一种降解BTK蛋白的化合物及其制备方法和应用
一种降解btk蛋白的化合物及其制备方法和应用
技术领域
1.本发明涉及有机化合物合成及医药应用领域,尤其涉及降解btk蛋白的化合物及其制备方法和应用。
背景技术:
2.b细胞淋巴瘤是一种临床治愈性和预后性均较差的非霍奇金淋巴瘤(non-hodgkin lymphoma,nhl),部分恶性b细胞淋巴瘤存在不可治愈性(参见armitage,j.o.;gascoyne,r.d.;lunning,m.a.;cavalli,f.,non-hodgkin lymphoma.lancet 2017,390(10091),298-310.)。b细胞受体(b cell receptor,bcr)信号通路是b细胞生长存活的特异性通路,其异常表达和过度活化是b细胞淋巴瘤发生的关键因素。btk(bruton’s tyrosine kinase)在bcr信号通路中起重要作用,能够激活下游信号级联中的pi3k、nf-κb等多个通路,促进b细胞存活、增殖和分化。依鲁替尼(ibrutinib,ibn)是2013年上市的第一个不可逆共价btk抑制剂,虽然临床取得不错的疗效,但易产生耐药性和副作用(参见liang,c.;tian,d.;ren,x.;ding,s.;jia,m.;xin,m.;thareja,s.,the development of bruton's tyrosine kinase(btk)inhibitors from 2012to 2017:a mini-review.eur.j.med.chem.2018,151,315-326.)。泛素-蛋白酶体系统(ubiquitin-proteasome system,ups)是真核生物体内降解泛素化蛋白的主要途径,蛋白降解靶向嵌合体技术是近年来迅速发展的新兴技术,通过ups将目标蛋白泛素化,进而被蛋白酶体识别降解,具有克服耐药性、提高选择性及催化降解靶蛋白等优势(参见li,x.;song,y.,proteolysis-targeting chimera(protacs)for targeted protein degradation and cancer therapy.j hematol oncol 2020,13(1),50.),可以为b细胞淋巴瘤的治疗提供新的策略。
技术实现要素:
3.本发明的目的是提供一种降解btk蛋白的化合物及其制备方法和应用,所述降解btk蛋白的化合物能有效降解淋巴瘤细胞的btk蛋白,抑制肿瘤细胞的增殖。具体地,本发明的技术方案如下所述:
4.在本发明的第一方面,提供了一种降解btk蛋白的化合物,结构如通式ⅰ所示:
[0005][0006]
其中,x选自亚甲基、吡咯烷基、苄基、哌啶基、吡啶基、嘧啶基、咪唑基或噁二唑基;
1-基)丁酰基)哌嗪-1-基)-n-(2-(2,6-二氧杂哌啶-3-基)-1,3-二氧异吲哚-4-基)戊酰胺(
ⅰ‑
17);
[0026]
2-(4-(5-(3-(4-氨基-3-(4-苯氧基苯基)-1h-吡唑并[3,4-d]嘧啶-1-基)吡咯烷-1-基)戊酰基)哌嗪-1-基)-n-(2-(2,6-二氧杂哌啶-3-基)-1,3-二氧异吲哚-4-基)乙酰胺(
ⅰ‑
18);
[0027]
2-(4-(4-(3-(4-氨基-3-(4-苯氧基苯基)-1h-吡唑并[3,4-d]嘧啶-1-基)吡咯烷-1-基)丁酰基)哌嗪-1-基)-n-(2-(2,6-二氧杂哌啶-3-基)-1,3-二氧异吲哚-4-基)乙酰胺(
ⅰ‑
19);
[0028]
2-(4-(5-(4-(4-氨基-3-(4-苯氧基苯基)-1h-吡唑并[3,4-d]嘧啶-1-基)哌啶-1-基)戊酰基)哌嗪-1-基)-n-(2-(2,6-二氧杂哌啶-3-基)-1,3-二氧异吲哚-4-基)乙酰胺(
