一种基于非活化烯烃远程羧基化合成二元羧酸类化合物的方法与流程
1.本发明涉及有机合成技术领域,具体涉及到一种基于非活化烯烃远程羧基化合成二元羧酸类化合物的方法。
背景技术:
2.烯烃类化合物的广泛存在使其在有机合成化学中占据着非常重要的地位,通过对烯烃双键的官能化可以合成多种多样的化合物,目前已被大量应用于药物化学、高分子化学和材料化学等领域。
3.近年来,随着化学家们的努力,活化烯烃的双官能团化取得了较大的进展,且已成为高效构建复杂化合物分子的有效手段,但非活化烯烃的双官能团化仍然面临着巨大挑战。由于非活化烯烃和co2的化学惰性使得非活化烯烃的双羧基化反应研究鲜有报道,因此,合成不同链长的二元羧酸面临更大的挑战。另一方面,二氧化碳作为一种来源广泛、廉价易得、可再生的优良的碳一合成子被广泛应用于各类化学合成中。鉴于此,提供一种利用二氧化碳实现非活化烯烃远程羧基化合成二元羧酸类化合物的方法也就显得十分的有意义。
技术实现要素:
4.针对上述的不足,本发明的目的是提供一种基于非活化烯烃远程羧基化合成二元羧酸类化合物的方法,可有效解决现有技术中利用二氧化碳实现非活化烯烃的远程双羧基化反应研究空白的问题,同时该方法具有操作方便、原料廉价易得、反应条件温和、底物普适性广和产物收率高的特点。
5.为达上述目的,本发明采取如下的技术方案:
6.本发明提供一种基于非活化烯烃远程羧基化合成二元羧酸类化合物的方法,包括以下步骤:
7.将烯烃化合物、光催化剂和碱加入反应容器中,然后在co2气氛下加入还原剂和溶剂,于可见光照射条件下,室温搅拌反应0.1~100h,对反应产物进行分离纯化,制得二元羧酸类化合物;其中,烯烃化合物、光催化剂、碱和还原剂的摩尔比为1:(0.001~0.5):(0.1~10):(1~10);
8.烯烃化合物的结构通式如下所示:
[0009][0010]
其中,r1为氢、酯基、羧基、酰胺基、氰基、芳基、杂芳基、炔基、烯基或烷基(烷基包
含甲基、乙基、异丙基、叔丁基、羟甲基、乙酰氧基甲基、三氟甲基等);r2为氢、酯基、羧基、酰胺基、氰基、芳基、杂芳基、炔基、烯基或烷基(烷基包含甲基、乙基、异丙基、叔丁基、羟甲基、乙酰氧基甲基、三氟甲基等);r3为氢、芳基、杂芳基、炔基、烯基、酯基、羧基、酰胺基、氰基或烷基(烷基包含甲基、乙基、异丙基、叔丁基、羟甲基、乙酰氧基甲基、三氟甲基等);r4为氢、芳基、杂芳基、酯基、羧基、酰胺基、氰基、氟、氯、溴、碘、硼基、硅基、膦基、硫醚基、烷氧基、酰氧基、芳氧基、胺基、炔基、烯基或烷基(烷基包含甲基、乙基、异丙基、叔丁基、羟甲基、乙酰氧基甲基、三氟甲基等);r5为氢、甲氧羰基、乙氧羰基或叔丁氧羰基或烷基(烷基包含甲基、乙基、异丙基、叔丁基、羟甲基、乙酰氧基甲基、三氟甲基等);r6为氢、芳基、杂芳基、炔基、烯基、酯基、羧基、酰胺基、氰基或烷基(烷基包含甲基、乙基、异丙基、叔丁基、羟甲基、乙酰氧基甲基、三氟甲基等);r7为氢、芳基、杂芳基、酯基、羧基、酰胺基、氰基、氟、氯、溴、碘、硼基、硅基、膦基、硫醚基、烷氧基、酰氧基、芳氧基、胺基、炔基、烯基或烷基(烷基包含甲基、乙基、异丙基、叔丁基、羟甲基、乙酰氧基甲基、三氟甲基等);r8为氢、芳基、杂芳基、炔基、烯基、酯基、羧基、酰胺基、氰基或烷基(烷基包含甲基、乙基、异丙基、叔丁基、羟甲基、乙酰氧基甲基、三氟甲基等);r9为氢、芳基、杂芳基、炔基、烯基、酯基、羧基、酰胺基、氰基或烷基(烷基包含甲基、乙基、异丙基、叔丁基、羟甲基、乙酰氧基甲基、三氟甲基等);r
10
为氢、芳基、杂芳基、酯基、羧基、酰胺基、氰基、氟、氯、溴、碘、硼基、硅基、膦基、硫醚基、烷氧基、酰氧基、芳氧基、胺基、炔基、烯基或烷基(烷基包含甲基、乙基、异丙基、叔丁基、羟甲基、乙酰氧基甲基、三氟甲基等);r
11
为氢或烷基;n为1或2或3;虚线环为芳环或杂芳环(如吡啶环、呋喃环或噻吩环),优选为苯环。
[0011]
进一步地,烯烃化合物、光催化剂、碱和还原剂的摩尔比为1:(0.005~0.1):(4.5~6.5):2.5。
[0012]
进一步地,烯烃化合物的结构式如下所示:
[0013]
[0014][0015]
进一步地,光催化剂为d-a型光催化剂或ir光催化剂。
[0016]
进一步地,光催化剂为4czipn、4dpaipn、3dpafipn、3dpa2fbn、5czbn、4czpn、dpz、4czpn-ph、4czpn-bu、4cztpn、4cztpn-bu、ir(dfcf3ppy)2(dtbbpy)pf6、fac-ir(df(ppy)3)、fac-ir(ppy)3和ir(ppy)2(dtbbpy)pf6中至少一种。
[0017]
进一步地,碱的碳酸盐为cs2co3、k2co3、na2co3或li2co3;碳酸氢盐为cshco3、khco3或nahco3;氟化盐为csf或kf;叔丁醇盐为ko
t
bu、nao
t
bu或lio
t
bu;磷酸盐为k3po4或na3po4;羧酸盐为csoac、koac、naoac、csopiv或kopiv;有机碱为dbu、tbd、dabco、tmg或dbn。
[0018]
进一步地,还原剂有机胺类化合物为cy2net、cy2nme、ipr2net、net3或pmp(五甲基哌啶)。
[0019]
进一步地,可见光的波长为400~560nm,可见光的功率为3~60w,优选为20~30w。
[0020]
进一步地,二氧化碳压力为0.5-30倍大气压,优选为1~5倍大气压。
[0021]
