,1h),7.01
–
6.90(m,8h),6.77
–
6.68(m,2h),6.14(d,j=7.8hz,1h),6.01(s,1h),4.53(q,j=16.3hz,2h),3.86(ddd,j=11.5,7.1,1.1hz,1h),3.48
–
3.39(m,1h),3.21(d,j=13.4hz,1h),3.04(t,j=8.7hz,1h),2.28(ddd,j=9.6,8.7,5.1hz,2h).
13
c-nmr(100mhz,acetone-d6),δ165.81(c),163.00(c),160.46(c),154.49(c),150.60(c),149.33(c),140.38(ch),135.32(c),133.93(c),131.74(ch),131.67(ch),131.59(ch),128.70(ch),128.63(ch),128.52(ch),125.77(c),123.47(ch),122.82(ch),117.55(ch),114.85(ch),114.64(ch),114.43(ch),106.155(ch),82.75(ch),56.48(ch2),48.77(c),48.72(ch2),43.56(ch2),38.05(ch2).ms(esi(+))calcd for c
30h24
clf2n3o[m+h]
+
:516.1;found:516.1。
[0035]
实施例4
[0036][0037]
淡黄色油状,1h-nmr(400mhz,acetone-d6),δ8.90(s,1h),8.69(d,j=2.4hz,1h),8.62
–
8.59(m,1h),7.14(d,j=7.3hz,1h),7.06
–
6.91(m,10h),6.16(d,j=7.9hz,1h),6.03(s,1h),4.54(q,j=16.3hz,2h),3.94
–
3.88(m,1h),3.42(dt,j=13.8,6.6hz,1h),3.22(d,j=13.4hz,1h),3.03(d,j=13.5hz,1h),2.29(dd,j=10.7,4.6hz,2h).13c-nmr(100mhz,acetone-d6),δ166.42(c),163.80(c),163.68(c),161.38(c),161.27(c),151.42(c),150.23(ch),146.69(ch),146.20(ch),143.54(c),136.09(c),134.67(c),132.54(ch),132.46(ch),132.39(c),129.50(ch),129.42(ch),129.32(c),124.27(ch),118.38(ch),115.65(ch),115.44(ch),115.44(ch),115.23(ch),106.95(ch),83.56(ch),57.28(ch2),49.57(c),49.39(ch2),44.35(ch2),38.89(ch2).ms(esi(+))calcd for c29h24f2n4o[m+h]+:483.1;found:483.1。
[0038]
实施例5
[0039][0040]
白色固体,1h-nmr(400mhz,acetone-d6),δ7.52(d,j=7.7hz,2h),7.17(d,j=7.2hz,1h),6.99(ddd,j=18.0,8.3,3.3hz,11h),6.73(t,j=7.4hz,1h),6.19
–
6.07(m,2h),4.52(dd,j=69.9,16.3hz,2h),3.87(s,3h),3.69(d,j=7.3hz,1h),3.42(d,j=3.8hz,1h),3.25(d,j=13.4hz,1h),3.06(d,j=13.4hz,1h),2.34
–
2.18(m,2h).13c-nmr(100mhz,acetone-d6),δ169.54(c),162.95(c),162.79(c),161.33(c),160.54(c),160.38(c),150.84(c),135.34(c),134.03(c),134.00(c),131.68(ch),131.61(ch),129.69(ch),129.69(ch),128.48(ch),128.45(ch),128.45(c),128.41(c),123.47(ch),117.28(ch),114.77(ch),114.56(2ch),114.34(ch),113.24(ch),113.24(ch),105.83(ch),82.02(ch),56.51(ch2),54.85(ch3),49.44(c),48.12(ch2),43.65(ch2),38.23(ch2).ms(esi(+))calcd for c32h28f2n2o2[m+h]+:511.2;found:511.2。