ⅰ‑
20);
[0029]
4-(4-(5-(4-(4-氨基-3-(4-苯氧基苯基)-1h-吡唑并[3,4-d]嘧啶-1-基)哌啶-1-基)戊酰基)哌嗪-1-基)-n-(2-(2,6-二氧杂哌啶-3-基)-1,3-二氧异吲哚-4-基)丁酰胺(
ⅰ‑
21);
[0030]
2-(4-(4-(4-(4-氨基-3-(4-苯氧基苯基)-1h-吡唑并[3,4-d]嘧啶-1-基)哌啶-1-基)丁酰基)哌嗪-1-基)-n-(2-(2,6-二氧杂哌啶-3-基)-1,3-二氧代异吲哚-4-基)乙酰胺(
ⅰ‑
22);
[0031]
5-(4-(3-(4-(4-氨基-3-(4-苯氧基苯基)-1h-吡唑并[3,4-d]嘧啶-1-基)哌啶-1-基)丙酰基)哌嗪-1-基)-n-(2-(2,6-二氧杂哌啶-3-基)-1,3-二氧异吲哚-4-基)戊酰胺(
ⅰ‑
23);
[0032]
4-(4-(4-(4-(4-氨基-3-(4-苯氧基苯基)-1h-吡唑并[3,4-d]嘧啶-1-基)哌啶-1-基)丁酰基)哌嗪-1-基)-n-(2-(2,6-二氧杂哌啶-3-基)-1,3-二氧异吲哚-4-基)丁酰胺(
ⅰ‑
24)。
[0033]
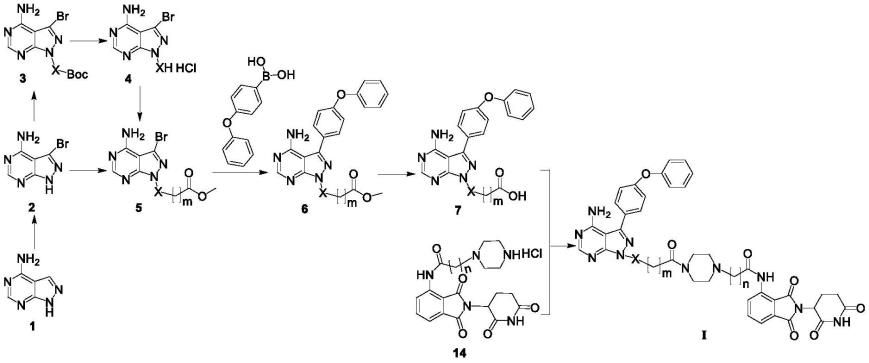
本发明的第二方面,提供了一种制备上述第一方面所述降解btk蛋白的化合物及其制备方法,其包括:以4-氨基吡唑并[3,4-d]嘧啶即化合物1作为起始原料,经溴代自由基反应得到中间体2;中间体2与n-boc-4-羟基x或1-boc-3-羟基x经光延反应,得到中间体3;中间体3脱去叔丁氧羰基保护基,得到中间体4;中间体2或4与溴取代的羧酸甲酯、乙酯经取代反应得中间体5;中间体5与4-苯氧基苯基硼酸经suzuki-miyaura反应得到中间体6;中间体6经过酯的水解得中间体7;中间体7与中间体14经酰胺缩合反应得通式(ⅰ)化合物,所述合成路线如下所示:
[0034][0035]
其中,x、m和n如上述第一方面中所定义。
[0036]
所述方法具体地包括如下:以4-氨基吡唑并[3,4-d]嘧啶即化合物1为起始原料,在dmf中与n-溴代丁二酰亚胺(nbs)经溴代自由基反应得到中间体2;中间体2与n-boc-4-羟基x或1-boc-3-羟基x经光延(mitsunobu)反应,得到中间体3;中间体3在四氢呋喃/浓盐酸下脱去叔丁氧羰基保护基,得到中间体4;中间体2或4与不同溴取代的羧酸甲酯、乙酯经亲核取代反应得中间体5;中间体5与4-苯氧基苯基硼酸在四(三苯基膦)钯催化下,经suzuki-miyaura反应得到中间体6;中间体6在碱性条件下经过酯的水解得中间体7;最终中间体7与中间体14经酰胺缩合反应得到式ⅰ化合物。
[0037]
进一步地,在本发明的实施方式中,式ⅰ目标化合物的制备方法具体包括:
[0038]
(1)将原料1溶于dmf中,加入nbs,80℃油浴反应7h,tlc检测基本反应完全,将反应液冷却到室温,倒入冰水中,搅拌析出大量黄色固体,抽滤,滤饼水洗,干燥,得中间体2。
[0039]
(2)将中间体2、n-boc-4-羟基哌啶或1-boc-3-羟基吡咯烷和三苯基膦溶于无水thf中,冰浴下缓慢滴加偶氮二甲酸二异丙酯(diad),搅拌10min,溶液由浑浊变澄清。tlc检测反应完全,加入乙酸乙酯萃取,合并有机相,加入nacl洗涤,无水硫酸钠干燥,过滤,减压蒸除溶剂,硅胶柱层析纯化(乙酸乙酯:石油醚=120:1-60:1)得中间体3a-3b。
[0040]
(3)将中间体3a-3b溶于无水thf中,加入适量的浓盐酸,室温反应4h,析出白色固体,tcl检测反应完全,抽滤,滤饼用乙酸乙酯洗涤,干燥,得中间体4a-4b。
[0041]