进一步地,溶剂浓度为0.01-10.0m,溶剂优选为dmso、nmp、dmf、dmac、thf、dcm、meoh或mecn等。
[0022]
进一步地,反应时间为2~60h。
[0023]
本发明的反应式如下所示:
[0024][0025]
其中,r1为氢、酯基、羧基、酰胺基、氰基、芳基、杂芳基、炔基、烯基或烷基(烷基包含甲基、乙基、异丙基、叔丁基、羟甲基、乙酰氧基甲基、三氟甲基等);r2为氢、酯基、羧基、酰胺基、氰基、芳基、杂芳基、炔基、烯基或烷基(烷基包含甲基、乙基、异丙基、叔丁基、羟甲基、乙酰氧基甲基、三氟甲基等);r3为氢、芳基、杂芳基、炔基、烯基、酯基、羧基、酰胺基、氰基或烷基(烷基包含甲基、乙基、异丙基、叔丁基、羟甲基、乙酰氧基甲基、三氟甲基等);r4为氢、芳基、杂芳基、酯基、羧基、酰胺基、氰基、氟、氯、溴、碘、硼基、硅基、膦基、硫醚基、烷氧基、酰氧基、芳氧基、胺基、炔基、烯基或烷基(烷基包含甲基、乙基、异丙基、叔丁基、羟甲基、乙酰氧基甲基、三氟甲基等);r5为氢、甲氧羰基、乙氧羰基或叔丁氧羰基或烷基(烷基包含甲基、乙基、异丙基、叔丁基、羟甲基、乙酰氧基甲基、三氟甲基等);r6为氢、芳基、杂芳基、炔基、烯基、酯基、羧基、酰胺基、氰基或烷基(烷基包含甲基、乙基、异丙基、叔丁基、羟甲基、乙酰氧基甲基、三氟甲基等);r7为氢、芳基、杂芳基、酯基、羧基、酰胺基、氰基、氟、氯、溴、碘、硼基、硅基、膦基、硫醚基、烷氧基、酰氧基、芳氧基、胺基、炔基、烯基或烷基(烷基包含甲基、乙基、异丙基、叔丁基、羟甲基、乙酰氧基甲基、三氟甲基等);r8为氢、芳基、杂芳基、炔基、烯基、酯基、羧基、酰胺基、氰基或烷基(烷基包含甲基、乙基、异丙基、叔丁基、羟甲基、乙酰氧基甲基、三氟甲基等);r9为氢、芳基、杂芳基、炔基、烯基、酯基、羧基、酰胺基、氰基或烷基(烷基包含甲基、乙基、异丙基、叔丁基、羟甲基、乙酰氧基甲基、三氟甲基等);r
10
为氢、芳基、杂芳基、酯基、羧基、酰胺基、氰基、氟、氯、溴、碘、硼基、硅基、膦基、硫醚基、烷氧基、酰氧基、芳氧基、胺基、炔基、烯基或烷基(烷基包含甲基、乙基、异丙基、叔丁基、羟甲基、乙酰氧基甲基、三氟甲基等);r
11
为氢或烷基;n为1或2或3;虚线环为芳环或杂芳环(如吡啶环、呋喃环或噻吩环),优选为苯环。
[0026]
本发明的反应机理如图1所示,具体过程为:以ir
iii
催化剂为例;首先,光激发ir
iii
催化剂生成[ir
iii
]
*
物种,进一步在光作用下,将二氧化碳还原为二氧化碳自由基负离子和ir
iv
物种,ir
iv
物种再被电子还原剂还原为ir
iii
物种;形成的二氧化碳自由基负离子对烯烃进行自由基加成,生成烷基碳自由基中间体(i);随后发生分子内1,n-氢迁移,生成更稳定的苄基自由基(ii);苄基自由基(ii)进一步被还原生成苄基碳负离子中间体;最后进攻二氧化碳、酸化后得到目标二元羧酸衍生物。
[0027]
综上所述,本发明具有以下优点:
[0028]
1、本发明提供了一种基于非活化烯烃远程羧基化合成二元羧酸类化合物的方法,在可见光催化下,以非活化烯烃化合物作为反应底物,二氧化碳作为羧酸源,同时加入光催化剂、还原剂和碱,制得二元羧酸类化合物;该方法具有操作方便和原料廉价易得的特点;
[0029]
2、本发明的制备方法对于非活化烯烃类底物,表现出优异的反应性,室温下实现了非活化烯烃远程羧基化反应,具有反应条件温和、底物普适性广和产物收率高的特点。
附图说明
[0030]
图1为本发明中反应机理图。
具体实施方式
[0031]
为了使本发明的目的、技术方案及优点更加清楚明白,以下结合实施例,对本发明进行进一步详细说明。应当理解,此处所描述的具体实施例仅用以解释本发明,并不用于限定本发明,即所描述的实施例仅仅是本发明一部分实施例,而不是全部的实施例。
[0032]
因此,以下对提供的本发明的实施例的详细描述并非旨在限制要求保护的本发明的范围,而是仅仅表示本发明的选定实施例。基于本发明的实施例,本领域技术人员在没有做出创造性劳动的前提下所获得的所有其他实施例,都属于本发明保护的范围。
[0033]
实施例1
[0034]
本例提供一种基于非活化烯烃远程羧基化合成二元羧酸类化合物的方法,具体过程如下:
[0035]
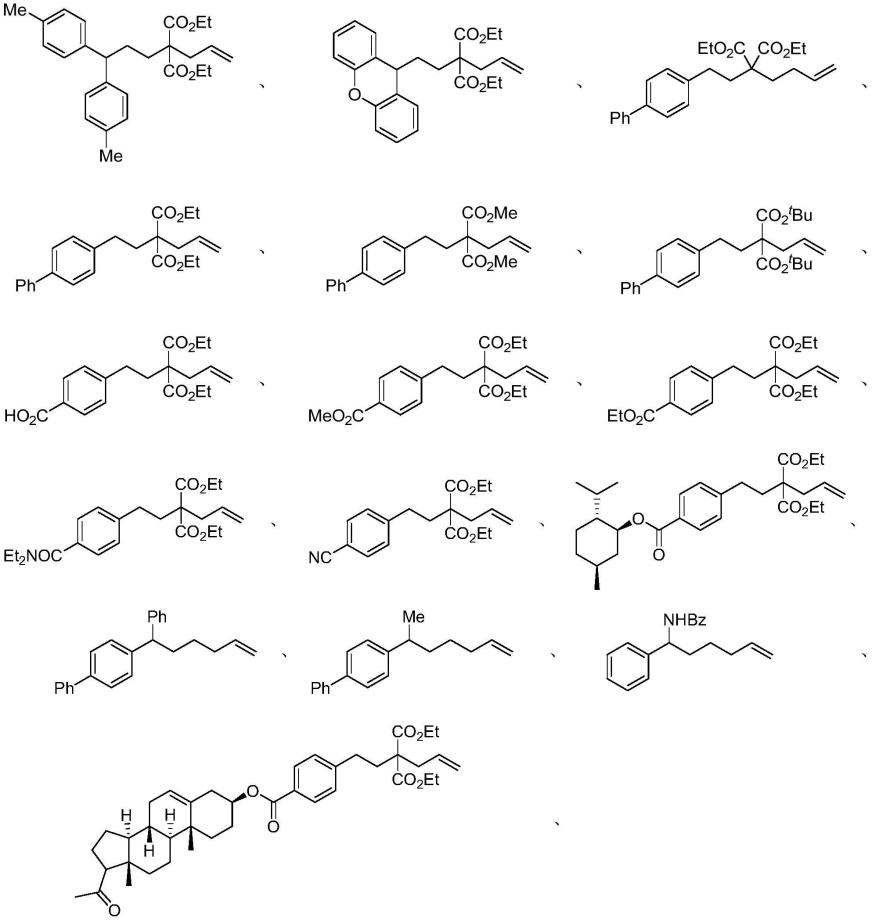
将配有搅拌子的25ml的schlenk反应管在真空下加热干燥好后,再加入0.2mmol非活化烯烃(若为固体反应底物此时加入;若为液体反应底物则在加入溶剂的前一步通过注射器加入)和光催化剂fac-ir(ppy)3(1mol%),随后置于手套箱,加入4.5当量cs2co3后封管拿出手套箱,在co2气氛的双排管下抽置换气三次,抽置换完毕后,于co2气流下加入2.5当量的dcyea(二环己基乙基胺)和2ml dmso;加完溶剂后,封管置于距30w蓝色leds 1cm处,在室温下搅拌48h,反应结束后,加入3ml的2n hcl和3ml乙酸乙酯,搅拌5min,随后加入15ml的水,用乙酸乙酯萃取6次,合并有机相,在旋转蒸发仪上旋干;固体残留物经过硅胶柱的分离,得到双羧基化衍生物的目标产物2。具体结果如下:
[0036]
[0037][0038]
注:上述结果均为分离结果,括号中为原料回收;[a]代表为4毫摩尔规模,反应时间为94h;[b]代表为使用6.5当量的cs2co3;[c]代表为使用5.5当量的cs2co3;[d]代表为通过tmsch2n2进行酯化;[e]代表为底物完全转化。
[0039]