[0041]
实施例6
[0042][0043]
白色固体,1h-nmr(400mhz,acetone-d6),δ7.91(d,j=8.3hz,2h),7.63(d,j=8.3hz,1h),7.52
–
7.41(m,3h),7.31(d,j=6.9hz,1h),7.14
–
7.08(m,3h),7.06
–
6.92(m,7h),6.69
–
6.63(m,1h),6.23
–
6.13(m,2h),4.65(t,j=21.4hz,2h),3.21(d,j=13.4hz,1h),3.16
–
2.99(m,3h),2.14(dd,j=6.1,1.8hz,2h).13c-nmr(100mhz,acetone-d6),δ169.14(c),163.05(c),162.96(c),160.64(c),160.56(c),150.85(c),135.60(c),135.23(c),133.96(c),133.56(c),131.92(ch),131.84(ch),131.76(c),129.42(ch),129.13(ch),128.76(ch),128.68(ch),128.56(ch),128.44(c),126.90(ch),126.39(ch),125.21(ch),124.86(ch),123.99(ch),123.52(ch),117.50(ch),114.96(ch),114.74(2ch),114.53(ch),105.85(ch),81.91(ch),57.16(ch2),48.76(c),48.03(ch2),43.56(ch2),38.17(ch2).ms(esi(+))calcd for c35h28f2n2o[m+h]+:531.2;found:531.2。
[0044]
实施例7
[0045][0046]
淡黄色油状固体,1h-nmr(400mhz,acetone-d6),δ8.31(d,j=4.1hz,1h),7.94(t,j=8.3hz,1h),7.45
–
7.37(m,1h),7.15(d,j=7.3hz,1h),7.06
–
6.90(m,9h),6.70(t,j=7.4hz,1h),6.14(d,j=7.9hz,1h),5.98(s,1h),4.52(q,j=16.4hz,2h),3.47(dd,j=11.2,5.8hz,1h),3.32
–
3.19(m,2h),3.05(d,j=13.4hz,1h),2.29(dt,j=11.3,4.7hz,2h).13c-nmr(100mhz,acetone-d6),δ163.91(c),163.01(c),162.90(c),160.60(c),160.49(c),160.03(c),157.67(c),150.75(ch),149.17(ch),149.02(ch),140.50(c),140.47(c),135.24(ch),133.84(ch),131.70(ch),131.62(ch),131.31(ch),128.60(c),128.52(c),123.53(ch),122.11(ch),122.07(ch),119.74(ch),117.62(c),114.87(ch),114.65(2ch),114.43(ch),106.04(ch),82.27(ch),57.12(ch2),48.66(c),47.83(ch2),
43.66(ch2),38.05(ch2).ms(esi(+))calcd for c30h24f3n3o[m+h]+:500.1;found:500.1。
[0047]
实施例8
[0048][0049]
黄色油状固体,1h-nmr(400mhz,acetone-d6),δ8.54(s,1h),7.73(d,j=7.7hz,1h),7.26(d,j=7.9hz,1h),7.13(d,j=7.1hz,1h),7.04
–
6.90(m,9h),6.70(t,j=7.4hz,1h),6.14(d,j=7.8hz,1h),6.06(s,1h),4.50(dd,j=46.0,16.5hz,2h),3.62(dd,j=15.8,8.9hz,1h),3.41(d,j=6.2hz,1h),3.23(d,j=13.4hz,1h),3.03(d,j=13.3hz,1h),2.51(s,3h),2.31
–
2.22(m,2h).