(4)将中间体2或中间体4a-4b溶于dmf中,加入不同溴末端取代的羧酸甲酯和k2co3,室温搅拌8h,反应完毕,反应液加入ea和水进行萃取,合并有机相,加入nacl洗涤,无水na2so4干燥,过滤,减压蒸除溶剂,硅胶柱层析(二氯甲烷:甲醇=120:1-60:1)得中间体5a-5i。
[0042]
(5)将中间体5a-5i、4-苯氧基苯硼酸、四(三苯基膦)钯和磷酸钾置于微波管中,加入1,4-二氧六环和水(4:1)溶解,超声脱去溶液中的氧气,120℃进行微波反应20min。tlc检测反应完全,反应液加入ea/水萃取,合并有机相,加入食盐水洗涤,无水na2so4干燥,过滤,减压蒸除溶剂,硅胶柱层析(二氯甲烷:甲醇=100:1-50:1)得中间体6a-6i。
[0043]
(6)将中间体6a-6i溶于无水乙醇中,加入3m naoh调节ph为10-11左右,室温反应6h,反应完毕,加入1m hcl调节溶液的ph为5-6,析出白色固体,抽滤,滤饼用水洗涤,干燥,得到中间体7a-7i。
[0044]
(7)将化合物8和化合物9置于醋酸溶液搅拌,加入醋酸钠,118℃油浴,回流反应
8h,tlc检测反应完全,将反应液冷却至室温,倒入冰水中,搅拌析出黑紫色亮晶固体,抽滤,滤饼用甲醇洗涤,干燥,得中间体10。
[0045]
(8)将中间体10溶于体积比为1:1的乙醇和二氯甲烷混合溶液中,加入钯碳(pd/c),h2保护,室温反应6h,反应完毕后,硅藻土过滤,二氯甲烷冲洗,合并滤液,减压蒸除溶剂,得中间体11。
[0046]
(9)将中间体11溶于无水四氢呋喃溶液,加入不同取代的卤代酰氯,n2保护下,60℃油浴回流反应6h,tlc检测反应完毕,减压蒸除thf,加入适量无水乙醚,超声搅拌,析出淡黄色固体,抽滤,滤饼用无水乙醚洗涤,干燥,得中间体12a-12d。
[0047]
(10)将中间体12a-12d溶于n-甲基吡咯烷酮(nmp)中,加入n-boc-哌嗪和碘化钠,滴加n,n-二异丙基乙胺(dipea),n2保护下,85℃油浴反应5h,tlc检测反应完毕,将反应液用ea/水萃取,合并有机相,加入nacl洗涤,无水na2so4干燥,过滤,减压蒸除溶剂,硅胶柱层析(二氯甲烷:甲醇=100:1-60:1)得中间体13a-13d。
[0048]
(11)将中间体13a-13d加入到饱和hcl的乙酸乙酯溶液,室温反应4h,析出白色固体,tcl检测反应完毕,抽滤,滤饼用乙酸乙酯洗涤,干燥,得中间体14a-14d。
[0049]
(12)将中间体7a-7i和缩合剂2-(7-氮杂苯并三氮唑)-n,n,n',n'-四甲基脲六氟磷酸酯(hatu)溶于无水dmf,滴加dipea,置于冰水浴中活化30min,得活化酯,再加入中间体14a-14d,n2保护下,室温反应8h,tlc检测反应完毕,将反应液倒入冰水中,析出白色固体,冷却至室温,抽滤,滤饼用水洗涤,干燥,得白色固体,之后经过硅胶柱层析(二氯甲烷:甲醇=100:1-50:1-20:1)得到目标化合物
ⅰ‑
1~
ⅰ‑
24。
[0050]
具体地,其合成路线如下所示:
[0051][0052]
上述合成路线的试剂及条件:(a)nbs,dmf,80℃,7h;(b)不同溴末端取代的羧酸甲酯,k2co3,dmf,r.t.,8h;(c)n-boc-4-羟基哌啶或1-boc-3-羟基吡咯烷,三苯基膦,diad,无
水thf,0℃,10min;(d)四氢呋喃:浓盐酸=1:1,r.t.,4h;(e)不同溴末端取代的羧酸甲酯,k2co3,dmf,r.t.,8h;(f)pd(pph3)4,三水磷酸钾,1,4-二氧六环:水=4:1,mw,120℃,20min;(g)无水甲醇,3m naoh,r.t.,6h;(h)ch3coona,ch3cooh,118℃,8h;(i)ch3ch2oh:ch2cl2=1:1,pd/c,h2,r.t.,6h;(j)不同取代的卤代酰氯,无水thf,n2,60℃,6h;(k)n-boc-哌嗪,nai,dipea,nmp,n2,85℃,5h;(l)饱和hcl的乙酸乙酯溶液,r.t.,4h;(q)dipea,hatu,n2,无水dmf,r.t.,8h。
