一种钯催化炔烃亲核甲基化反应构建甲基杂环化合物的方法
1.本发明属于杂环化合物合成领域,具体涉及一种钯催化炔烃亲核甲基化反应构建甲基杂环化合物的方法。
技术背景
2.甲基杂环化合物是一类重要的有机合成砌块,广泛存在于各种生理活性药物中(参考文献一:(a)phytother.res.2017,31,441-448.(b)eur.j.med.chem.2015,97,483-504.(c)eur.j.med.chem.2015,97,561-581.)。例如,bazedoxifene是一种具有口服活性且能透过血脑屏障的非甾体类雌激素受体调节剂,可用于预防妇女绝经后的骨质疏松(参考文献二:j.med.chem.2001,44,1654-1657.)。雌激素拮抗剂zindoxifene可用于治疗乳腺癌(参考文献三:cancer treat.rev.1984,11,147-153.)。afn-1252是fabi高效抑制剂,能抑制金黄色葡萄球菌(参考文献四:antimicrob.agents chemother.2009,53,3544-3548.)。由此可见,发展一些高效的方法来合成甲基杂环化合物并提升相关产业的技术水平具有重要的科学价值和应用前景。
3.现有的甲基杂环化合物合成方法主要包括:芳基镁、锌、锡及芳基硼等试剂的甲基化反应(参考文献五:(a)j.am.chem.soc.1984,106,158-163.(b)tetrahedron lett.1986,27,6013-6016.(c)chem.lett.1999,28,1241-1242.(d)acta.chem.scand.1995,49,683-688.(e)chem.eur.j.1997,3,2039-2042.(f)chem.lett.2017,46,711-714.(g)j.am.chem.soc.2018,140,17197-17202.(h)org.lett.2019,21,1337-1341),芳烃卤代物及其等价体的甲基化反应(参考文献六:(a)j.org.chem.1997,62,8681-8686.(b)tetrahedron lett.2000,41,7555-7558.(c)tetrahedron lett.2000,41,6237-6240.(d)org.lett.2007,9,5629-5631.(e)org.lett.2012,14,3316-3319.(f)angew.chem.,int.ed.2016,55,9743-9747.(g)org.lett.2018,20,6345-6348.(h)org.lett.2018,20,2902-2905.(h)org.lett.2018,20,2902-2905.(i)j.am.chem.soc.2020,142,7683-7689.(j)j.am.chem.soc.2022,144,1045-1055.(k)j.am.chem.soc.2021,143,4524-4530.(l)j.am.chem.soc.2016,138,5016-5019.(m)j.am.chem.soc.2016,138,8084-8087.),及芳烃c-h键活化甲基化反应(参考文献七:(a)j.am.chem.soc.1984,106,5759-5760.(b)j.am.chem.soc.2006,128,12634-12635.(c)j.am.chem.soc.2008,130,2900-2901.(d)angew.chem.,int.ed.2011,50,1109-1113.(e)org.lett.2015,17,2812-2815.(f)j.am.chem.soc.2015,137,7660-7663.(g)rsc.adv.2015,5,70329-70332.(h)angew.chem.,int.ed.2016,55,3162-3165.(i)j.am.chem.soc.2016,138,10132-10135.(j)angew.chem.,int.ed.2016,55,1484-1488.(k)org.lett.2017,19,5458-5461.(l)acs catal.2018,8,6440-6445.(m)j.am.chem.soc.2019,141,15986-15993.(n)nat.chem.2020,12,511-519.(o)angew.chem.,int.ed.2013,52,7317-7320.(p)chem 2017,2,688-702.(q)nature 2015,525,87-90.(r)org lett 2016,18,5628-5631.)。虽然,上述甲基化反应表现出优良的反应活性,但是有些体系并不适合构建3-甲基吲哚、4-甲基
异喹啉及3-甲基苯并呋喃等甲基取代的杂环芳基化合物,表现出反应活性低、官能团兼容性差及底物适用范围窄等。因此,发展新型且高效的体系构建3-甲基吲哚、4-甲基异喹啉及3-甲基苯并呋喃等甲基取代的杂环芳基化合物是急需解决的研究课题,具有重要的理论意义和实际应用价值。
技术实现要素:
4.本发明的目的是提供一种操作简便实用,原料易得,产率好,且反应条件温和的合成甲基杂环化合物的方法。
5.为实现上述目的,本发明以金属钯为催化剂,4,5-双二苯基膦-9,9-二甲基氧杂蒽为配体,磷酸钾作为碱,四氢呋喃作为溶剂,氧气作为氧化剂及分子筛作为干燥剂,反应得到所述甲基杂环化合物,实现钯催化炔烃亲核甲基化构建甲基杂环化合物,所述方法的反应式和条件如下:
[0006][0007]
式中:
[0008]
r1为对甲苯磺酰基或叔丁基;
[0009]
r2为苯或含有取代基的苯环,或为烷基或含有取代基的烷基;所述取代基为甲基、甲氧基、叔丁基、硝基、乙酰基、卤素、苄氧基、乙酰氧基、叔丁基二甲基硅氧基、苯甲酰氧基、吗啉基、三甲基硅基中的一种。
[0010]
x为氨基,羟基或亚胺基;
[0011]
ar为苯或含有取代基的苯环,所述取代基为甲基、甲氧基、叔丁基、卤素中的一种或二种。
[0012]
基于以上技术方案,优选的,当x为氨基时,所述方法的反应式和条件如下:
[0013][0014]
式中,r1为对甲苯磺酰基或叔丁基,优选的,r1为对甲苯磺酰基;r2为苯或含有取代基的苯环,或为烷基或含有取代基的烷基,优选的,r2为含有取代基的苯环时,取代基选自甲基、甲氧基、叔丁基、硝基、乙酰基、卤素;r2为烷基时,r2为环丙烷基、叔丁基;r2为含有取代基的烷基时,取代基选自苄氧基、乙酰氧基、叔丁基二甲基硅氧基、苯甲酰氧基、吗啉基、三甲基硅基;ar为苯或含有取代基的苯环,优选的,ar为含有取代基的苯环时,取代基选自甲基、甲氧基、叔丁基、卤素。
[0015]
当x为亚胺基时,所述方法的反应式和条件如下:
[0016][0017]
式中,r1为对甲苯磺酰基或叔丁基,优选的,r1为叔丁基;r2为苯或含有取代基的苯环,或为烷基或含有取代基的烷基,优选的,r2为含有取代基的苯环时,取代基选自甲氧基、卤素;r2为烷基时,r2为正戊烷基;r2为含有取代基的烷基时,取代基为叔丁基二甲基硅氧基。
[0018]
当x为羟基时,所述方法的反应式和条件如下:
[0019][0020]
式中,r2为苯或含有取代基的苯环,或为烷基或含有取代基的烷基,优选的,r2为含有取代基的苯环时,取代基选自甲基、甲氧基、卤素;r2为烷基时,r2为三甲基硅基;r2为含有取代基的烷基时,取代基为叔丁基二甲基硅氧基;ar为苯或含有取代基的苯环,优选的,ar为含有取代基的苯环时,取代基选自甲基、甲氧基、叔丁基、卤素。