13
c-nmr(100mhz,acetone-d6),δ167.97(c),163.00(c),162.86(c),160.58(c),160.45(c),160.27(c),150.80(ch),148.06(c),135.40(ch),133.94(c),131.71(c),131.64(ch),131.43(ch),129.31(ch),128.55(ch),128.48(c),123.54(2ch),122.29(ch),117.42(ch),114.84(ch),114.63(2ch),114.40(ch),105.93(ch),82.27(ch),56.71(ch2),49.17(c),48.39(ch2),43.69(ch2),38.28(ch2),23.65(ch3).ms(esi(+))calcd for c
31h27
f2n3o[m+h]
+
:496.2;found:496.2。
[0050]
实施例9
[0051][0052]
淡黄色油状固体,1h-nmr(400mhz,acetone-d6),δ8.56(d,j=4.4hz,1h),7.90(td,j=7.7,1.1hz,1h),7.72(d,j=7.8hz,1h),7.45(dd,j=6.8,5.5hz,1h),7.13(d,j=7.3hz,1h),7.07
–
6.91(m,10h),6.12(d,j=7.9hz,1h),6.03(s,1h),4.60(d,j=16.3hz,1h),4.48(d,j=16.3hz,1h),3.90(dd,j=11.5,6.9hz,1h),3.42(td,j=11.4,5.7hz,1h),3.22(d,j=13.4hz,1h),3.03(d,j=13.4hz,1h),2.25(dd,j=14.1,5.4hz,2h).
13
c-nmr nmr(100mhz,acetone-d6),δ167.41(c),162.99(c),162.86(c),160.58(c),160.46(c),154.36(c),150.73(c),148.09(ch),136.94(ch),135.39(c),133.98(c),131.75(ch),
131.67(ch),128.71(ch),128.64(ch),128.46(ch),125.17(ch),125.05(c),123.89(ch),123.58(ch),123.45(ch),117.44(ch),114.84(ch),114.62(2ch),114.41(ch),106.05(ch),82.63(ch),56.45(ch2),48.71(c),47.10(ch2),43.65(ch2),38.21(ch2).ms(esi(+))calcd for c
30h25
f2n3o[m+h]
+
:482.2;found:482.2。
[0053]
实施例10
[0054][0055]
白色固体,1h-nmr(400mhz,acetone-d6),δ8.46(d,j=5.0hz,1h),7.50
–
7.35(m,2h),7.15(d,j=7.3hz,1h),7.05
–
6.91(m,9h),6.71(t,j=7.4hz,1h),6.17(d,j=7.9hz,1h),5.98(s,1h),4.52(dd,j=39.4,16.5hz,2h),3.61
–
3.51(m,1h),3.43
–
3.32(m,1h),3.24(d,j=13.4hz,1h),3.04(d,j=13.4hz,1h),2.30(dd,j=9.4,5.0hz,2h).
13
c-nmr(100mhz,acetone-d6)δ166.27(c),162.98(c),162.85(c),160.57(c),160.45(c),151.27(c),150.68(c),150.26(c),147.26(ch),135.33(c),135.30(c),133.82(c),133.79(c),131.65(ch),131.58(ch),131.24(ch),128.55(ch),128.52(ch),128.44(c),123.49(ch),122.06(ch),120.64(ch),117.52(ch),114.84(ch),114.62(ch),114.60(ch),114.39(ch),105.95(ch),82.38(ch),56.83(ch2),48.67(c),48.35(ch2),43.51(ch2),38.10(ch2).ms(esi(+))calcd for c
30h24
clf2n3o[m+h]
+
:516.1;found:516.1。
[0056]
实施例11
[0057][0058]
淡黄色固体,1h-nmr(400mhz,acetone-d6),δ8.78(s,1h),8.49(s,1h),7.14(d,j=7.3hz,1h),7.05
–
6.92(m,10h),6.14(d,j=7.9hz,1h),6.04(s,1h),4.53(dd,j=36.9,16.3hz,2h),3.92(dd,j=11.4,6.8hz,1h),3.16
–
3.08(m,2h),2.57(s,3h),2.48(s,1h),2.28(dd,j=12.5,5.6hz,2h).