[0053]
本发明的第三方面,一种药物组合物,其包含上述第一方面所述的一种降解btk蛋白的化合物。
[0054]
本发明的第四方面,一种药物制剂,其包括有效成分和药学上可接受的辅料和/或载体,所述有效成分包含上述降解btk蛋白的化合物,或包含上述药物组合物。
[0055]
本发明的第五方面,上述第一方面中所述的降解btk蛋白的化合物或上述第三方面中所述的药物组合物在制备降解btk蛋白的化合物药物中的应用。
[0056]
本发明的第六方面,上述第一方面中所述的一种降解btk蛋白的化合物或上述第三方面中所述的药物组合物在制备抗肿瘤药物中的应用。
[0057]
本发明的第七方面,上述第一方面中所述的一种降解btk蛋白的化合物或上述第三方面中所述的药物组合物在制备治疗b细胞淋巴瘤的药物中的应用。
[0058]
本发明具有以下有益效果:本发明所述的一种降解btk蛋白的化合物对肿瘤细胞具有优良的抗增殖活性,其中化合物
ⅰ‑
7、
ⅰ‑
8、
ⅰ‑
10、
ⅰ‑
16、
ⅰ‑
17、
ⅰ‑
18对b细胞淋巴瘤细胞株jeko-1的抑制率大于75%,化合物
ⅰ‑
21、
ⅰ‑
23和
ⅰ‑
24对b细胞淋巴瘤细胞株jeko-1的抑制率大于80%;显著优于对照药依鲁替尼(ibn),在相同测试条件下,ibn对b细胞淋巴瘤细胞珠jeko-1的抑制率小于64%;进一步测定部分化合物对肿瘤细胞的半数生长抑制浓度(ic
50
值),结果显示化合物
ⅰ‑
7、
ⅰ‑
21、
ⅰ‑
23和
ⅰ‑
24抑制jeko-1细胞的ic
50
值分别为4.6μm、4.1μm、3.6μm和4.2μm,阳性对照药ibn的ic
50
值为4.7μm;化合物
ⅰ‑
21和
ⅰ‑
23对k562细胞的半数生长抑制浓度(ic
50
值)分别为8μm和7μm,阳性对照药ibn的ic
50
值为10μm;化合物
ⅰ‑
21和
ⅰ‑
23对hel细胞的生长抑制作用是阳性对照药ibn的两倍(ic
50
值分别为16μm、15μm和31μm)。本发明所述的化合物对b细胞淋巴瘤细胞具有显著降解btk蛋白的效果,其中化合物
ⅰ‑
6、
ⅰ‑
7、
ⅰ‑
8、
ⅰ‑
10、
ⅰ‑
11、
ⅰ‑
14、
ⅰ‑
15、
ⅰ‑
16、
ⅰ‑
17、
ⅰ‑
18、
ⅰ‑
21、
ⅰ‑
23和
ⅰ‑
24可有效降解btk蛋白,特别是化合物
ⅰ‑
7、
ⅰ‑
21和
ⅰ‑
23对b细胞淋巴瘤细胞珠jeko-1显示浓度依赖性和时间依赖性地显著降解btk蛋白。具体的是,在化合物浓度为5μm时处理jeko-1细胞24h,
ⅰ‑
7对btk蛋白最大降解量(d
max
)为73%,
ⅰ‑
21对btk蛋白最大降解量(d
max
)为92%,
ⅰ‑
23对btk蛋白最大降解量(d
max
)为94%,化合物
ⅰ‑
7、化合物
ⅰ‑
21和化合物
ⅰ‑
23对btk的半数降解浓度(dc
50
)分别为0.45μm、0.25μm和0.10μm。在相同实验条件下,对照药依鲁替尼和泊马度胺均未显示出对btk蛋白的降解作用。
附图说明
[0059]
构成本发明的一部分的说明书附图用来提供对本发明的进一步理解,本发明的示意性实施例及其说明用于解释本发明,并不构成对本发明的不当限定。
[0060]
图1为目标化合物的蛋白质印迹法检测结果,其中ibn表示依鲁替尼,pomalidomide表示泊马度胺。
具体实施方式
[0061]