[0021]
有益效果
[0022]
本发明具有以下优点
[0023]
1.原料简单易得,操作简单;
[0024]
2.反应活性高,原料转化完全;
[0025]
3.反应条件温和;
[0026]
4.构建杂环骨架的同时引入了甲基,合成效率高。
具体实施方式
[0027]
下面通过实施例详述本发明,但本发明并不限于下述的实施例,下述实施例中所用的钯催化剂及配体为市售且无需任何处理,下述实施例中所用的底物1a参照文献合成[(a)tetrahedron lett.2008,49,7213-7216.(b)j.am.chem.soc.2015,137,10144-10147.],下述实施例中所用的底物1b参考文献合成[(a)j.am.chem.soc.2015,137,10144-10147.(b)angew.chem.int.ed.2019,58,5075-5079.],下述实施例中所用的底物1c参考文献合成[angew.chem.int.ed.2016,55,11882-11886.],下述实施例中所用的甲基硼酸均为市售且无需任何处理。
[0028]
实施例1
[0029]
条件的优化改变催化剂以及有机溶剂的种类
[0030]
氮气氛围下,向反应瓶中投入金属钯催化剂(10mol%),4,5-双二苯基膦-9,9-二甲基氧杂蒽(11mmol%),炔烃底物1(0.1mmol),甲基硼酸2(0.3mmol),磷酸钾(1.5eq.),分子筛(100.0mg),用反应所用溶剂将其溶解,然后用氧气球置换管中气氛后再将此反
应瓶放入50℃油浴中,反应10小时,将反应液冷却至室温,直接柱层析分离得到纯的产物,反应式如下:
[0031][0032]
表1.炔烃亲核甲基化反应条件优化
[0033][0034]
产率为核磁收率,通过以1,3,5-三甲氧基苯为内标来确定。
[0035]
实施例2
[0036]
氮气氛围下,向反应瓶中投入三氟醋酸钯(5mol%或者10mol%),4,5-双二苯基膦-9,9-二甲基氧杂蒽(5.5mol%或者11mmol%),炔烃底物1a(0.1mmol),甲基硼酸2(0.3mmol),磷酸钾(1.5eq.),分子筛(100.0mg),用反应所用溶剂四氢呋喃将其溶解,然后用氧气球置换管中气氛后再将此反应瓶放入50℃油浴中,反应10小时,将反应液冷却至室温,直接柱层析分离得到纯的产物,反应式如下:
[0037][0038]
产率为分离收率。
[0039]
实施例3
[0040]
氮气氛围下,向反应瓶中投入三氟醋酸钯(5mol%或者10mol%),4,5-双二苯基膦-9,9-二甲基氧杂蒽(5.5mol%或者11mmol%),炔烃底物1a(0.1mmol),甲基硼酸2(0.3mmol),磷酸钾(1.5eq.),分子筛(100.0mg),用反应所用溶剂四氢呋喃将其溶解,然后用氧气球置换管中气氛后再将此反应瓶放入50℃油浴中,反应10小时,将反应液冷却至室温,直接柱层析分离得到纯的产物,反应式如下:
[0041][0042]
产率为分离收率。
[0043]
实施例4
[0044]
氮气氛围下,向反应瓶中投入三氟醋酸钯(10mol%),4,5-双二苯基膦-9,9-二甲基氧杂蒽(11mmol%),炔烃底物1a(0.1mmol),甲基硼酸2(0.3mmol),磷酸钾(1.5eq.),分子筛(100.0mg),用反应所用溶剂四氢呋喃将其溶解,然后用氧气球置换管中气氛后再将此反应瓶放入50℃油浴中,反应10小时,将反应液冷却至室温,直接柱层析分离得到纯的产物,反应式如下:
[0045][0046]
产率为分离收率。
[0047]
实施例5
[0048]
氮气氛围下,向反应瓶中投入三氟醋酸钯(10mol%),4,5-双二苯基膦-9,9-二甲基氧杂蒽(11mmol%),炔烃底物1b(0.1mmol),甲基硼酸2(0.3mmol),磷酸钾(1.5eq.),分子筛(100.0mg),用反应所用溶剂四氢呋喃将其溶解,然后用氧气球置换管中气氛后再将此反应瓶放入50℃油浴中,反应10小时,将反应液冷却至室温,直接柱层析分离得到纯的产物,反应式如下:
[0049][0050]
产率为分离收率。
[0051]
实施例6
[0052]
氮气氛围下,向反应瓶中投入三氟醋酸钯(10mol%),4,5-双二苯基膦-9,9-二甲基氧杂蒽(11mmol%),炔烃底物1c(0.1mmol),甲基硼酸2(0.3mmol),磷酸钾(1.5eq.),分子筛(100.0mg),用反应所用溶剂1,4-二氧六环将其溶解,然后用氧气球置换管中气氛后再将此反应瓶放入50℃油浴中,反应10小时,将反应液冷却至室温,直接柱层析分离得到纯的产物,反应式如下:
[0053][0054]
产率为分离收率。
[0055]
实施例7
[0056]
在氮气保护下,将化合物1ai’(438.0mg,1.0mmol,1.0eq.),2(180.0mg,3.0mmol,3.0eq.),4,5-双二苯基膦-9,9-二甲基氧杂蒽(64.0mg,0.11mmol,11mol%),三氟醋酸钯(33.0mg,0.1mmol,5mol%),分子筛(1000.0mg),磷酸钾(0.318g,1.5mmol,1.5eq.)和四氢呋喃(20.0ml)依次加入100ml反应瓶中。随后将氧气球插入反应管中,置换管中气氛2-3次,将反应体系置于50℃下搅拌反应10h。待反应降至室温薄层色谱法(tlc)监测到反应原料全部消失,停止反应并用少量水淬灭。用乙酸乙酯萃取,合并有机相并用饱和食盐水洗涤,随后用无水硫酸钠干燥,减压蒸馏除去溶剂,通过柱色谱法(用乙酸乙酯和石油醚作为淋洗剂)纯化得到375.0mg目标产物3ai’,产率为83%。
[0057]
在氮气保护下,将化合物3ai’(135.5mg,0.3mmol,1.0eq),氢氧化钾(50.5mg,0.9mmol,3.0eq.),乙醇(6.0ml)依次加入到25ml反应管中。然后,将反应体系在100℃下搅拌12h。薄层色谱法(tlc)监测到反应原料全部消失,停止反应并用适量水淬灭,用乙酸乙酯萃取,合并有机相并用饱和食盐水洗涤,随后用无水硫酸钠干燥,减压蒸馏除去溶剂,通过柱色谱法(用乙酸乙酯和石油醚作为淋洗剂)纯化得到28.2mg目标产物4,产率为95%。
[0058]
在氮气保护下,将化合物4(29.7mg,0.1mmol),二氯甲烷(2.0ml)依次加入到25ml反应管中。然后,将反应体系在0℃下搅拌5min。随后向反应管中缓慢滴加三溴化硼的二氯甲烷溶液(1m,0.9ml,0.9mmol),滴加完毕后将反应体系转至室温搅拌1.5h。薄层色谱法(tlc)监测到反应原料全部消失,停止反应并用适量甲醇淬灭。减压蒸馏除去溶剂,通过柱色谱法(用乙酸乙酯和石油醚作为淋洗剂)纯化得到20.4mg目标产物孕烷x受体拮抗剂5,产率为80%。
[0059][0060]
实施例8
[0061]
在氮气保护下,将化合物1aj’(407.0mg,1.0mmol,1.0eq.),2(180.0mg,3.0mmol,3.0eq.),4,5-双二苯基膦-9,9-二甲基氧杂蒽(64.0mg,0.11mmol,11mol%),三氟醋酸钯(33.0mg,0.1mmol,5mol%),分子筛(1000.0mg),磷酸钾(318.0mg,1.5mmol,1.5eq.)和四氢呋喃(20.0ml)依次加入100ml反应瓶中。随后将氧气球插入反应管中,置换管中气氛2-3次,将反应体系置于50℃下搅拌反应10h。待反应降至室温薄层色谱法(tlc)监测到反应原料全部消失,停止反应并用少量水淬灭。用乙酸乙酯萃取,合并有机相并用饱和食盐水洗涤,随后用无水硫酸钠干燥,减压蒸馏除去溶剂,通过柱色谱法(用乙酸乙酯和石油醚作为淋洗剂)纯化得到384.0mg目标产物3aj’,产率为91%。