13
c-nmr(100mhz,acetone-d6),δ165.90(c),162.94(c),162.82(c),160.53(c),160.41(c),155.49(c),150.57(ch),146.36(c),144.30(ch),
142.23(c),135.27(c),135.24(c),133.88(c),133.85(c),131.71(ch),131.64(ch),131.55(ch),128.65(ch),128.57(ch),128.47(c),123.45(ch),117.52(ch),114.82(ch),114.60(2ch),114.38(ch),106.07(ch),82.64(ch),56.36(ch2),48.66(c),48.60(ch2),43.46(ch2),38.01(ch2),20.76(ch3).ms(esi(+))calcd for c
30h26
f2n4o[m+h]
+
:497.2;found:497.2。
[0059]
实施例12
[0060][0061]
白色固体,1h-nmr(400mhz,acetone-d6),δ8.64(d,j=5.4hz,2h),7.38(d,j=5.5hz,2h),7.13(d,j=7.3hz,1h),6.97(ddt,j=20.5,17.7,5.7hz,9h),6.70(t,j=7.4hz,1h),6.15(d,j=7.8hz,1h),6.00(s,1h),4.51(dd,j=39.1,16.4hz,2h),3.59
–
3.49(m,1h),3.35(td,j=10.8,6.8hz,1h),3.23(d,j=13.4hz,1h),3.03(d,j=13.4hz,1h),2.35
–
2.24(m,2h).
13
c-nmr(100mhz,acetone-d6),δ167.79(c),163.00(c),162.87(c),160.59(c),160.47(c),150.74(c),150.10(2ch),143.74(c),135.34(c),133.91(c),131.71(ch),131.63(ch),131.36(ch),128.58(c),128.47(ch),123.54(ch),121.46(2ch),117.52(ch),114.88(ch),114.67(ch),114.64(ch),114.43(ch),105.99(ch),82.33(ch),56.85(ch2),48.87(c),48.47(ch2),43.63(ch2),38.15(ch2).ms(esi(+))calcd for c
30h25
f2n3o[m+h]
+
:482.2;found:482.2。
[0062]
实施例13
[0063][0064]
淡黄色油状,1h-nmr(400mhz,acetone-d6),δ8.44(dd,j=4.7,1.9hz,1h),7.72(s,1h),7.47(dd,j=7.3,4.9hz,1h),7.16(dd,j=7.3,0.6hz,1h),7.06
–
6.90(m,9h),6.71
–
6.63(m,1h),6.16(d,j=7.6hz,1h),5.96(s,1h),4.58(dd,j=55.3,16.3hz,2h),3.33(ddd,j=10.9,6.5,1.8hz,1h),3.21(dd,j=37.5,13.3hz,2h),3.05(d,j=13.4hz,1h),
2.34
–
2.23(m,2h).
13
c-nmr(100mhz,acetone-d6),δ165.36(c),163.02(c),162.91(c),160.61(c),160.51(c),150.76(c),150.22(ch),146.36(c),137.16(ch),135.29(c),133.81(c),133.07(c),131.79(ch),131.71(ch),131.38(ch),128.71(ch),128.63(ch),128.60(c),123.51(ch),123.21(ch),117.62(ch),114.90(ch),114.71(2ch),114.50(ch),105.94(ch),81.94(ch),57.32(ch2),48.56(c),47.44(ch2),43.53(ch2),38.26(ch2).ms(esi(+))calcd for c
30h24
clf2n3o[m+h]
+
:516.1;found:516.1。
[0065]
实施例14
[0066][0067]
淡黄色固体,1h-nmr(400mhz,acetone-d6),δ8.49(s,1h),7.93(d,j=8.1hz,1h),7.51(d,j=8.2hz,1h),7.28
–
6.83(m,11h),6.71(t,j=7.4hz,1h),6.16(d,j=7.8hz,1h),6.04(s,1h),4.51(dd,j=42.1,16.5hz,2h),3.71
–
3.61(m,1h),3.45(dt,j=17.0,8.4hz,1h),3.23(d,j=13.4hz,1h),3.04(d,j=13.4hz,1h),2.38
–
2.24(m,2h).