上述实验结果表明,不同取代基修饰地长链烯烃底物,不管是富电子基团、贫电子基团和电中性基团都能兼容,且都能以中等偏上的产率得到目标双羧基化产物。多种官能团或取代基在该反应体系中都能兼容,包括:氟、甲基甲氧基、苯基、羧基、酰胺基。此外,该体系还能兼容二苯并吡喃衍生地长链烯烃和单芳基取代地烯烃等,且反应效果佳。
[0040]
实施例2
[0041]
本例提供一种基于非活化烯烃远程羧基化合成二元羧酸类化合物的方法,具体过程如下:
[0042]
将配有搅拌子的25ml的schlenk反应管在真空下加热干燥好后,再加入0.2mmol非活化烯烃(若为固体反应底物此时加入;若为液体反应底物则在加入溶剂的前一步通过注射器加入)和光催化剂fac-ir(ppy)3(0.5mol%),随后置于手套箱,加入5当量cs2co3后封管拿出手套箱,在co2气氛的双排管下抽置换气三次,抽置换完毕后,于co2气流下加入2.5当量
的dcyea和2ml dmso;加完溶剂后,将反应液封管置于距30w蓝色leds 1cm处,在室温下搅拌48h,反应结束后,加入3ml的2n hcl和3ml乙酸乙酯,搅拌5min,随后加入15ml的水,用乙酸乙酯萃取6次,合并有机相,在旋转蒸发仪上旋干;固体残留物经过硅胶柱的分离,得到羧酸目标产物5或6。具体结果如下:
[0043][0044]
注:上述结果均为分离结果,括号中为原料回收;[a]代表为酸化后柱分离;[b]代表为fac-ir(ppy)3(1mol%);[c]代表为fac-ir(ppy)3(1mol%),cs2co3(4.5当量)。
[0045]
上述实验结果表明,本发明的反应能兼容许多常见的官能团,例如:羧基,酯基、酰胺基团、氰基,都能以中等偏上的产率得到相应的双羧基化产物。此外,天然产物衍生的底物如孕烯醇酮、薄荷醇,也能以中等的产率得到目标产物。对于当底物中不存在桥联基团的柔性烯烃也能得到相应的目标产物。值得注意的是,当迁移后的自由基处在胺基α位时,能够构建含远端羧基的α-氨基酸衍生物。
[0046]
实施例3
[0047]
本例提供一种基于非活化烯烃远程羧基化合成二元羧酸类化合物的方法,具体过程如下:
[0048]
将配有搅拌子的25ml的schlenk反应管在真空下加热干燥好后,再加入0.2mmol非活化烯烃(若为固体反应底物此时加入;若为液体反应底物则在加入溶剂的前一步通过注射器加入)和光催化剂fac-ir(ppy)3(1mol%),随后置于手套箱,加入4.5当量cs2co3后封管拿出手套箱,在co2气氛的双排管下抽置换气三次,抽置换完毕后,于co2气流下加入2.5当量的dcyea和2ml dmso;加完溶剂后,封管置于距30w蓝色leds 1cm处,在室温下搅拌48h,反应
结束后,加入3ml的2n hcl和3ml乙酸乙酯,搅拌5min,随后加入15ml的水,用乙酸乙酯萃取6次,合并有机相,在旋转蒸发仪上旋干;固体残留物经过硅胶柱的分离,得到双羧基化衍生物的目标产物9或10。具体结果如下:
[0049][0050]
注:上述结果均为分离结果,括号中为原料回收;[a]代表为cs2co3(5.5当量);[b]代表为1hnmr测定9l:9c=7.1:1,7l:7c=1:1;[c]代表为fac-ir(ppy)3(1mol%)cs2co3(5当量)。
[0051]
以上实验结果表明:当选用含非活化烯烃的苄胺衍生物为底物时,可以得到一系列官能团化的α氨基酸衍生物。反应对于给电子基团、电中性基团和拉电子基团都能兼容,且对于常见的各种官能团,如卤素氟原子、羧基、酯基、酰胺基团、甲氧基和苯氧基等都能兼容。值得注意的是,反应中对于含氯原子的底物而言,也能兼容,但也会存在少量脱氯产物,推测原因可能是与所形成的co
2-直接还原c
–
cl键有关。此外,反应受位阻影响小,邻位取代基团都能以中的偏上的产率得到相应的目标产物。反应也能兼容二取代的底物,并且能构建全碳α氨基酸。对于二取代烯烃而言,尽管转化率不高,但也能以较好的选择性得到中等产率的目标产物。令人高兴的是,对于不含胺基取代的底物而言,也能以较好的产率得到双羧基化产物。
[0052]
实施例4
[0053]
本例以2-烯丙基-2-(3,3-二苯丙基)丙二酸二乙酯作为反应底物,通过改变反应条件,考察对反应产率的影响。具体过程如下:
[0054][0055]
注:上述中产率和转化率是以dmap为内标的核磁产率,括号中为分离产率。pc1=ir(ppy)2(dtbbpy)pf6,pc2=3dpafipn。
[0056]
从上述实验结果可以看出,在本发明反应条件下对应羧酸的核磁收率高达82%,一系列控制实验表明,铱光催化剂、碱、还原剂、光照和二氧化碳都是必不可少的,缺少任意一项,均不能得到目标产物。dmso、cs2co3、dcyea对反应有明显促进用。当使用其他光催化剂、溶剂、碱或者还原剂时,产率明显下降。
[0057]
对本发明所制得的产物进行了核磁共振和质谱表征分析,核磁和质谱表征数据结果与所得产物一致。具体表征数据如下:
[0058]
5,5-双(乙氧羰基)-2,2-二苯基壬二酸
[0059]
13
c nmr(101mhz,cd3od)δ175.68,175.31,171.35,142.97,128.66,127.46,126.43,60.90,59.53,56.97,33.30,32.12,30.72,26.93,18.84,12.95;hrms(esi-):calculated for c
27h31o8-[m-h]-483.2024,found 483.2126.