下面结合具体实施例,进一步阐述本发明。应理解,这些实施例仅用于说明本发明而不用于限制本发明的范围。下列实施例中未注明具体条件的实验方法,通常按照常规条件或按照制造厂商所建议的条件。
[0062]
除非另行定义,文中所使用的所有专业与科学用语与本领域熟练人员所熟悉的意义相同。本发明所使用的试剂或原料均可通过常规途径购买获得,如无特殊说明,本发明所使用的试剂或原料均按照本领域常规方式使用或者按照产品说明书使用。此外,任何与所记载内容相似或均等的方法及材料皆可应用于本发明方法中。
[0063]
为了使得本领域技术人员能够更加清楚地了解本公开的技术方案,以下将结合具体的实施例与对比例详细说明本公开的技术方案。
[0064]
实施例1:中间体2的制备
[0065]
将原料4-氨基吡唑并[3,4-d]嘧啶(15.0g,111.0mmol)置于250ml茄形瓶中,加入55ml n,n-二甲基甲酰胺(dmf)溶液,搅拌至溶解。室温条件下加入n-溴代丁二酰亚胺(23.7g,133.2mmol),反应瓶置于80℃油浴,加热反应6h,溶液由黄棕色浑浊液体变为红褐色透明液体。tlc检测基本反应完全,将反应液冷却至室温,倒入300ml冰水中淬灭,搅拌下析出大量黄棕色固体,抽滤,滤饼水洗,干燥得中间体2,黄棕色固体20.8g,产率86.9%。mp:272-274℃;1h nmr(400mhz,dmso-d6)δ13.76(s,1h),8.16(s,1h),7.94(s,1h),6.85(s,1h)。
[0066]
实施例2:中间体3的制备
[0067]
取中间体2(3.0g,14.0mmol)、n-boc-4-羟基哌啶或1-boc-3-羟基吡咯烷(21.0mol)和三苯基膦(8.1g,30.8mol)于100ml茄形瓶中,加入30ml无水四氢呋喃,置于0℃冰浴中,搅拌溶解,缓慢滴加diad(8.5g,42.1mol),冰浴下反应10min,溶液由红褐色浑浊逐渐变澄清。tcl检测反应完毕,加入ea/水萃取(30ml
×
3),合并有机相,加入食盐水(20ml)洗涤,无水硫酸钠干燥,过滤,减压蒸除溶剂,硅胶柱层析纯化,石油醚:乙酸乙酯=80:1~20:1~5:1,得中间体3a-3b。
[0068]
实施例3:中间体4的制备
[0069]
将中间体3a-3b置于250ml茄形瓶,直接加入20ml无水thf搅拌溶解,将5ml浓盐酸缓慢滴加茄形瓶中,室温反应4h,析出白色固体,tlc检测反应完毕,直接抽滤,滤饼用乙酸乙酯洗,干燥,得中间体4a-4b。
[0070]
实施例4:中间体5的制备
[0071]
取中间体2(11.98mmol)分别加入3-溴丙酸甲酯、4-溴丁酸甲酯、5-溴戊酸甲酯、6-溴己酸甲酯(14.38mmol),或取中间体4a-4b,分别加入3-溴丙酸甲酯、4-溴丁酸甲酯、5-溴戊酸甲酯(14.38mmol),再加入k2co3粉末(29.98mmol)置于100ml茄形瓶中,加入30ml dmf溶液搅拌溶解,室温反应8h。tcl检测反应完毕,将反应液倒入60ml冷水淬灭,乙酸乙酯萃取(30ml
×
3),有机相合并,饱和nacl溶液(30ml)洗涤,无水na2so4干燥,过滤,减压蒸除溶剂,硅胶柱层析纯化,二氯甲烷:甲醇=120:1~60:1~40:1,得中间体5a-5i。
[0072]
实施例5:中间体6的制备
[0073]
取中间体5a-5i(10mmol),4-苯氧基苯基硼酸(16mmol),四(三苯基膦)钯(pd(pph3)4,0.522mmol)和磷酸钾三水(k3po4·
3h2o,21mmol)于35ml微波管中,加入1,4-二氧六环/水(4:1)溶剂搅拌溶解,超声脱去溶液中的氧气,微波反应设置反应温度时间分别为