13
c-nmr(100mhz,acetone-d6),δ166.61(c),162.97(c),162.83(c),160.56(c),160.42(c),152.22(c),150.72(c),148.80(c),138.48(ch),135.32(c),133.84(c),131.66(ch),131.58(ch),131.45(ch),131.29(ch),128.54(c),128.50(ch),128.42(c),123.85(ch),123.50(ch),117.46(ch),114.82(ch),114.61(ch),114.58(ch),114.37(ch),105.93(ch),82.31(ch),56.71(ch2),49.03(c),48.33(ch2),43.57(ch2),38.12(ch2).ms(esi(+))calcd for c
30h24
clf2n3o[m+h]
+
:516.1;found:516.1。
[0068]
实施例15
[0069][0070]
白色固体,1h-nmr(400mhz,acetone-d6),δ8.43(d,j=1.9hz,1h),8.06(d,j=1.9hz,1h),7.14(d,j=7.3hz,1h),7.03
–
6.92(m,9h),6.71(t,j=7.4hz,1h),6.17(d,j=7.9hz,1h),6.02(s,1h),4.50(dd,j=42.6,16.5hz,2h),3.73
–
3.66(m,1h),3.46(td,j=
10.8,6.7hz,1h),3.23(d,j=13.4hz,1h),3.02(d,j=13.4hz,1h),2.34
–
2.26(m,2h).
13
c-nmr(100mhz,acetone-d6),δ165.29(c),163.01(c),162.88(c),160.60(c),160.47(c),150.74(ch),149.47(c),146.38(c),138.08(c),135.34(ch),133.83(c),132.91(c),131.69(ch),131.62(ch),131.28(c),129.71(ch),128.60(2ch),128.50(c),123.54(ch),117.55(ch),114.88(ch),114.66(2ch),114.43(ch),106.00(ch),82.45(ch),56.84(ch2),48.99(c),48.39(ch2),43.64(ch2),38.22(ch2).ms(esi(+))calcd for c
30h23
cl2f2n3o[m+h]+:550.1;found:550.1。
[0071]
实施例16
[0072][0073]
淡黄色固体,1h-nmr(400mhz,acetone-d6),δ8.46(d,j=9.7hz,2h),7.62(s,1h),7.14(d,j=7.2hz,1h),7.08
–
6.90(m,9h),6.70(t,j=7.3hz,1h),6.16(d,j=7.8hz,1h),6.05(s,1h),4.51(dd,j=49.1,16.5hz,2h),3.63
–
3.54(m,1h),3.40(dt,j=17.1,8.4hz,1h),3.23(d,j=13.4hz,1h),3.04(d,j=13.4hz,1h),2.33(d,j=11.6hz,3h),2.30
–
2.22(m,2h).
13
c nmr(100mhz,acetone-d6),δ167.88(c),162.97(c),162.83(c),160.55(c),160.42(c),151.42(c),150.76(ch),145.61(ch),135.38(ch),135.08(c),133.92(c),132.77(c),131.70(ch),131.68(ch),131.60(2ch),131.38(c),128.52(ch),128.45(c),123.49(ch),117.39(ch),114.81(ch),114.59(2ch),114.37(ch),105.87(ch),82.17(ch),56.67(ch2),49.03(c),48.30(ch2),43.58(ch2),38.18(ch2),17.24(ch3).ms(esi(+))calcd for c
31h27
f2n3o[m+h]
+
:496.2;found:496.2。
[0074]
实施例17
[0075][0076]
褐色油状,1h-nmr(600mhz,cdcl3),δ8.63(s,2h),7.71(d,j=7.9hz,1h),7.29(dd,j=7.6,4.9hz,1h),7.03(td,j=7.7,1.2hz,1h),6.94
–
6.78(m,9h),6.70(t,j=7.3hz,
1h),6.17(d,j=7.8hz,1h),5.96(s,1h),4.52(d,j=16.3hz,1h),4.38(d,j=16.3hz,1h),3.53(dd,j=10.8,7.4hz,1h),3.37(td,j=11.5,5.4hz,1h),3.10(d,j=13.5hz,1h),2.86(d,j=13.5hz,1h),2.21(dd,j=12.2,5.3hz,1h),2.09(td,j=12.1,7.5hz,1h).