[0060]
5,5-双(甲氧羰基)-2,2-二苯基壬二酸
[0061]
mhz,cd3od)δ175.64,175.30,171.77,142.91,128.63,127.47,126.45,59.51,57.09,
51.44,33.19,32.13,30.80,27.08,18.86;hrms(esi-):calculated for c
24h27o6-[m-h-co2]-411.1813,found 411.1810.
[0062]
5,5-双(叔丁氧羰基)-2,2-二苯基壬二酸
[0063]
cd3od)δ175.61,175.32,170.70,143.13,128.71,127.44,126.42,80.91,59.48,57.75,33.45,32.12,30.39,26.62,26.41,18.73;hrms(esi-):calculated for c
30h39o6-[m-h-co2]-492.2752,found 492.2752.
[0064]
5,5-双(乙酰氧基甲基)-2,2-二苯基壬二酸
[0065]
1.18
–
1.05(m,2h);
13
c nmr(101mhz,cd3od)δ175.84,175.66,171.21,143.31,128.81,127.49,126.44,65.17,59.77,39.25,33.77,30.98,29.95,25.70,19.38,17.65;hrms(esi-):calculated for c
26h31o6-[m-h-co2]-439.2126,found 439.2129.
[0066]
5,5-双(羟甲基)-2,2-二苯基壬二酸
[0067]
13
c nmr(101mhz,cd3od)δ176.65,176.18,143.34,128.86,127.38,126.29,64.72,60.03,41.39,34.11,30.99,29.44,25.58,17.75;hrms(esi-):calculated for c
22h27o4-[m-h-co2]-355.1915,found 355.1914.
[0068]
5-(乙氧羰基)-2,2,5-三苯基壬二酸
[0069]
cd3od)δ176.07,175.81,175.67,143.10,143.01,142.20,128.76,128.75,127.95,127.45,126.42,126.39,126.12,60.52,59.72,53.30,33.71,33.57,32.27,29.29,19.17,12.93;hrms(esi-):calculated for c
29h31o4-[m-h-co2]-443.2228,found 443.2225.
[0070]
2-([1,1'-联苯]-4-基)-5,5-双(乙氧羰基)-2-苯基壬二酸
[0071]
0.9hz,6h);
13
c nmr(101mhz,cdcl3)δ180.76,180.29,171.20,171.19,141.95,141.04,140.50,139.85,129.44,128.98,128.76,128.07,127.35,127.19,127.05,126.64,61.22,59.73,56.99,34.30,31.89,30.54,26.53,18.91,14.00;hrms(esi-):calculated for c
32h35o6-[m-h-co2]-515.2439,found 515.2444.
[0072]
2-(4-羧苯基)-5,5-双(乙氧羰基)-2-苯基壬二酸
[0073]
cd3od)δ175.37,175.01,171.37,168.20,148.47,142.48,128.97,128.92,128.87,128.56,127.75,126.79,61.03,59.77,57.04,33.27,32.10,30.83,27.07,18.91,12.99;hrms(esi-):calculated for c
27h31o8-[m-h-co2]-483.2024,found 483.2021.
[0074]
2-(4-(二乙基氨基甲酰基)苯基)-5,5-双(乙氧基羰基)-2-苯基壬二酸
[0075]
135.11,129.07,128.56,127.71,126.73,125.50,61.00,59.58,57.04,43.63,39.51,33.31,32.14,30.89,27.20,18.94,13.05,13.03,11.71;hrms(esi-):calculated for c
31h40
no
7-[m-h-co2]-538.2810,found 538.2804.
[0076]
5,5-双(乙氧羰基)-2-(4-氟苯基)-2-苯基壬二酸
[0077]
175.44,175.32,171.38,161.59(d,j=245.0hz),142.90,139.06(d,j=3.3hz),130.63(d,j=8.0hz),128.53,127.64,126.62,114.07(d,j=21.4hz),60.99,59.07,57.04,33.31,32.31,30.85,27.10,18.92,13.00;
19
f nmr(376mhz,cd3od)δ-118.02;hrms(esi-):calculated for c
26h30
fo
6-[m-h-co2]-457.2032,found 457.2031.
[0078]
5,5-双(乙氧羰基)-2-(2-氟苯基)-2-苯基壬二酸
[0079]
4.4hz,1h),1.35
–
1.27(m,2h),1.22(dt,j=8.9,7.1hz,6h);
13
c nmr(101mhz,cd3od)δ175.36,175.29,171.32,160.99(d,j=246.9hz),140.06,130.57(d,j=11.9hz),130.36(d,j=3.9hz),128.81(d,j=8.9hz),128.38,127.72,126.96,123.22(d,j=3.2hz),115.53(d,j=23.4hz),61.03(d,j=1.8hz),57.09,56.64,33.41,31.21,29.34,27.29,19.13,13.02,12.98;
19
f nmr(376mhz,cd3od)δ-109.59;hrms(esi-):calculated for c
26h30
fo
6-[m-h-co2]-457.2032,found 457.2028.
[0080]
4,4-二乙基1,7-二甲基-1-苯基-1-(邻甲苯基)庚烷-1,4,4,7-四羧酸酯
[0081]
2.18(m,3h),1.94
–
1.81(m,5h),1.74
–
1.66(m,2h),1.35
–
1.24(m,2h),1.21(t,j=7.1hz,3h),1.17(t,j=7.1hz,3h);
13
c nmr(101mhz,cdcl3)δ174.48,173.26,171.28,171.24,141.92,140.67,137.19,132.29,128.78,128.68,127.90,127.13,126.72,125.46,61.20,58.85,57.07,52.32,51.51,34.04,31.98,31.62,27.52,20.95,19.29,14.05,14.00;hrms(esi+):calculated for c
30h38
nao
8+
[m+na]
+
549.2459,found 549.2455.
[0082]
1,4-二乙基1,7-二甲基1,1-二-对甲苯基庚烷-1,4,4,7-四羧酸酯
[0083]
mhz,cdcl3)δ174.52,173.31,171.34,139.65,136.42,128.67,128.63,61.15,59.24,57.09,52.31,51.50,34.04,32.43,31.33,27.20,20.96,19.25,14.02;hrms(esi+):calculated for c
31h40
nao
8+
[m+na]
+
563.2615,found 563.2612.
[0084]
5,5-双(乙氧羰基)-2-(2-氟苯基)-2-(4-氟苯基)壬二酸
[0085]
=13.8,3.8hz,1h),1.49(dtd,j=26.3,13.0,12.1,5.7hz,2h),1.14(t,j=7.1hz,3h),1.11(t,j=7.1hz,3h);
13
c nmr(101mhz,cdcl3)δ180.13,180.09,171.13,171.08,162.12(d,j=247.3hz),160.71(d,j=248.4hz),135.03(d,j=3.3hz),130.64(d,j=3.3hz),
130.54(d,j=8.0hz),129.48(d,j=11.7hz),129.30(d,j=8.8hz),123.65(d,j=3.4hz),116.10(d,j=22.9hz),115.16(d,j=21.5hz),61.30,56.98,56.52,33.96,30.57,29.10,26.35,18.86,13.97,13.91;
19
fnmr(376mhz,cdcl3)δ-107.62,-114.81;hrms(esi-):calculated for c
27h29
f2o
8-[m-h]-519.1836,found 519.1828.