120℃和20min。反应完毕后,tlc检测反应完全,将反应液用乙酸乙酯/水萃取(25ml
×
3),有机相合并,nacl溶液(20ml)洗涤,无水硫酸钠干燥,过滤,减压蒸除溶剂,硅胶柱层析纯化,二氯甲烷:甲醇=120:1~100:1~50:1,得中间体6a-6i。
[0074]
实施例6:中间体7的制备
[0075]
取中间体6a-6i(16mmol)置于100ml的茄形瓶中,加入25ml无水乙醇溶解,滴加1ml 3m naoh溶液,搅拌使溶液呈碱性,ph约为10~11,室温反应6h。取样点板,tcl检测反应完毕,减压蒸除溶剂,加入15ml纯化水溶解,滴加1m hcl调节溶液ph约为5~6,搅拌,析出白色固体,抽滤,滤饼水洗,得中间体7a-7i。
[0076]
实施例7:中间体10的制备
[0077]
将化合物8(3g,18.4mmol)和化合物9(3.3g,20.2mmol)置于250ml茄形瓶中,用40ml醋酸溶液溶解,加入醋酸钠(1.8g,22.0mmol),反应瓶置于118℃回流,加热反应8h。tlc检测反应完全,将反应液倒入200ml冰水中,析出黑紫色亮晶固体,抽滤,滤饼用甲醇洗涤,干燥,得中间体10,黑紫色固体4.16g,产率88.32%。mp:274-276℃;1h nmr(400mhz,dmso)δ11.19(s,1h),8.36(d,j=8.0hz,1h),8.25(d,j=7.4hz,1h),8.13(t,j=7.7hz,1h),5.22(dd,j=12.7,5.1hz,1h),3.02
–
2.80(m,1h),2.72
–
2.59(m,1h),2.58
–
2.49(m,1h),2.17
–
2.03(m,1h)。
[0078]
实施例8:中间体11的制备
[0079]
取中间体10(4g,13.2mmol)和钯碳(pd/c,400mg)置于250ml茄形瓶中,加入35ml乙醇和二氯甲烷(1:1)溶液,搅拌溶解,h2保护,室温反应6h。tlc检测反应完毕,硅藻土过滤,合并滤液,减压蒸除有机溶剂,得中间体11,黄色固体3.3g,产率93%。mp:278-280℃;1h nmr(400mhz,dmso-d6)δ11.10(s,1h),7.47(t,j=7.7hz,1h),7.01(t,j=6.9hz,2h),6.53(s,2h),5.05(dd,j=12.9,5.1hz,1h),2.95
–
2.82(m,1h),2.70
–
2.52(m,2h),2.09
–
1.95(m,1h)。
[0080]
实施例9:中间体12的制备
[0081]
取中间体11(3.7mmol)分别加入氯乙酰氯、4-氯丁酰氯、5-氯戊酰氯、6-溴己酰氯(10.98mmol)于100ml茄形瓶中,加入25ml无水四氢呋喃溶液,搅拌溶解,n2保护,60℃回流,加热反应6h。tlc检测反应完毕,减压蒸除溶剂,加入15ml无水乙醚,超声搅拌,析出淡黄色固体,抽滤,滤饼用无水乙醚洗涤,干燥,得中间体12a-12d。
[0082]
实施例10:中间体13的制备
[0083]
取中间体12a-12d(16mmol),n-boc-哌嗪(79mmol)和碘化钠(3.176mmol)于250ml茄形瓶中,加入20ml n-甲基吡咯烷酮(nmp)溶液,搅拌溶解,滴加n,n-二异丙基乙胺(dipea,16mmol),n2保护,85℃油浴,加热反应8h。tlc检测反应完毕,反应液用乙酸乙酯/水萃取(30ml
×
3),合并有机相,食盐水(30ml)洗涤,无水na2so4干燥,过滤,减压蒸除溶剂,硅胶柱层析纯化,二氯甲烷:甲醇=120:1~100:1~50:1,得中间体13a-13d。
[0084]
实施例11:中间体14的制备
[0085]