13
c-nmr(150mhz,cdcl3),δ168.08(c),162.68(c),161.06(c),151.37(c),150.61(ch),148.43(ch),135.30(ch),134.44(c),132.73(c),132.71(c),131.70(ch),131.36(ch),131.31(c),130.55(ch),128.97(ch),128.31(ch),128.26(c),123.48(ch),123.25(ch),117.65(ch),115.18(ch),115.04(ch),114.97(ch),114.83(ch),106.27(ch),82.31(ch),56.68(ch2),49.34(c),48.67(ch2),44.25(ch2),38.37(ch2).ms(esi(+))calcd for c
30h25
f2n3o[m+h]
+
:482.2;found:482.2。
[0077]
实施例18
[0078][0079]
针状白色固体,1h-nmr(400mhz,acetone-d6),δ8.03(dd,j=4.8,1.6hz,1h),7.46(d,j=5.5hz,1h),7.14(d,j=7.2hz,1h),7.05
–
6.87(m,9h),6.70(t,j=7.4hz,1h),6.56(dd,j=7.4,4.9hz,1h),6.08(dd,j=36.5,26.1hz,4h),4.43(t,j=21.1hz,2h),3.67(s,1h),3.48
–
3.17(m,2h),3.04(d,j=13.4hz,1h),2.34
–
2.19(m,2h).
13
c nmr(100mhz,acetone-d6)δ169.11(c),162.98(c),162.86(c),160.57(c),160.45(c),157.98(c),150.84(ch),150.16(c),136.77(ch),135.24(c),133.95(c),133.92(ch),131.66(ch),131.58(ch),131.43(ch),128.48(ch),128.40(c),123.51(ch),117.43(ch),114.79(ch),114.57(ch),114.54(ch),114.33(ch),112.69(ch),111.71(ch),105.87(ch),82.12(ch),70.40(ch2),56.75(c),48.17(ch2),43.66(ch2),38.37(ch2).ms(esi(+))calcd for c
30h26
f2n4o[m+h]
+
:497.2;found:497.2。
[0080]
实施例19
[0081]
[0082]
褐色固体,1h-nmr(400mhz,acetone-d6),δ8.45(dd,j=4.7,1.5hz,1h),7.73(s,1h),7.47(dd,j=7.1,5.1hz,1h),7.17(d,j=7.3hz,1h),6.96(ddd,j=45.0,25.2,17.9hz,10h),6.70(t,j=7.4hz,1h),6.17(d,j=7.6hz,1h),5.96(s,1h),4.58(dd,j=54.6,16.4hz,2h),3.36
–
3.02(m,4h),2.37
–
2.25(m,2h).
13
c-nmr(100mhz,acetone-d6),δ165.34(c),162.99(c),162.88(c),160.58(c),160.48(c),150.73(c),150.18(ch),137.11(ch),135.26(c),133.80(c),133.04(ch),131.74(ch),131.66(c),131.34(ch),128.66(ch),128.59(ch),128.54(c),123.46(ch),123.15(ch),117.56(ch),114.84(ch),114.65(ch),114.63(ch),114.44(ch),105.89(ch),81.88(ch),57.25(ch2),48.49(c),47.36(ch2),43.45(ch2),38.17(ch2).ms(esi(+))calcd for c
32h26
f2n2o2[m+h]
+
:509.2;found:509.2。
[0083]