[0062]
在氮气保护下,将化合物3aj’(380.0mg,0.9mmol,1.0eq),氢氧化钾(151.5mg,2.7mmol,3.0eq.),乙醇(2.0ml)依次加入到25ml反应管中。然后,将反应体系在100℃下搅拌12h。薄层色谱法(tlc)监测到反应原料全部消失,停止反应并用适量水淬灭,用乙酸乙酯萃取,合并有机相并用饱和食盐水洗涤,随后用无水硫酸钠干燥,减压蒸馏除去溶剂,通过柱色谱法(用乙酸乙酯和石油醚作为淋洗剂)纯化得到235.8mg目标产物6,产率为98%。
[0063][0064]
实施例9
[0065]
在氮气保护下,将化合物6(26.7mg,0.1mmol,1.0eq),氢氧化钾(50.5mg,0.3mmol,3.0eq.),n,n-二甲基甲酰胺(2.0ml)依次加入到25ml反应管中。然后,将反应体系在室温下搅拌1h。随后向体系内加入化合物7(32.1mg,0.12mmol,1.2eq.),继续搅拌10h。薄层色谱法(tlc)监测到反应原料全部消失,停止反应并用适量水淬灭,用乙酸乙酯萃取,合并有机相后用无水硫酸钠干燥,减压蒸馏除去溶剂,通过柱色谱法(用乙酸乙酯,石油醚和三乙胺作为淋洗剂)纯化得到48.9mg目标产物8,产率为89%。
[0066]
在氮气保护下,将化合物8(24.9mg,0.05mmol),氯仿(1.0ml)依次加入到25ml反应管中。然后,将反应体系在-30℃下搅拌5min。随后向反应管中缓慢滴加三溴化硼的二氯甲烷溶液(1m,0.3ml,0.3mmol),滴加完毕后将反应体系转至-10℃搅拌16h。薄层色谱法(tlc)监测到反应原料全部消失,停止反应并用适量甲醇淬灭。减压蒸馏除去溶剂,通过柱色谱法(用二氯甲烷和甲醇作为淋洗剂)纯化得到8.0mg目标产物bazedoxifene 9,产率为34%。
[0067][0068]
实施例10
[0069]
在氮气保护下,将化合物6(80.0mg,0.3mmol,1.0eq),氢化钠(10.8mg,0.45mmol,1.5eq.),n,n-二甲基甲酰胺(5.0ml),碘乙烷(56.1mg,0.36mmol,1.2eq.),依次加入到25ml反应管中。然后,将反应体系在室温下搅拌12h。薄层色谱法(tlc)监测到反应原料全部消失,停止反应并用适量水淬灭,用乙酸乙酯萃取,合并有机相并用饱和食盐水洗涤,随后用无水硫酸钠干燥,减压蒸馏除去溶剂,通过柱色谱法(用乙酸乙酯和石油醚作为淋洗剂)纯
化得到75.0mg目标产物10,产率为85%。
[0070]
在氮气保护下,将化合物10(29.5mg,0.1mmol,1.0eq),二氯甲烷(2.0ml)依次加入到25ml反应管中。然后,将反应体系在-60℃下搅拌5min。随后向反应管中缓慢滴加三溴化硼的二氯甲烷溶液(1m,0.4ml,0.4mmol),滴加完毕后将反应体系转至室温搅拌12h。薄层色谱法(tlc)监测到反应原料全部消失,停止反应并用适量碳酸氢钠水溶液淬灭。用二氯甲烷萃取,减压蒸馏除去溶剂,所得粗产品直接进行下一步反应。
[0071]
将粗产品溶于1ml吡啶中加入25ml封管,随后向管内加入乙酸酐(61.3mg,0.6mmol),将反应管置于110℃油浴中搅拌2h。薄层色谱法(tlc)监测到反应原料全部消失,停止反应并用适量稀盐酸淬灭。用二氯甲烷萃取,减压蒸馏除去溶剂,通过柱色谱法(用乙酸乙酯和石油醚作为淋洗剂)纯化得到32.3mg目标产物zindoxifene 11,两步反应总产率92%。
[0072][0073]
实施例11
[0074]
在氮气保护下,将化合物3co(310.0mg,1.5mmol,1.0eq.),四丁基氟化铵的四氢呋喃溶液(1.0m,1.8ml,1.8mmol),四氢呋喃(10.0ml)依次加入50ml反应瓶中。反应体系在室温下搅拌12h后,薄层色谱法(tlc)监测到反应原料全部消失,加水淬灭反应。用乙酸乙酯萃取,合并有机相,随后用无水硫酸钠干燥,减压蒸馏除去溶剂,所得粗产品直接用于下一步反应。
[0075]
将粗产品溶于四氢呋喃(10.0ml)中转移至50ml反应瓶,随后将反应瓶置于-78℃下搅拌10min,向管内滴加正丁基锂的正己烷溶液(2.5m,0.4ml,1.6mmol),滴加完毕后,反应体系继续在该温度下搅拌1h。然后,向反应体系中缓慢滴加n,n-二甲基甲酰胺(0.12ml,1.6mmol,2.0eq.),滴加完毕后,反应体系继续在该温度下搅拌。薄层色谱法(tlc)监测反应,反应完成后,加入适量饱和氯化铵溶液淬灭。用乙酸乙酯萃取,减压蒸馏除去溶剂,通过柱色谱法(用乙酸乙酯和石油醚作为淋洗剂)纯化得到192.2mg目标产物12,两步反应总产率92%。
[0076]
在氮气保护下,将甲胺盐酸盐(20.3mg,0.3mmol,1.5eq.),碳酸钾(20.7mg,0.15mmol,0.75eq.),甲醇(2.0ml)依次加入25ml反应管中,在0℃下搅拌0.5h后,化合物12(32.2mg,0.2mmol,1.0eq.)加入到反应体系中,随后将反应体系升至室温搅拌1h。将反应体系再次冷却至室温,将硼氢化钠(11.4mg,0.3mmol,1.5eq.)加入反应管中,加料完毕后反应体系升至室温搅拌3h。薄层色谱法(tlc)监测到反应原料全部消失,将加水淬灭反应。用乙
酸乙酯萃取,合并有机相,随后用无水硫酸钠干燥,减压蒸馏除去溶剂,通过柱色谱法(用乙酸乙酯,石油醚和三乙胺作为淋洗剂)纯化得到26.4mg目标产物13,反应产率75%。根据文献报道,化合物13经过一系列转化可得到afn-1252 14。
[0077][0078]
本发明上述实施例中合成的具体物质的核磁数据如下:
[0079]
3-methyl-2-phenyl-1-tosyl-1h-indole(3aa):the reaction was conducted at6h),7.12
–
6.96(m,2h),2.29(s,3h),2.03(s,3h).
13
c nmr(100mhz,cdcl3)δ144.5,137.4,136.9,135.3,132.0,131.8,131.6,129.4,128.6,127.6,127.0,125.2,124.1,120.0,119.2,116.4,21.7,9.7.
[0080]
3,5-dimethyl-2-phenyl-1-tosyl-1h-indole(3ab):the reaction was conducted3h),7.38
–
7.31(m,2h),7.30
–
7.25(m,2h),7.22
–
7.15(m,2h),7.08
–
6.99(m,2h),2.44(s,3h),2.28(s,3h),2.01(s,3h).
13
c nmr(100mhz,cdcl3)δ144.4,137.0,135.6,135.2,133.8,132.3,131.9,131.5,129.4,128.5,127.6,127.0,126.5,120.0,119.2,116.2,21.7,21.6,9.7.hrms(esi-qeplus)m/z:[m+na]
+
calcd for c
23h21
nnao2s 398.1185;found 398.1184.