称取中间体13a-13d(8.01mmol)置于100ml茄形瓶中,加入15ml饱和hcl的乙酸乙酯溶液,搅拌溶解,室温下反应4h,逐渐析出白色固体。取样点板,tlc检测反应完全,抽滤,滤饼用乙酸乙酯洗涤,干燥,得中间体(14a-14d)。
[0086]
实施例12:目标化合物
ⅰ‑
1至
ⅰ‑
24的制备
[0087]
取中间体7a-7i(5.05mmol)和缩合剂hatu(5.05mmol)于100ml茄形瓶中,加入15ml无水dmf,滴加n,n-二异丙基乙胺(14mmol),搅拌溶解,置于冰水浴(0℃)中活化30min,后再加入中间体14a-14d(4.59mmol),n2保护,室温反应8h。tlc检测反应完毕,将反应液倒入150ml冰水浴中,析出白色固体,冷却至室温,抽滤,滤饼用冷水洗涤,干燥,得白色固体,固体用二氯甲烷溶解拌样,硅胶柱层析纯化,二氯甲烷:甲醇=100:1~60:1~20:1,得到目标终产物
ⅰ‑
1至
ⅰ‑
24。
[0088]
ⅰ‑
1:2-(4-(3-(4-氨基-3-(4-苯氧基苯基)-1h-吡唑并[3,4-d]嘧啶-1-基)丙酰基)哌嗪-1-基)-n-(2-(2,6-二氧杂哌啶-3-基)-1,3-二氧异吲哚-4-基)乙酰胺
[0089]
白色固体,产率47.4%,mp:166-168℃。1h-nmr(400mhz,cdcl3)δ11.14(s,1h),9.74(s,1h),8.87(d,j=8.4hz,1h),8.42(s,1h),7.72(t,j=7.9hz,1h),7.64(d,j=8.0hz,2h),7.57(d,j=7.2hz,1h),7.39(t,j=7.6hz,2h),7.23
–
7.10(m,3h),7.08(d,j=7.7hz,2h),5.04
–
4.93(m,1h),4.80(t,j=7.1hz,2h),3.78(s,2h),3.58(s,2h),3.21(s,2h),3.17
–
3.08(m,1h),3.07
–
2.98(m,1h),2.95
–
2.87(m,1h),2.86
–
2.73(m,2h),2.59(s,4h),2.25
–
2.11(m,1h);
13
c-nmr(100mhz,cdcl3)δ171.99(s),169.73(s),168.88(s),168.69(s),168.41(s),166.92(s),158.56(s),157.95(s),156.29(s),155.74(s),154.36(s),144.11(s),136.85(s),136.29(s),131.42(s),129.99(s),129.89(s),127.59(s),125.10(s),124.09(s),119.56(s),119.10(s),118.68(s),116.13(s),98.41(s),61.70(s),53.34(s),53.10(s),49.35(s),45.28(s),43.54(s),41.51(s),32.80(s),31.53(s),22.68(s).hrms(esi):calcd for c
39h36n10
o7[m+h]
+
757.2841,found 757.2838。
[0090]
ⅰ‑
2:4-(4-(3-(4-氨基-3-(4-苯氧基苯基)-1h-吡唑并[3,4-d]嘧啶-1-基)丙酰基)哌嗪-1-基)-n-(2-(2,6-二氧杂哌啶-3-基)-1,3-二氧异吲哚-4-基)丁酰胺
[0091]
白色固体,产率43.5%,mp:162-164℃。1h nmr(400mhz,cdcl3)δ9.52(s,1h),8.82(d,j=8.6hz,1h),8.42(s,1h),7.71(t,j=8.0hz,1h),7.63(d,j=7.9hz,2h),7.55(d,j=7.2hz,1h),7.39(t,j=7.3hz,2h),7.19