[0086]
5,5-双(乙氧羰基)-2,2-双(4-氟苯基)壬二酸
[0087]
1.18(t,j=7.1hz,6h);
13
c nmr(101mhz,cd3od)δ1175.32,175.20,171.34,161.64(d,j=245.2hz),138.98(d,j=3.3hz),130.48(d,j=8.0hz),114.20(d,j=21.6hz),61.02,58.56,57.01,33.27,32.42,30.83,27.12,18.91,12.98;
19
f nmr(376mhz,cd3od)δ-117.84;hrms(esi-):calculated for c
26h29
f2o
6-[m-h-co2]-475.1938,found 475.1935.
[0088]
5,5-双(乙氧羰基)-2-(4-氟苯基)-2-(4-甲氧基苯基)壬二酸
[0089]
175.30,171.34,161.49(d,j=244.9hz),158.51,139.31(d,j=3.4hz),134.63,130.53(d,j=8.0hz),129.58,113.98(d,j=21.4hz),112.87,60.95,58.33,56.96,54.25,33.26,32.34,30.73,26.98,18.86,12.96;
19
f nmr(376mhz,cd3od)δ-118.18;hrms(esi-):calculated for c
27h32
fo
7-[m-h-co2]-487.2138,found 487.2134.
[0090]
9-(6-羧基-3,3-双(乙氧基羰基)己基)-9h-吨-9-羧酸
[0091]
175.59,175.24,171.14,150.73,128.63,126.80,123.13,121.10,116.30,60.96,56.70,49.27,34.35,33.26,30.74,26.16,18.81,12.95;hrms(esi-):calculated for c
27h29o9-[m-h]-497.1817,found497.1814.
[0092]
3,3-二乙基1,7-二甲基-1-([[1,1'-联苯]-4-基)庚烷-1,3,3,7-四羧酸酯
[0093]
4.12
–
4.05(m,1h),3.96(dq,j=10.8,7.1hz,1h),3.74(dd,j=7.6,5.0hz,1h),3.67(s,
3h),3.66(s,3h),2.84(dd,j=14.7,7.6hz,1h),2.40(dd,j=14.7,5.1hz,1h),2.29(t,j=7.5hz,2h),1.92(tt,j=14.3,7.2hz,2h),1.71
–
1.54(m,2h),1.32
–
1.10(m,8h);
13
c nmr(101mhz,cdcl3)δ173.93,173.78,171.19,170.95,140.67,140.40,138.41,128.78,128.42,127.40,127.34,127.05,61.38,61.25,56.83,52.23,51.49,46.99,35.91,33.68,32.78,25.04,23.59,14.02,13.90;hrms(esi+):calculated for c
29h36
nao
8+
[m+na]
+
535.2302,found 535.2273.
[0094]
3,3-二乙基1,6-二甲基-1-([[1,1'-联苯]-4-基)己烷-1,3,3,6-四羧酸酯
[0095]
j=10.8,7.1hz,1h),3.95(dq,j=10.8,7.1hz,1h),3.80(dd,j=7.7,5.1hz,1h),3.69(s,3h),3.67(s,3h),2.86(dd,j=14.8,7.7hz,1h),2.44(dd,j=14.8,5.1hz,1h),2.38
–
2.23(m,2h),2.04
–
1.86(m,2h),1.61
–
1.43(m,2h),1.27(t,j=7.1hz,3h),1.21(t,j=7.2hz,3h);
13
c nmr(101mhz,cdcl3)δ173.88,173.31,171.03,170.76,140.62,140.34,138.24,128.74,128.39,127.35,127.30,127.01,61.46,61.29,56.59,52.23,51.59,46.78,35.72,33.86,32.47,19.50,13.99,13.85;hrms(esi+):calculated for c
28h34
nao
8+
[m+na]
+
521.2146,found 521.2131.
[0096]
1-([1,1'-联苯]-4-基)四甲基-1,3,3,6-四羧酸酯
[0097]
hz,1h),3.71(s,3h),3.69(s,3h),3.67(s,3h),3.58(s,3h),2.86(dd,j=14.8,7.9hz,1h),2.44(dd,j=14.8,5.1hz,1h),2.31(td,j=7.3,3.0hz,2h),2.07
–
1.88(m,2h),1.59
–
1.43(m,2h);
13
c nmr(101mhz,cdcl3)δ173.82,173.30,171.46,171.18,140.56,140.36,138.07,128.76,128.37,127.40,127.33,127.01,56.57,52.62,52.38,52.27,51.64,46.75,35.93,33.75,32.71,19.52;hrms(esi+):calculated for c
26h31o8+
[m+h]
+
470.2013,found 470.2015.
[0098]
1,3-二叔丁基1,6-二甲基-1-([[1,1'-联苯]-4-基)己烷-1,3,3,6-四羧酸酯
[0099]
14.9,7.7hz,1h),2.30(dd,j=14.9,4.7hz,1h),2.24
–
2.06(m,2h),1.83
–
1.66(m,2h),1.54
–
1.43(m,10h),1.40
–
1.26(m,10h);
13
c nmr(101mhz,cdcl3)δ174.07,173.34,170.33,170.21,140.75,140.35,138.92,128.75,128.40,127.45,127.29,127.06,81.72,81.61,58.08,52.21,51.46,46.90,35.24,34.07,31.92,27.87,27.81,26.92,19.48;hrms(esi+):calculated for c
32h42
nao
8+
[m+na]
+
577.2772,found 577.2771.
[0100]
3,3-二乙基1,6-二甲基-1-(4-(甲氧基羰基)苯基)己烷-1,3,3,6-四羧酸酯
[0101]
7.39(d,j=7.9hz,2h),4.22
–
4.02(m,3h),3.97
–
3.87(m,4h),3.83(dd,j=7.6,5.1hz,1h),3.68(s,3h),3.63(s,3h),2.83(dd,j=14.8,7.9hz,1h),2.42
–
2.24(m,3h),2.01
–
1.84(m,2h),1.58
–
1.39(m,2h),1.24(t,j=7.3hz,3h),1.19(t,j=7.3hz,3h);
13
c nmr(101mhz,cdcl3)δ173.32,173.27,170.92,170.65,166.72,144.42,129.96,129.31,128.07,61.51,61.34,56.58,52.31,52.10,51.58,47.20,35.66,33.80,32.59,19.48,13.97,13.84;hrms(esi+):calculated for c
24h32
nao
10+
[m+na]
+
503.1388,found 503.1377.
[0102]
4,4-双(乙氧羰基)-2-(4-(乙氧羰基)苯基)辛二酸
[0103]
hz,2h),4.06(dq,j=10.8,7.2hz,1h),3.91(dq,j=10.8,7.1hz,1h),3.82(dd,j=7.7,5.0hz,1h),2.80(dd,j=14.8,7.7hz,1h),2.38
–
2.21(m,3h),1.95(qdd,j=14.1,11.5,5.1hz,2h),1.59
–
1.46(m,1h),1.40(t,j=7.1hz,3h),1.25(t,j=7.1hz,3h),1.19(t,j=7.1hz,3h);
13
cnmr(101mhz,cd3od)δ175.27,174.57,170.95,170.74,166.26,145.26,129.35,129.23,127.94,61.18,61.00,60.75,56.44,47.13,35.34,33.14,32.17,19.16,13.18,12.91,12.75;hrms(esi-):calculated for c
22h29o8-[m-h-co2]-421.1868,found 421.1870.