[0081]
5-methoxy-3-methyl-2-phenyl-1-tosyl-1h-indole(3ac):the reaction was7.47
–
7.40(m,3h),7.39
–
7.31(m,2h),7.29
–
7.23(m,2h),7.09
–
7.01(m,2h),7.00
–
6.93(m,1h),6.88
–
6.80(m,1h),3.85(s,3h),2.28(s,3h),2.01(s,3h).
13
c nmr(100mhz,cdcl3)δ157.2,144.4,137.9,135.0,133.3,131.9,131.8,131.5,129.3,128.6,127.6,127.0,120.2,117.6,113.5,101.9,55.8,21.7,9.8.hrms(esi-qeplus)m/z:[m+na]
+
calcd for c
23h21
nnao3s 414.1134;found 414.1134.
[0082]
5-chloro-3-methyl-2-phenyl-1-tosyl-1h-indole(3ad):the reaction was7.48
–
7.41(m,3h),7.40
–
7.37(m,1h),7.36
–
7.29(m,3h),7.28
–
7.24(m,2h),7.11
–
7.02(m,2h),2.31(s,3h),2.00(s,3h).
13
c nmr(100mhz,cdcl3)δ144.9,137.7,137.4,135.2,131.6,131.2,131.0,130.4,129.6,128.8,127.7,127.1,124.6,119.9,119.4,116.5,21.8,9.6.hrms(esi-qeplus)m/z:[m+na]
+
calcd for c
22h18
clnnao2s 418.0639;found 418.0633.
[0083]
acetate 50/1);1h nmr(400mhz,cdcl3)δ8.30
–
8.23(m,1h),7.48
–
7.41(m,3h),7.38
–
7.31(m,2h),7.30
–
7.23(m,2h),7.13
–
7.01(m,4h),2.30(s,3h),2.00(s,3h).
13
c nmr(100mhz,cdcl3)δ160.3(d,j
c-f
=239.8hz),144.8,138.7,134.9,133.6,133.3(d,j
c-f
=9.4hz),131.5,131.4,129.5,128.8,127.7,127.0,119.8(d,j
c-f
=4.0hz),117.7(d,j
c-f
=9.2hz),112.8(d,j
c-f
=25.1hz),104.9(d,j
c-f
=23.7hz),21.8,9.7.
19
f nmr(376mhz,cdcl3)δ-118.79.hrms(esi-qeplus)m/z:[m+na]
+
calcd for c
22h18
fnnao2s 402.0934;found 402.0930.
[0084]
3,6-dimethyl-2-phenyl-1-tosyl-1h-indole(3af):the reaction was conducted7.36
–
7.31(m,2h),7.30
–
7.26(m,3h),7.15
–
7.11(m,1h),7.09
–
7.02(m,2h),2.54(s,3h),2.30(s,3h),2.01(s,3h).
13
c nmr(100mhz,cdcl3)δ144.4,137.8,136.2,135.4,135.2,131.9,131.6,129.8,129.4,128.4,127.6,127.0,125.5,120.0,118.8,116.6,22.3,21.7,9.7.hrms(esi-qeplus)m/z:[m+na]
+
calcd for c
23h21
nnao2s 398.1185;found 398.1184.
[0085]
6-chloro-3-methyl-2-phenyl-1-tosyl-1h-indole(3ag):the reaction was3h),7.36
–
7.24(m,6h),7.14
–
7.03(m,2h),2.32(s,3h),2.00(s,3h).
13
c nmr(100mhz,cdcl3)δ144.9,137.7,137.4,135.2,131.6,131.2,131.0,130.4,129.6,128.8,127.7,127.1,124.6,119.9,119.4,116.5,21.8,9.6.hrms(esi-qeplus)m/z:[m+na]
+
calcd for c
22h18
clnnao2s 418.0639;found 418.0642.
[0086]
7-fluoro-3-methyl-2-phenyl-1-tosyl-1h-indole(3ah):the reaction was
2.34(s,3h),2.08(s,3h).
13
c nmr(100mhz,cdcl3)δ151.5(d,j
c-f
=251.6hz),144.6,139.8,136.7(d,j
c-f
=2.9hz),135.4,132.2,130.7,129.4,128.5,127.9,127.5(d,j
c-f
=1.5hz),125.4(d,j
c-f
=7.2hz),120.3(d,j
c-f
=1.8hz),115.2(d,j
c-f
=3.6hz),112.7(d,j
c-f
=21.7hz),21.8,10.0.
19
f nmr(376mhz,cdcl3)δ-116.45.hrms(esi-qeplus)m/z:[m+na]
+
calcd for c
22h18
fnnao2s 402.0934;found 402.0931.
[0087]
5-chloro-7-fluoro-3-methyl-2-phenyl-1-tosyl-1h-indole(3ai):the reaction1h),7.39
–
7.34(m,2h),7.20
–
7.17(m,1h),7.16
–
7.12(m,2h),7.10
–
7.05(m,1h),2.36(s,3h),2.04(s,3h).
13
c nmr(100mhz,cdcl3)δ150.9(d,j
c-f
=256.3hz),144.9,141.3,137.2(d,j
c-f
=3.8hz),135.2,131.7,130.7,130.2(d,j
c-f
=9.1hz),129.5,128.8,128.0,127.5(d,j
c-f
=0.8hz),123.9(d,j
c-f
=9.5hz),119.6(d,j
c-f
=2.0hz),115.2(d,j
c-f
=3.9hz),113.3(d,j
c-f
=25.3hz),21.8,9.9.
19
f nmr(376mhz,cdcl3)δ-113.73.hrms(esi-qeplus)m/z:[m+na]
+
calcd for c
22h17
clfnnao2s 436.0545;found 436.0552.
[0088]
4,6-difluoro-3-methyl-2-phenyl-1-tosyl-1h-indole(3aj):the reaction was1h),7.40
–
7.34(m,3h),7.17
–
7.09(m,2h),6.91
–
6.79(m,2h),2.35(s,3h),2.03(s,3h).
13
c nmr(100mhz,cdcl3)δ160.0(dd,j
c-f
=242.8,10.2hz),151.2(dd,j
c-f
=255.2,13.1hz),144.9,141.6,137.0(dd,j
c-f
=10.7,4.4hz),134.9,131.8,130.7,129.5,128.8,128.0,127.5(d,j
c-f
=0.3hz),121.9(dd,j
c-f
=9.4,2.3hz),120.4(dd,j
c-f
=3.8,2.4hz),101.9,101.6,101.6,101.4,101.4,101.4,101.2,101.2,,21.8,9.9.
19
f nmr(376mhz,cdcl3)δ=-112.06(d,j=6.5hz),-115.05(d,j=6.0hz).hrms(esi-qeplus)m/z:[m+na]
+
calcd for c
22h17
f2nnao2s 420.0840;found 420.0834.
[0089]
7-chloro-5-fluoro-3-methyl-2-phenyl-1-tosyl-1h-indole(3ak):the reaction7.34
–
7.27(m,2h),7.18
–
7.10(m,3h),7.07
–
7.00(m,2h),6.98
–
6.92(m,1h),2.35(s,3h),2.01(s,3h).
13
c nmr(100mhz,cdcl3)δ160.4(d,j
c-f
=244.9hz),144.7,143.2,138.4(d,j
c-f
=10.0hz),134.5,133.9(d,j
c-f
=2.1hz),131.5,130.6,129.0,128.7,127.9,127.6,125.6(d,j
c-f
=11.6hz),121.7(d,j
c-f
=4.1hz),115.4(d,j
c-f
=27.4hz),104.3(d,j
c-f
=
23.2hz),21.8,10.0.