实施例20
[0084]
所得化合物对植物病原菌的抑制活性测定,结果见表2。其中t-1~t-19对应实施例1~19得化合物。
[0085]
表2化合物对植物病原真菌的mic
[0086]
[0087][0088]
注:
“‑”
表示无活性。mic:最小抑制浓度,s.s.:油菜菌核病菌,a.s.:番茄早疫病菌,v.d.:棉花黄萎病菌,c.o.:黄瓜枯萎菌,c.j.:核桃腐霉,c.l.:玉米弯孢
[0089]
由表可知,吲哚并吡咯环衍生物t-2对油菜菌核病菌(sclerotinia sclerotiorum)有优越的抑菌活性,其最小抑菌浓度均为1.95μg/ml。另外化合物t-4、t-11和t-14对油菜菌核病菌的抑制作用也很显著,与阳性对照两性霉素的效果相当,均为3.9μg/ml;化合物t-7、t-8、t-13和t-15对油菜菌核病菌的抑制作用与阳性对照多菌灵的效果一致,为7.8μg/ml。
[0090]
化合物t-2、t-4和t-11对番茄早疫病菌(alternaria solani)表现出优于两组阳性对照的高度抑菌活性,它们的最小抑菌活性分别为3.9μg/ml、7.8μg/ml和7.8μg/ml。化合物t-3、t-7、t-8、t-14、t-15、t-16表现出优于阳性对照多菌灵的抑菌活性,其中t-15、t-16对番茄早疫病菌的抑菌活性与对照两性霉素的效果相当,它们的最小抑菌浓度为15.16μg/ml。
[0091]
化合物t-2对棉花黄萎病菌(verticillium dahliae)有显著的抑菌活性,它们的最小抑菌浓度为1.95μg/ml。化合物t-11和t-15的抑菌活性也很优越,均高于两组阳性对
照,它们的最小抑菌浓度分别为3.9μg/ml和7.8μg/ml。化合物t-4、t-7、t-13、t-14、t-16表现出优于对照两性霉素的生物活性,它们的最小抑菌浓度分别为15.63μg/ml、15.63μg/ml、15.63μg/ml、15.63μg/ml、31.25μg/ml。
[0092]
化合物t-2、t-11、t-15对黄瓜枯萎病菌(colletotrichum orbiculare)表现出优于两组阳性对照的抑菌活性,它们的最小抑菌浓度均为15.63μg/ml。化合物t-3、t-4、t-7、t-8、t-14也表现出优于两组阳性对照的抑菌活性,它们的最小抑菌浓度均为31.3μg/ml。
[0093]
化合物t-4、t-15、t-16对核桃腐霉(cytospora juglandis)有显著的生物活性,均高于两组阳性对照组,且t-15对核桃腐霉的抑菌活性更佳,它们的最小抑菌浓度为3.9μg/ml。化合物t-2、t-7、t-13、t-14对核桃腐霉的抑菌活性比对照组的两性霉素高,最小抑菌浓度分别为31.3μg/ml、62.5μg/ml、62.5μg/ml、31.3μg/ml。
[0094]
化合物t-1、t-2、t-3、t-4、t-7、t-14、t-15对玉米弯孢病菌(curvularia lunata)有杰出的生物活性,优于两组阳性对照组,最小抑菌浓度分别为31.25μg/ml、15.63μg/ml、31.3μg/ml、31.3μg/ml、31.3μg/ml、31.3μg/ml、15.63μg/ml。化合物t-11、t-12与阳性对照两性霉素的生物活性相当,最小抑菌浓度均为62.5μg/ml。
[0095]
通过比较这些化合物对不同植物病原真菌的抑菌活性发现,化合物t-2,它对油菜菌核病菌、番茄早疫病菌、棉花黄萎、黄瓜枯萎和玉米弯孢病菌的抑制作用均强于两组阳性对照,且对油菜菌核病菌和棉花黄萎病菌的最小抑菌浓度高达1.95μg/ml,它对核桃腐霉的抑制作用强于对照两性霉素而与多菌灵相当;化合物t-4对番茄早疫病菌、黄瓜枯萎、核桃腐霉和玉米弯孢的抑制作用强于两组对照,且对其它2种植物病原真菌都有抑制作用并且强于或等于其中一个对照;化合物t-4对棉花黄萎、黄瓜枯萎、核桃腐霉和玉米弯孢病菌的抑菌效果优于两组对照,同样对其它2种植物病原真菌都有抑制作用并且强于或等于其中一个对照,且对核桃腐霉的最小抑制活性达3.9μg/ml。然而,化合物t-5、t-6、t-9、t-10、t-17、t-18t-19六种植物病原真菌的抑制作用较差,有的甚至有的没有抑制作用。
相关技术
网友询问留言
已有0条留言
- 还没有人留言评论。精彩留言会获得点赞!
1