[0104]
3,3-二乙基1,6-二甲基-1-(4-(二乙基氨基甲酰基)苯基)己烷-1,3,3,6-四羧酸酯
[0105]
hz,1h),3.78(dd,j=8.5,4.3hz,1h),3.68(s,3h),3.62(s,3h),3.58
–
3.46(m,2h),3.33
–
3.20(m,2h),2.80(dd,j=14.7,8.5hz,1h),2.39
–
2.26(m,3h),2.02
–
1.83(m,2h),1.58
–
1.38(m,2h),1.29
–
1.18(m,9h),1.18
–
1.07(m,3h);
13
c nmr(101mhz,cdcl3)δ173.53,173.29,170.94,170.82,170.70,140.38,136.33,127.96,126.66,61.47,61.30,56.52,52.20,51.59,46.92,43.19,39.14,35.70,33.78,32.53,19.42,14.22,13.96,13.85,12.84;hrms(esi+):calculated for c
27h39
no
9+
[m+h]
+
522.2698,found 522.2697.
[0106]
2-(4-氰基苯基)-4,4-双(乙氧基羰基)辛二酸
[0107]
hz,1h),2.80(dd,j=14.8,7.7hz,1h),2.36
–
2.23(m,3h),2.04
–
1.88(m,2h),1.58
–
1.47(m,1h),1.45
–
1.36(m,1h),1.25(t,j=7.1hz,3h),1.20(t,j=7.1hz,3h);
13
c nmr(101mhz,
cd3od)δ175.27,174.11,170.88,170.65,145.51,132.12,128.88,118.08,110.81,61.22,61.03,56.35,35.21,33.06,32.17,19.09,12.89,12.74;hrms(esi-):calculated for c
21h24
no
8-[m-h]-418.1507,found 418.1505.
[0108]
4,4-双(乙氧羰基)-2-(4-(((3s,8s,9s,10r,13s,14s)-17-乙酰基-10,13-二甲基-2,3,4,7,8,9,10,11,12,13,14,15,16,17-十四氢-1h-环戊[a]菲基-3-基)羰基)苯基)辛二酸
[0109]
hz,1h),2.85(dd,j=14.9,7.5hz,1h),2.56(t,j=8.9hz,1h),2.46(d,j=8.1hz,2h),2.43
–
2.29(m,3h),2.26
–
1.87(m,11h),1.83
–
1.37(m,12h),1.31
–
1.18(m,7h),1.15(t,j=7.1hz,3h),1.11
–
0.94(m,5h),0.66(s,3h);
13
cnmr(101mhz,cdcl3)δ209.87,178.83,178.38,170.82,170.61,165.62,143.83,139.63,130.13,130.01,128.12,122.50,74.54,63.70,61.59,61.41,56.85,56.60,49.90,47.19,44.04,38.80,38.13,37.04,36.66,35.10,33.60,32.28,31.84,31.80,31.56,27.83,24.50,22.85,21.07,19.38,19.09,13.98,13.82,13.24;hrms(esi-):calculated for c
41h55o9-[m-h-co2]-691.3852,found 691.3849.
[0110]
4,4-双(乙氧基羰基)-2-(4-((((2-异丙基-5-甲基环己基)氧基)羰基)苯基)辛二酸7.37(d,j=8.1hz,2h),4.91(td,j=10.8,4.4hz,1h),4.21
–
4.08(m,2h),4.07
–
3.97(m,1h),3.88
–
3.79(m,1h),3.75(dt,j=7.9,4.1hz,1h),2.87(ddd,j=15.0,7.5,2.0hz,1h),2.46
–
2.27(m,3h),2.16
–
2.06(m,1h),1.95(tp,j=21.1,6.8,6.1hz,3h),1.72(dt,j=12.6,3.0hz,2h),1.65
–
1.50(m,3h),1.40(dq,j=13.0,6.5,6.1hz,1h),1.21(t,j=7.1hz,3h),1.17
–
1.03(m,5h),0.91(dd,j=9.0,6.8hz,7h),0.77(d,j=6.9hz,3h);
13
c nmr(101mhz,cdcl3)δ179.02,178.58,170.77,170.75,170.59,170.57,165.66,143.75,143.68,130.14,129.98,128.11,128.07,74.90,61.55,61.38,61.37,56.59,56.57,47.20,47.10,40.87,34.93,34.79,34.26,33.55,32.00,31.94,31.40,26.50,26.46,23.61,22.01,20.72,20.70,18.97,16.51,16.47,13.95,13.76;hrms(esi-):calculated for c
30h43o8-[m-h-co2]-531.2963,found 531.2957.
[0111]
2,2-二苯基辛二酸
[0112]
2h),1.39
–
1.27(m,2h),1.18
–
1.08(m,2h);
13
c nmr(101mhz,cd3od)δ176.33,176.16,143.42,128.66,127.32,126.21,59.98,37.75,33.33,29.15,24.84,24.40;hrms(esi-):calculated for c
20h21o4-[m-h]-325.1445,found 325.1442.
[0113]
2-([[1,1'-联苯]-4-基)-2-苯基辛二酸
[0114]
1h),2.44
–
2.31(m,2h),2.18(t,j=7.4hz,2h),1.50(p,j=7.4hz,2h),1.37
–
1.25(m,2h),1.12(tq,j=12.4,7.8,6.2hz,2h);
13
c nmr(101mhz,cd3od)δ176.27,176.20,143.40,142.54,140.50,139.26,129.21,128.66,128.41,127.40,126.88,126.49,126.48,126.27,125.83,59.78,37.75,33.36,29.19,24.90,24.42;hrms(esi-):calculated for c
25h25o2-[m-h-co2]-357.1860,found 357.1861.
[0115]
2-([[1,1'-联苯]-4-基)-2-甲基辛二酸
[0116]
j=13.3,9.3,6.6hz,1h),1.98
–
1.89(m,1h),1.66
–
1.50(m,5h),1.42
–
1.32(m,2h),1.30
–
1.20(m,2h);
13
c nmr(101mhz,cd3od)δ178.53,176.23,143.34,140.62,139.31,128.43,126.87,126.50,126.42,126.31,49.67,38.80,33.44,29.33,24.50,24.26,22.02;hrms(esi-):calculated for c
21h23o4-[m-h]-339.1602,found 339.1600.
[0117]
2-苯甲酰胺基-2-苯基辛二酸
[0118]
2.92
–
2.82(m,1h),2.69
–
2.59(m,1h),2.28(t,j=7.3hz,2h),1.63(p,j=7.2hz,2h),1.51
–
1.38(m,3h),1.33
–
1.25(m,1h);
13
c nmr(101mhz,cd3od)δ176.11,174.16,167.09,140.03,134.39,131.54,128.39,127.95,127.18,126.70,125.85,65.54,33.29,32.51,28.68,24.44,23.77;hrms(esi-):calculated for c
21h22
no
5-[m-h]-368.1503,found 368.1506.