19
f nmr(376mhz,cdcl3)δ-115.73.hrms(esi-qeplus)m/z:[m+na]
+
calcd for c
22h17
clfnnao2s 436.0545;found 436.0546.
[0090]
2-(4-methoxyphenyl)-3-methyl-1-tosyl-1h-indole(3al):the reaction was1h),7.43
–
7.34(m,2h),7.32
–
7.23(m,5h),7.09
–
7.01(m,2h),7.00
–
6.92(m,2h),3.89(s,3h),2.29(s,3h),2.02(s,3h).
13
c nmr(100mhz,cdcl3)δ159.9,144.5,137.3,136.8,135.4,132.9,132.0,129.4,127.0,125.0,124.1,123.9,119.5,119.1,116.4,113.1,55.5,21.7,9.7.
[0091]
3-methyl-2-(p-tolyl)-1-tosyl-1h-indole(3am):the reaction was conducted at2h),7.32
–
7.27(m,3h),7.26
–
7.22(m,4h),7.07
–
7.00(m,2h),2.44(s,3h),2.28(s,3h),2.03(s,3h).
13
c nmr(100mhz,cdcl3)δ144.5,138.4,137.3,137.0,135.2,132.1,131.4,129.4,128.8,128.4,127.0,125.0,124.1,119.8,119.1,116.4,21.7,21.7,9.7.hrms(esi-qeplus)m/z:[m+na]
+
calcd for c
23h21
nnao2s 398.1185;found 398.1184.
[0092]
2-(4-(tert-butyl)phenyl)-3-methyl-1-tosyl-1h-indole(3an):the reaction was1h),7.43
–
7.40(m,3h),7.38
–
7.33(m,1h),7.30
–
7.25(m,5h),7.07
–
6.99(m,2h),2.29(s,3h),2.04(s,3h),1.40(s,9h).
13
c nmr(100mhz,cdcl3)δ151.4,144.4,137.4,137.1,135.3,132.1,131.2,129.3,128.6,127.1,125.0,124.5,124.0,119.7,119.1,116.4,34.9,31.6,21.7,9.8.hrms(esi-qeplus)m/z:[m+na]
+
calcd for c
26h27
nnao2s 440.1655;found 440.1652.
[0093]
2-(4-fluorophenyl)-3-methyl-1-tosyl-1h-indole(3ao):the reaction was1h),7.45
–
7.36(m,2h),7.34
–
7.24(m,5h),7.17
–
7.09(m,2h),7.08
–
7.02(m,2h),2.30(s,3h),2.02(s,3h).
13
c nmr(100mhz,cdcl3)δ163.0(d,j
c-f
=246.8hz),144.7,137.4,135.7,135.3,133.3(d,j
c-f
=38.1hz),131.8,129.5,127.7(d,j
c-f
=3.3hz),126.9,125.3,124.2,120.2,119.2,116.4,114.8(d,j
c-f
=21.6hz),21.7,9.6.
19
f nmr(376mhz,cdcl3)δ-112.83.hrms(esi-qeplus)m/z:[m+na]
+
calcd for c
22h18
fnnao2s 402.0934;found 402.0935.
[0094]
2-(4-chlorophenyl)-3-methyl-1-tosyl-1h-indole(3ap):the reaction was
1h),7.45
–
7.36(m,4h),7.34
–
7.30(m,1h),7.29
–
7.26(m,4h),7.10
–
7.01(m,2h),2.30(s,3h),2.03(s,3h).
13
c nmr(100mhz,cdcl3)δ144.7,137.5,135.6,135.2,134.7,132.8,131.9,130.2,129.5,128.0,127.0,125.5,124.3,120.5,119.3,116.5,21.8,9.7.hrms(esi-qeplus)m/z:[m+na]
+
calcd for c
22h18
clnnao2s 418.0639;found 418.0611.
[0095]
1-(4-(3-methyl-1-tosyl-1h-indol-2-yl)phenyl)ethan-1-one(3aq):the reaction8.35
–
8.29(m,1h),8.08
–
8.02(m,2h),7.53
–
7.47(m,2h),7.46
–
7.37(m,2h),7.35
–
7.27(m,3h),7.09
–
7.03(m,2h),2.69(s,3h),2.30(s,3h),2.06(s,3h).
13
c nmr(100mhz,cdcl3)δ198.0,144.8,137.7,136.8,136.7,135.7,134.8,132.1,131.6,129.5,127.7,126.9,125.7,124.5,121.5,119.5,116.6,26.9,21.8,9.8.hrms(esi-qeplus)m/z:[m+na]
+
calcd for c
24h21
nnao3s 426.1134;found 426.1131.
[0096]
3-methyl-2-(4-nitrophenyl)-1-tosyl-1h-indole(3ar):the reaction was3h),7.62
–
7.54(m,2h),7.48
–
7.41(m,2h),7.37
–
7.31(m,1h),7.30
–
7.24(m,2h),7.10
–
7.05(m,2h),2.31(s,3h),2.09(s,3h).
13
c nmr(100mhz,cdcl3)δ147.7,145.1,138.7,137.9,134.6,132.1,131.9,129.6,126.9,126.2,124.7,123.1,122.9,122.5,119.7,116.7,21.8,9.8.hrms(esi-qeplus)m/z:[m-h]-calcd for c
22h17
n2o4s 405.0915;found 405.0906.
[0097]
3-methyl-2-(m-tolyl)-1-tosyl-1h-indole(3as):the reaction was conducted at1h),7.39
–
7.22(m,6h),7.17
–
7.08(m,2h),7.08
–
7.01(m,2h),2.40(s,3h),2.29(s,3h),2.03(s,3h).
13
c nmr(100mhz,cdcl3)δ144.5,137.4,137.1,137.0,135.4,132.3,132.0,131.6,129.4,128.6,127.5,127.1,125.1,124.0,119.7,119.2,116.4,21.7,21.7,9.7.hrms(esi-qeplus)m/z:[m+na]
+
calcd for c
23h21
nnao2s 398.1185;found 398.1183.
[0098]
2-(3-chlorophenyl)-3-methyl-1-tosyl-1h-indole(3at):the reaction was7.45
–
7.35(m,4h),7.34
–
7.24(m,4h),7.23
–
7.20(m,1h),7.10
–
7.04(m,2h),2.31(s,3h),2.04(s,
3h).
13
c nmr(100mhz,cdcl3)δ144.8,137.5,135.2,133.5,131.7,131.3,129.9,129.5,128.9,128.7,127.0,125.5,124.2,120.6,119.4,116.4,21.8,9.6.hrms(esi-qeplus)m/z:[m+na]
+
calcd for c
22h18
clnnao2s 418.0639;found 418.0641.
[0099]
2-(2-methoxyphenyl)-3-methyl-1-tosyl-1h-indole(3au):the reaction was7.45
–
7.42(m,2h),7.40
–
7.36(m,2h),7.35
–
7.30(m,1h),7.29
–
7.24(m,1h),7.15
–
7.13(m,1h),7.09
–
6.98(m,3h),6.97
–
6.93(m,1h),3.72(s,3h),2.29(s,3h),1.99(s,3h).
13
c nmr(100mhz,cdcl3)δ158.7,144.2,137.1,136.1,133.3,133.1,131.7,130.6,129.3,127.1,124.7,123.5,120.8,119.8,119.4,119.1,115.7,110.8,55.5,21.7,9.5.hrms(esi-qeplus)m/z:[m+na]
+
calcd for c
23h21
nnao3s 414.1134;found 414.1130.
[0100]
2-(2-fluorophenyl)-3-methyl-1-tosyl-1h-indole(3av):the reaction was7.49
–
7.42(m,2h),7.40
–
7.27(m,5h),7.26
–
7.21(m,1h),7.20
–
7.14(m,1h),7.10
–
7.04(m,2h),2.28(s,3h),2.03(s,3h).
13
c nmr(100mhz,cdcl3)δ160.8(d,j
c-f
=247.1hz),144.7,137.4,135.3,133.7(d,j
c-f
=2.7hz),131.6,131.0(d,j
c-f
=8.5hz),130.2,129.5,127.0,125.3,124.0,123.5(d,j
c-f
=3.6hz),121.4,119.9(d,j
c-f
=15.1hz),119.3,116.0,115.7(d,j
c-f
=21.7hz),21.7,9.6(d,j
c-f
=0.9hz).
19
f nmr(376mhz,cdcl3)δ-111.42.hrms(esi-qeplus)m/z:[m+na]
+
calcd for c
22h18
fnnao2s 402.0934;found 402.0928.
[0101]
2-(2-(benzyloxy)ethyl)-3-methyl-1-tosyl-1h-indole(3aw):the reaction was7.59
–
7.52(m,2h),7.41
–
7.35(m,1h),7.33
–
7.21(m,7h),7.16
–
7.09(m,2h),4.52(s,2h),3.79(t,j=6.9hz,2h),3.32(t,j=7.0hz,2h),2.30(s,3h),2.16(s,3h).
13
c nmr(100mhz,cdcl3)δ144.7,138.8,136.9,136.3,133.5,131.6,129.9,128.5,127.7,127.7,126.4,124.5,123.6,118.9,118.7,115.3,73.2,70.1,27.6,21.7,9.3.hrms(esi-qeplus)m/z:[m+na]
+
calcd for c
25h25
nnao3s 442.1447;found 442.1445.
[0102]
2-(3-methyl-1-tosyl-1h-indol-2-yl)ethyl acetate(3ax):the reaction was7.61
–
7.53(m,2h),7.42
–
7.37(m,1h),7.33
–
7.23(m,2h),7.19
–
7.12(m,2h),4.38(t,j=6.7hz,2h),3.34(t,j=6.7hz,2h),2.32(s,3h),2.17(s,3h),2.03(s,3h).
13
c nmr(100mhz,cdcl3)δ171.2,144.8,136.8,136.1,132.4,131.4,130.0,126.4,124.8,123.7,119.4,118.8,
115.3,64.0,26.3,21.7,21.2,9.2.hrms(esi-qeplus)m/z:[m+na]
+
calcd for c
20h21
nnao4s 394.1083;found 394.1078.
[0103]
2-(2-((tert-butyldimethylsilyl)oxy)ethyl)-3-methyl-1-tosyl-1h-indole(3ay):8.20
–
8.14(m,1h),7.59
–
7.52(m,2h),7.40
–
7.34(m,1h),7.30
–
7.20(m,2h),7.17
–
7.10(m,2h),3.92(t,j=6.7hz,2h),3.21(t,j=6.6hz,2h),2.31(s,3h),2.17(s,3h),0.85(s,9h),-0.03(s,6h).
13
c nmr(100mhz,cdcl3)δ144.6,136.8,136.3,133.8,131.7,129.9,126.4,124.4,123.6,119.1,118.6,115.3,63.3,30.5,26.1,21.7,18.5,9.5,-5.2.hrms(esi-qeplus)m/z:[m+na]
+
calcd for c
24h33
nnao3ssi 466.1843;found 466.1834.
[0104]
2-(3-methyl-1-tosyl-1h-indol-2-yl)ethyl benzoate(3az):the reaction was8.03
–
7.95(m,2h),7.63
–
7.57(m,2h),7.56
–
7.50(m,1h),7.45
–
7.35(m,3h),7.34
–
7.28(m,1h),7.27
–
7.22(m,1h),7.19
–
7.12(m,2h),4.65(t,j=6.6hz,2h),3.48(t,j=6.6hz,2h),2.31(s,3h),2.17(s,3h).
13
c nmr(100mhz,cdcl3)δ166.7,144.8,136.9,136.2,133.1,132.4,131.4,130.4,130.0,129.8,128.5,126.4,124.8,123.7,119.5,118.9,115.3,64.4,26.4,21.7,9.3.hrms(esi-qeplus)m/z:[m+na]
+
calcd for c
25h23
nnao4s 456.1240;found456.1234.
[0105]
2-(tert-butyl)-3-methyl-1-tosyl-1h-indole(3aa’):the reaction was conducted(m,1h),7.14
–
7.08(m,2h),6.97
–
6.90(m,2h),2.22(s,6h),1.66(s,9h).
13
c nmr(100mhz,cdcl3)δ147.6,143.8,139.9,135.4,132.7,128.6,127.0,124.9,124.7,124.6,118.5,118.2,36.1,32.1,21.7,12.9.hrms(esi-qeplus)m/z:[m+na]
+
calcd for c
20h23
nnao2s 364.1342;found 364.1340.
[0106]
2-cyclopropyl-3-methyl-1-tosyl-1h-indole(3ab’):the reaction was7.67
–
7.59(m,2h),7.39
–
7.33(m,1h),7.31
–
7.20(m,2h),7.18
–
7.12(m,2h),2.33(s,3h),2.21(s,3h),2.06
–
1.93(m,1h),1.06
–
0.97(m,2h),0.69
–
0.61(m,2h).
13
c nmr(100mhz,cdcl3)δ144.4,137.6,136.9,136.9,131.5,129.7,126.6,124.7,123.4,118.6,118.4,115.1,21.7,10.1,8.5,8.2.hrms(esi-qeplus)m/z calcd for c
19h19
nnao2s[m+na]
+
348.1029,found 348.1027.
[0107]
4-((3-methyl-1-tosyl-1h-indol-2-yl)methyl)morpholine(3ac’):the reaction1h),7.35
–
7.28(m,1h),7.26
–
7.21(m,1h),7.21
–
7.12(m,2h),3.88(s,2h),3.53(br s,4h),2.50(br s,4h),2.34(s,3h),2.23(s,3h).
13
c nmr(100mhz,cdcl3)δ144.4,137.4,136.6,132.2,130.3,129.5,127.3,125.0,123.2,119.2,119.1,114.9,66.9,53.4,52.1,21.7,9.5.hrms(esi-qeplus)m/z:[m+h]
+
calcd for c
21h25
n2o3s 385.1580;found 385.1577.
[0108]
3-methyl-1-tosyl-2-(trimethylsilyl)-1h-indole(3ad’):the reaction was7.46
–
7.41(m,2h),7.38
–
7.34(m,1h),7.26
–
7.21(m,1h),7.19
–
7.13(m,1h),7.08
–
7.02(m,2h),2.32(s,3h),2.24(s,3h),0.52(s,9h).
13
c nmr(100mhz,cdcl3)δ144.1,139.5,138.1,135.2,133.7,133.4,129.4,126.7,125.5,123.6,119.2,115.6,21.7,12.1,2.7.hrms(esi-qeplus)m/z:[m+na]
+
calcd for c
19h23
nnao2ssi 380.1111;found 380.1108.
[0109]
2-(3-methyl-1-tosyl-1h-indol-2-yl)ethyl 3-(n,n-dipropylsulfamoyl)benzoa-1h),8.14
–
8.05(m,2h),7.88
–
7.81(m,2h),7.62
–
7.54(m,2h),7.39
–
7.37(m1h),7.35
–
7.22(m,2h),7.20
–
7.12(m,2h),4.69(t,j=6.5hz,2h),3.50(t,j=6.5hz,2h),3.15
–
3.04(m,4h),2.32(s,3h),2.16(s,3h),1.55
–
1.49(m,4h),0.86(t,j=7.4hz,6h).