[0119]
4-(2-(苯甲酰胺基(羧基)甲基)苯基)丁酸
[0120]
hz,2h),2.36(t,j=7.4hz,2h),2.02
–
1.91(m,2h);
13
c nmr(101mhz,cd3od)δ175.82,172.93,168.64,140.63,134.73,133.80,131.44,129.79,128.19,128.10,127.43,127.26,
126.36,53.40,33.14,31.80,26.27;hrms(esi-):calculated for c
18h18
no
3-[m-h,-co2]-296.1292,found 296.1287.
[0121]
4-(4-(苯甲酰胺基(羧基)甲基)-[1,1'-联苯]-3-基)丁酸
[0122]
6.04(s,1h),2.97(t,j=8.0hz,2h),2.44(t,j=7.2hz,2h),2.06(tt,j=15.5,7.5hz,2h);
13
cnmr(101mhz,cd3od)δ175.87,172.89,168.65,141.27,141.09,140.48,133.83,133.80,131.47,128.47,128.38,128.12,127.99,127.27,127.13,126.62,124.94,53.25,33.10,31.96,26.25;hrms(esi-):calculated for c
25h22
no
5-[m-h]-416.1503,found 416.1501.
[0123]
4-(2-(苯甲酰胺基(羧基)甲基)-5-氟苯基)丁酸
[0124]
hz,1h),5.97(s,1h),2.90(td,j=7.5,3.1hz,2h),2.42(t,j=7.3hz,2h),2.10
–
1.89(m,2h);
13
c nmr(101mhz,cd3od)δ175.67,172.69,168.55,162.48(d,j=245.7hz),143.52(d,j=7.4hz),133.63,131.46,130.92(d,j=3.2hz),129.44(d,j=8.8hz),128.08,127.21,115.95(d,j=21.5hz),112.94(d,j=21.6hz),52.83,32.96,31.71,25.90;
19
f nmr(376mhz,cd3od)δ-115.92;hrms(esi-):calculated for c
19h17
fno
5-[m-h]-358.1096,found 358.1096.
[0125]
4-(2-(苯甲酰胺基(羧基)甲基)-5-甲基苯基)丁酸
[0126]
1.8hz,1h),7.03(dd,j=8.0,1.8hz,1h),5.90(s,1h),2.80(dd,j=8.8,7.1hz,2h),2.35(t,j=7.4hz,2h),2.30(s,3h),2.01
–
1.87(m,2h);
13
c nmr(101mhz,cd3od)δ175.82,173.09,168.58,140.38,138.03,133.74,131.56,131.37,130.38,128.04,127.34,127.21,126.97,53.15,33.10,31.75,26.29,19.74;hrms(esi-):calculated for c
20h20
no
5-[m-h]-354.1347,found 354.1347.
[0127]
4-(苯甲酰胺基(羧)甲基)-3-(3-羧丙基)苯甲酸
[0128]
(td,j=7.2,2.0hz,2h),2.44(t,j=7.3hz,2h),2.17
–
1.95(m,2h);
13
c nmr(101mhz,cd3od)δ175.65,172.17,168.54,168.02,141.01,140.02,133.59,131.49,130.85,130.40,128.09,127.57,127.47,127.22,53.20,33.03,31.72,26.01;hrms(esi-):calculated for c
19h18
no
5-[m-h-co2]-340.1190,found 340.1188.
[0129]
4-(2-(苯甲酰胺基(羧基)甲基)-5-(乙氧羰基)苯基)丁酸
[0130]
1h),7.47
–
7.40(m,2h),6.05(s,1h),4.35(q,j=7.1hz,2h),2.96(td,j=7.3,2.1hz,2h),2.42(t,j=7.3hz,2h),2.02(dq,j=9.0,7.3hz,2h),1.38(t,j=7.1hz,3h);
13
c nmr(101mhz,cd3od)δ175.69,172.25,168.51,166.38,141.17,140.39,133.67,131.54,130.59,130.13,128.15,127.67,127.25,127.22,60.85,53.34,33.09,31.76,26.04,13.21;hrms(esi-):calculated for c
21h22
no
5-[m-h-co2]-368.1503,found 368.1500.
[0131]
4-(2-(苯甲酰胺基(羧基)甲基)-5-(二乙基氨基甲酰基)苯基)丁酸
[0132]
7.12(m,2h),5.87(d,j=7.4hz,1h),3.41(s,2h),3.19(s,2h),2.78(t,j=7.9hz,2h),2.28(t,j=7.5hz,2h),1.98
–
1.73(m,2h),1.25
–
0.91(m,6h);
13
c nmr(101mhz,d
6-dmso)δ175.62,172.38,171.84,168.56,141.34,136.74,136.52,133.70,131.52,128.14,127.88,127.44,127.26,124.12,53.18,43.56,39.45,32.99,31.68,26.07,13.03,11.67;hrms(esi-):calculated for c
23h27
n2o
4-[m-h-co2]-395.1976,found 395.1975.
[0133]
4-(2-(苯甲酰胺基(羧基)甲基)-5-甲氧基苯基)丁酸
[0134]
5.86(s,1h),3.77(s,3h),2.81(t,j=8.0hz,2h),2.36(t,j=7.3hz,2h),2.09
–
1.80(m,2h);
13
cnmr(101mhz,cd3od)δ175.82,173.24,168.64,159.75,142.18,133.84,131.40,128.74,128.09,127.25,126.66,115.02,111.77,54.29,53.02,33.08,31.99,26.17;hrms(esi-):calculated for c
20h20
no
6-[m-h]-370.1296,found 370.1291.
[0135]
4-(2-(苯甲酰胺基(羧基)甲基)-4-甲氧基苯基)丁酸
[0136]
hz,1h),6.88(dd,j=8.5,2.8hz,1h),5.96(s,1h),3.79(s,3h),2.83(t,j=7.8hz,2h),2.38(t,j=7.3hz,2h),2.09
–
1.91(m,2h);
13
c nmr(101mhz,cd3od)δ175.88,172.84,168.65,158.32,135.58,133.73,132.41,131.39,130.75,128.04,127.24,113.58,112.87,54.27,53.41,33.03,31.04,26.39;hrms(esi-):calculated for c
20h20
no
6-[m-h]-370.1296,found 370.1292.
[0137]
4-(2-(苯甲酰胺基(羧基)甲基)-4-苯氧基苯基)丁酸
[0138]
7.05(t,j=7.3hz,1h),6.99
–
6.91(m,2h),6.88(dd,j=8.4,2.6hz,1h),5.95(s,1h),2.84(dd,j=9.4,6.5hz,2h),2.38(t,j=7.3hz,2h),1.97(p,j=7.4hz,2h);
13
c nmr(101mhz,cd3od)δ175.84,172.57,168.64,157.27,155.77,136.57,135.53,133.76,131.46,131.10,129.46,128.11,127.26,122.94,118.39,118.32,117.84,53.42,33.13,31.18,26.30;hrms(esi-):calculated for c
24h22
no
4-[m-h,-co2]-388.1554,found 388.1554.