13
c nmr(100mhz,cdcl3)δ165.4,144.9,144.4,136.9,136.0,133.7,132.1,131.3,130.4,130.0,127.2,126.4,124.9,123.8,119.6,118.9,115.3,65.1,50.1,26.3,22.1,21.7,11.3,9.3.hrms(esi-qeplus)m/z:[m+na]
+
calcd for c
31h36
n2nao6s
2 619.1907;found 619.1908.
[0110]
2-(3-methyl-1-tosyl-1h-indol-2-yl)ethyl 2-(6-methoxynaphthalen-2-yl)pro-7.55
–
7.53(m,1h),7.52
–
7.46(m,2h),7.32
–
7.18(m,4h),7.13
–
7.03(m,4h),4.40(t,j=6.5 hz,2h),3.91(s,3h),3.79(q,j=7.1hz,1h),3.33
–
3.21(m,2h),2.27(s,3h),1.94(s,3h),1.53(d,j=7.2hz,3h).
13
c nmr(100mhz,cdcl3)δ174.8,157.8,144.7,136.9,136.1,135.8,133.8,132.3,131.4,129.9,129.5,129.1,127.3,126.4,126.4,126.1,124.7,123.6,
mmol7.74
–
7.65(m,1h),7.57
–
7.48(m,1h),3.06
–
2.96(m,2h),2.61(s,3h),1.82
–
1.69(m,2h),1.50
–
1.32(m,4h),0.93(t,j=7.0hz,3h).
13
c nmr(100mhz,cdcl3)δ153.5,150.1,136.2,130.2,128.2,127.1,125.9,123.5,123.2,36.2,32.2,29.9,22.9,14.3,13.8.hrms(esi-qeplus)m/z:[m+h]
+
calcd for c
15h20
n 214.1590;found 214.1584.
[0119]
3-(2-((tert-butyldimethylsilyl)oxy)ethyl)-4-methylisoquinoline(3bg):thecdcl3)δ9.08(s,1h),8.01
–
7.96(m,1h),7.94
–
7.88(m,1h),7.73
–
7.66(m,1h),7.57
–
7.50(m,1h),4.05(t,j=6.9hz,2h),3.25(t,j=6.9hz,2h),2.65(s,3h),0.83(s,9h),-0.07(s,6h).
13
c nmr(100mhz,cdcl3)δ150.3,150.2,136.0,130.2,128.2,127.2,126.1,125.0,123.3,63.5,39.2,26.1,18.5,14.1,-5.2.hrms(esi-qeplus)m/z:[m+h]
+
calcd for c
18h28
nosi 302.1935;found 302.1928.
[0120]
3-methyl-2-phenylbenzofuran(3ca):the reaction was conducted at 0.1 mmol7.32
–
7.24(m,2h),2.49(s,3h).
13
c nmr(100mhz,cdcl3)δ154.0,150.9,131.6,131.4,128.8,128.1,126.9,124.5,122.5,119.5,111.5,111.2,9.7.
[0121]
5-fluoro-3-methyl-2-phenylbenzofuran(3cb):the reaction was conducted at7.83
–
7.76(m,2h),7.52
–
7.44(m,2h),7.42
–
7.34(m,2h),7.21
–
7.15(m,1h),7.05
–
6.95(m,1h),2.44(s,3h).
13
c nmr(100mhz,cdcl3)δ159.4(d,j
c-f
=236.4hz),152.8,150.2,132.3(d,j
c-f
=10.1hz),131.3,128.9,128.4,127.0,112.1(d,j
c-f
=26.0hz),111.7(d,j
c-f
=9.5hz),111.7(d,j
c-f
=3.8hz),105.1(d,j
c-f
=24.6hz),9.7.
19
f nmr(376mhz,cdcl3)δ-121.32.
[0122]
3,5-dimethyl-2-phenylbenzofuran(3cc):the reaction was conducted at 0.17.13
–
7.06(m,1h),2.47(s,3h),2.46(s,3h).
13
c nmr(100mhz,cdcl3)δ152.4,151.0,132.0,131.8,131.5,128.8,128.0,126.9,125.8,119.4,111.3,110.7,21.6,9.7.
[0123]
5-(tert-butyl)-3-methyl-2-phenylbenzofuran(3cd):the reaction was
0.17.20
–
7.13(m,1h),7.12
–
7.05(m,1h),2.57(s,3h),2.47(s,3h).
13
c nmr(100mhz,cdcl3)δ153.0,150.6,131.9,130.8,128.8,128.0,126.9,125.5,122.6,121.4,117.0,111.8,15.2,9.8.hrms(esi-qeplus)m/z:[m+h]
+
calcd for c
16h15
o 223.1117;found 223.1117.
[0129]
2-(2-fluorophenyl)-3-methylbenzofuran(3cj):the reaction was conducted at2.32(d,j=2.7hz,3h).
13
c nmr(100mhz,cdcl3)δ159.7(d,j
c-f
=249.6hz),154.7,146.5,130.9(d,j
c-f
=3.3hz),130.7,130.6(d,j
c-f
=8.1hz),124.7,124.4(d,j
c-f
=3.4hz),122.6,119.8,119.4(d,j
c-f
=14.2hz),116.5(d,j
c-f
=22.0hz),114.4,111.3,9.2(d,j
c-f
=6.4hz).
19
f nmr(376mhz,cdcl3)δ-111.93.hrms(esi-qeplus)m/z:[m+h]
+
calcd for c
15h12
fo 227.0867;found 227.0866.
[0130]
2-(4-fluorophenyl)-3-methylbenzofuran(3ck):the reaction was conducted(m,1h),7.33
–
7.22(m,2h),7.21
–
7.12(m,2h),2.45(s,3h).
13
c nmr(100mhz,cdcl3)δ162.6(d,j
c-f
=246.9hz),153.9,150.1,131.3,128.7(d,j
c-f
=8.2hz),127.9(d,j
c-f
=3.6hz),124.6,122.7,119.5,115.9(d,j
c-f
=21.6hz),111.1,9.6.
19
f nmr(376mhz,cdcl3)δ-113.13.
[0131]
3-methyl-2-(p-tolyl)benzofuran(3cl):the reaction was conducted at 0.1(m,1h),7.32
–
7.20(m,4h),2.46(s,3h),2.41(s,3h).
13
c nmr(100mhz,cdcl3)δ153.9,151.2,138.1,131.5,129.6,128.8,126.9,124.3,122.5,119.4,111.1,110.8,21.6,9.7.
[0132]
2-(4-methoxyphenyl)-3-methylbenzofuran(3cm):the reaction was(m,1h),7.48
–
7.43(m,1h),7.31
–
7.20(m,2h),7.06
–
6.98(m,2h),3.87(s,3h),2.45(s,3h).
13
c nmr(100mhz,cdcl3)δ159.6,153.8,151.1,131.5,128.4,124.4,124.1,122.5,119.2,114.3,111.0,109.9,55.6,9.6.
[0133]
tert-butyldimethyl((3-methylbenzofuran-2-yl)methoxy)silane(3cn):the
1h),7.29
–
7.24(m,1h),7.23
–
7.19(m,1h),4.78(s,2h),2.25(s,3h),0.92(s,9h),0.12(s,6h).
13
c nmr(100mhz,cdcl3)δ154.3,151.7,130.2,124.4,122.3,119.6,112.4,111.3,56.9,26.1,18.7,8.2,-5.0.hrms(esi-qeplus)m/z:[m-t
bu]
+
calcd for c
12h15
o2si 219.0836;found 219.0833.
[0134]
trimethyl(3-methylbenzofuran-2-yl)silane(3co):the reaction was(m,1h),7.23
–
7.19(m,1h),2.31(s,3h),0.38(s,9h).
13
c nmr(100mhz,cdcl3)δ157.6,157.6,130.1,125.3,124.4,122.0,119.4,111.3,9.1,-0.9.