[0139]
4-(3-(苯甲酰胺基(羧基)甲基)-[1,1'-联苯]-4-基)丁酸
[0140]
(d,j=8.0hz,1h),7.35
–
7.30(m,1h),6.07(s,1h),2.94(dd,j=9.3,6.5hz,2h),2.43(t,j=7.3hz,2h),2.11
–
1.98(m,2h);
13
c nmr(101mhz,cd3od)δ175.80,172.99,168.71,140.39,139.61,139.44,135.21,133.73,131.40,130.29,128.42,128.05,127.27,126.93,126.57,126.42,126.08,53.44,33.10,31.49,26.22;hrms(esi-):calculated for c
25h22
no
5-[m-h]-416.1503,found 416.1501.
[0141]
4-(2-(苯甲酰胺基(羧基)甲基)-4-氯苯基)丁酸
[0142]
7.47
–
7.40(m,3h),7.29
–
7.21(m,2h),5.97(s,1h),2.86(td,j=7.5,2.9hz,2h),2.38(t,j=7.3hz,2h),2.08
–
1.87(m,2h);
13
c nmr(101mhz,cd3od)δ175.71,172.28,168.57,139.37,137.15,133.63,131.85,131.53,131.28,128.13,128.06,127.43,127.28,53.14,33.04,31.27,26.05;hrms(esi-):calculated for c
18h17
clno
3-[m-h,-co2]-330.0902,found 330.0904.
[0143]
4-(2-(苯甲酰胺基(羧基)甲基)-6-甲基苯基)丁酸
[0144]
1.97
–
1.83(m,2h);
13
c nmr(101mhz,cd3od)δ175.77,173.17,168.61,139.16,136.93,134.88,133.80,131.44,130.30,128.11,127.26,126.06,125.24,53.70,33.61,28.37,25.00,18.79;hrms(esi-):calculated for c
20h20
no
5-[m-h]-354.1347,found 354.1343.
[0145]
4-(2-(苯甲酰胺基(羧基)甲基)-4,5-二甲氧基苯基)丁酸
[0146]
(s,3h),3.80(s,3h),2.82(t,j=7.9hz,2h),2.37(t,j=7.3hz,2h),2.03
–
1.90(m,2h);
13
cnmr(101mhz,cd3od)δ175.94,173.32,168.60,148.86,147.46,133.81,133.40,131.36,128.04,127.23,126.60,113.07,111.06,55.03,54.93,53.26,33.00,31.49,26.43;hrms
(esi-):calculated for c
21h22
no
7-[m-h]-400.1402,found 400.1399.
[0147]
4-(2-(苯甲酰胺基(羧基)甲基)-5-氟-3-甲基苯基)丁酸
[0148][0149]1h nmr(400mhz,cd3od)δ7.87
–
7.76(m,2h),7.54
–
7.47(m,1h),7.45
–
7.38(m,2h),7.27(dd,j=7.5,1.7hz,1h),7.18
–
7.08(m,2h),6.00(s,1h),2.86(t,j=8.5hz,2h),2.44(t,j=7.3hz,2h),2.37(s,3h),1.97
–
1.83(m,2h);
13
c nmr(101mhz,cd3od)δ175.77,173.17,168.61,139.16,136.93,134.88,133.80,131.44,130.30,128.11,127.26,126.06,125.24,53.70,33.61,28.37,25.00,18.79;hrms(esi-):calculated for c
20h19
fno
5-[m-h]-372.1253,found 372.1257.4-(2-(苯甲酰胺基(羧基)(苯基)甲基)苯基)丁酸
[0150]
(t,j=7.2hz,2h),1.69
–
1.55(m,1h),1.47
–
1.34(m,1h);
13
c nmr(101mhz,cd3od)δ175.62,173.50,167.69,140.82,138.53,138.07,134.14,131.59,130.38,130.16,128.32,128.30,127.59,127.40,127.33,127.05,124.34,70.16,33.36,32.09,25.87;hrms(esi-):calculated for c
24h22
no
3-[m-h,-co2]-372.1605,found 372.1602.
[0151]
4-(2-(苯甲酰胺基(羧基)甲基)苯基)-3-甲基丁酸
[0152]
j=12.7,6.2hz,2h),2.68(ddd,j=13.8,7.6,4.1hz,2h),2.36(ddt,j=15.0,9.5,4.2hz,4h),2.16(ddd,j=15.0,9.0,4.5hz,2h),1.01(d,j=6.4hz,3h),0.96(d,j=6.3hz,3h);
13
c nmr(101mhz,cd3od)δ175.36,175.32,173.00,172.97,168.69,168.66,139.45,139.39,135.02,135.00,133.76,133.75,131.37,130.65,130.59,128.04,128.03,127.90,127.51,127.34,127.25,127.23,126.48,126.42,53.39,53.33,40.63,40.59,39.42,39.19,31.75,31.67,18.59,18.54;hrms(esi-):calculated for c
19h20
no
3-[m-h,-co2]-310.1449,found 310.1446.
[0153]
4-(4-(羧基(新戊酰胺基)甲基)-[1,1'-联苯]-3-基)丁酸
[0154]
2.43(t,j=7.2hz,2h),2.04(p,j=7.5hz,2h);
13
c nmr(101mhz,cd3od)δ179.25,175.81,172.99,141.03,140.99,140.44,134.10,128.43,128.26,127.51,127.06,126.56,124.82,52.81,38.14,33.10,31.89,26.24,26.11;hrms(esi-):calculated for c
23h26
no
5-[m-h]-395.1816,found 398.1811.
[0155]
4-(2-(1-([[(1,1'-联苯]-4-基)-1-羧乙基)苯基)丁酸
[0156]
2h);
13
c nmr(101mhz,cd3od)δ177.55,175.88,143.24,142.47,140.85,140.51,139.38,130.07,128.39,128.37,127.32,126.87,126.73,126.52,126.06,125.34,55.14,33.63,32.24,26.94,25.89;hrms(esi-):calculated for c
25h23o4-[m-h]-387.1602,found 387.1607.
[0157]
4-(4-(2-羧基丙烷-2-基)-[1,1'-联苯]-3-基)丁酸
[0158]
(t,j=7.4hz,2h),2.08
–
1.91(m,2h),1.61(s,6h);
13
c nmr(101mhz,cd3od)δ180.85,175.94,141.63,140.62,140.39,139.34,128.37,128.29,126.78,126.42,125.51,124.05,45.61,33.69,31.44,26.73,26.37;hrms(esi-):calculated for c
20h21o4-[m-h]-325.1445,found 325.1442.
[0159]
4-(2-(1-羧基-1-苯基乙基)苯基)丁酸
[0160]
2h),2.13(t,j=7.5hz,2h),1.95(s,3h),1.80
–
1.63(m,2h);
13
c nmr(101mhz,cd3od)δ177.58,175.85,144.12,142.55,140.82,130.01,127.83,127.55,127.33,126.68,126.30,125.27,55.33,33.59,32.21,26.90,25.80;hrms(esi-):calculated for c
19h19o4-[m-h]-311.1289,found 311.1291.
[0161]
以上内容仅仅是对本发明结构所作的举例和说明,所属本领域的技术人员不经创造性劳动即对所描述的具体实施例做的修改或补充或采用类似的方式替代仍属本专利的保护范围。
相关技术
网友询问留言
已有0条留言
- 还没有人留言评论。精彩留言会获得点赞!
1