[0135]
2-(3,4-dimethoxyphenyl)-5-methoxy-3-methyl-1-tosy10/1);1h nmr(400mhz,cdcl3)δ8.24
–
8.18(m,1h),7.29
–
7.24(m,2h),7.07
–
7.01(m,2h),7.00
–
6.95(m,1h),6.94
–
6.90(m,1h),6.89
–
6.82(m,3h),3.96(s,3h),3.87(s,3h),3.86(s,3h),2.30(s,3h),2.01(s,3h).
13
c nmr(100mhz,cdcl3)δ157.1,149.3,148.0,144.4,137.7,135.1,133.2,131.8,129.3,127.0,124.0,124.0,119.7,117.5,115.1,113.3,110.0,101.8,56.1,56.0,55.8,21.7,9.9.hrms(esi-qeplus)m/z:[m+na]
+
calcd for c
25h25
nnao5s 474.1346;found 474.1344.
[0136]
2-(3,4-dimethoxyphenyl)-5-methoxy-3-methyl-1h-indole(4):the reaction7.89(s,1h),7.28
–
7.25(m,1h),7.14
–
7.10(m,1h),7.09
–
7.06(m,1h),7.04
–
7.01(m,1h),6.99
–
6.95(m,1h),6.88
–
6.82(m,1h),3.94(s,3h),3.93(s,3h),3.89(s,3h),2.42(s,3h).
13
c nmr(100mhz,cdcl3)δ154.3,149.3,148.7,135.3,131.0,130.7,126.5,120.5,112.3,111.6,111.5,111.2,107.9,100.9,56.2,9.9.
[0137]
4-(5-hydroxy-3-methyl-1h-indol-2-yl)benzene-1,2-diol(5):the reaction was(m,1h),7.09
–
7.08(m,1h),6.98
–
6.95(m,1h),6.90
–
6.85(m,2h),6.66
–
6.64(m,1h),2.35(s,3h).
13
c nmr(100mhz,cd3od)δ149.7,145.0,144.4,135.5,131.0,130.6,125.8,119.2,
115.1,114.7,110.6,110.5,104.9,102.0,8.7.
[0138]
5-methoxy-2-(4-methoxyphenyl)-3-methyl-1-tosyl-1h-indole(3aj’):themhz,cdcl3)δ8.24
–
8.16(m,1h),7.28
–
7.22(m,4h),7.08
–
7.00(m,2h),6.99
–
6.92(m,3h),6.86
–
6.79(m,1h),3.89(s,3h),3.85(s,3h),2.29(s,3h),2.00(s,3h).
13
c nmr(100mhz,cdcl3)δ159.9,157.1,144.4,137.8,135.1,133.3,132.8,131.8,129.3,127.0,124.0,119.7,117.6,113.3,113.1,101.9,55.9,55.5,21.7,9.8.hrms(esi-qeplus)m/z:[m+na]
+
calcd for c
24h23
nnao4s 444.1240;found 444.1235.
[0139]
5-methoxy-2-(4-methoxyphenyl)-3-methyl-1h-indole(6):the reaction was7.85(s,1h),7.52
–
7.44(m,2h),7.27
–
7.19(m,1h),7.04
–
6.97(m,3h),6.87
–
6.81(m,1h),3.88(s,3h),3.85(s,3h),2.39(s,3h).
13
c nmr(100mhz,cdcl3)δ159.1,154.2,135.2,131.0,130.6,129.1,126.1,114.4,112.1,111.5,107.7,100.9,56.1,55.6,9.9.
[0140]
1-(4-(2-(azepan-1-yl)ethoxy)benzyl)-5-methoxy-2-(4-methoxyphenyl)-3-me7.09
–
7.03(m,2h),6.96
–
6.90(m,2h),6.87
–
6.83(m,2h),6.82
–
6.78(m,1h),6.78
–
6.71(m,2h),5.11(s,2h),4.00(t,j=6.2hz,2h),3.88(s,3h),3.84(s,3h),2.91(t,j=6.2hz,2h),2.80
–
2.71(m,4h),2.25(s,3h),1.70
–
1.54(m,8h).
13
c nmr(100mhz,cdcl3)δ159.5,158.0,154.2,138.5,132.1,131.9,131.0,129.3,127.4,124.6,114.8,114.0,111.8,111.1,108.5,100.9,66.4,56.4,56.2,56.0,55.5,47.3,27.8,27.2,9.7.
[0141]
bazedoxifene(9):the reaction was conducted at 0.05mmol scale,8.0mg,5.10(s,2h),3.92(t,j=6.2hz,2h),2.77(t,j=6.0hz,2h),2.67
–
2.60(m,4h),2.10(s,3h),1.58
–
1.45(m,8h).
13
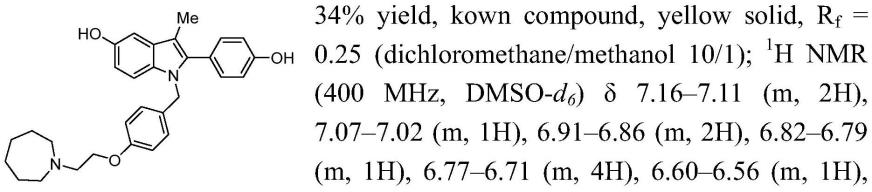
c nmr(100mhz,dmso-d6)δ157.9,157.8,151.4,138.5,131.8,131.8,131.1,131.1,131.1,127.8,127.8,115.9,114.8,111.2,106.9,103.0,66.6,56.5,55.6,28.3,
27.0,9.9.
[0142]
1-ethyl-5-methoxy-2-(4-methoxyphenyl)-3-methyl-1h-indole(10):themhz,cdcl3)δ7.39
–
7.31(m,2h),7.31
–
7.24(m,1h),7.11
–
7.01(m,3h),6.97
–
6.87(m,1h),4.05(q,j=7.1hz,2h),3.93(s,3h),3.92(s,3h),2.24(s,3h),1.22(t,j=7.1hz,3h).
13
c nmr(100mhz,cdcl3)δ159.3,153.9,137.8,131.7,131.2,128.9,124.8,113.9,111.5,110.2,108.2,100.8,56.1,55.4,38.7,15.5,9.4.
[0143]
zindoxifene(11):the reaction was conducted at 0.1mmol scale,32.3mg,7.28
–
7.26(m,1h),7.24
–
7.18(m,2h),6.97
–
6.91(m,1h),4.04(q,j=7.1hz,2h),2.34(s,3h),2.33(s,3h),2.18(s,3h),1.20(t,j=7.2hz,3h).
13
c nmr(100mhz,cdcl3)δ170.8,169.6,150.6,144.2,137.7,134.1,131.7,129.9,129.0,121.8,115.8,111.2,110.1,109.5,39.0,21.4,15.6,9.4.
[0144]
3-methylbenzofuran-2-carbaldehyde(12):the reaction was conducted at 1.51h),2.64(s,3h).
13
c nmr(100mhz,cdcl3)δ179.9,155.6,148.3,129.6,128.9,123.8,122.0,112.8,8.63.
[0145]
n-methyl-1-(3-methylbenzofuran-2-yl)methanamine(13):the reaction was7.51
–
7.46(m,1h),7.41
–
7.35(m,1h),7.26
–
7.17(m,2h),3.82(s,2h),2.37(s,3h),2.23(s,3h).
13
c nmr(100mhz,cd3od)δ155.0,151.0,130.4,124.6,122.7,119.7,113.3,111.0,34.7,7.2.hrms(esi-qeplus)m/z:[m+h]
+
calcd for c
11h14
no 176.1070;found 176.1068.
相关技术
网友询问留言
已有0条留言
- 还没有人留言评论。精彩留言会获得点赞!
1