–
7.12(m,3h),7.08(d,j=7.8hz,2h),4.99
–
4.90(m,1h),4.77(t,j=7.4hz,2h),3.58(s,2h),3.36(s,2h),3.08
–
2.98(m,2h),2.95
–
2.87(m,1h),2.85
–
2.73(m,2h),2.51(dd,j=10.4,6.2hz,2h),2.44
–
2.29(m,6h),2.20
–
2.14(m,1h),1.97
–
1.86(m,2h);
13
c nmr(100mhz,cdcl3)δ172.31(s),171.68(s),169.23(s),168.67(s),168.57(s),166.75(s),158.52(s),157.93(s),156.34(s),155.86(s),154.30(s),144.01(s),137.77(s),136.46(s),131.22(s),129.98(s),129.93(s),127.67(s),125.24(s),124.05(s),119.53(s),119.11(s),118.50(s),115.28(s),98.45(s),56.64(s),53.17(s),52.36(s),49.41(s),45.28(s),43.55(s),41.46(s),35.59(s),32.73(s),31.52(s),22.69(s),22.10(s).hrms(esi):calcd for c
41h40n10
o7[m+h]
+
785.3154,found 785.3146。
[0092]
ⅰ‑
3:5-(4-(3-(4-氨基-3-(4-苯氧基苯基)-1h-吡唑并[3,4-d]嘧啶-1-基)丙酰基)哌嗪-1-基)-n-(2-(2,6-二氧杂哌啶-3-基)-1,3-二氧异吲哚-4-基)戊酰胺
[0093]
白色固体,产率45%,mp:160-162℃。1h nmr(400mhz,cdcl3)δ10.30(s,1h),9.44(s,1h),8.80(d,j=8.4hz,1h),8.42(s,1h),7.71(t,j=7.9hz,1h),7.63(d,j=7.9hz,2h),7.54(d,j=7.2hz,1h),7.38(t,j=7.4hz,2h),7.19
–
7.11(m,3h),7.07(d,j=7.9hz,2h),5.00
–
4.90(m,1h),4.78(t,j=7.0hz,2h),3.59(t,j=16.6hz,2h),3.38(s,2h),3.01(t,j=7.0hz,2h),2.95
–
2.86(m,1h),2.84
–
2.70(m,2h),2.48(t,j=6.9hz,2h),2.40
–
2.33
(m,4h),2.31(s,2h),2.20
–
2.14(m,1h),1.82
–
1.74(m,2h),1.60
–
1.52(m,2h);
13
c nmr(100mhz,cdcl3)δ172.13(s),171.58(s),169.29(s),168.61(s),168.58(s),166.73(s),158.52(s),157.88(s),156.35(s),155.87(s),154.39(s),143.99(s),137.77(s),136.46(s),131.15(s),129.98(s),129.92(s),127.70(s),125.25(s),124.05(s),119.53(s),119.13(s),118.52(s),115.34(s),98.46(s),57.64(s),53.14(s),52.70(s),49.35(s),45.35(s),43.57(s),41.51(s),37.68(s),32.75(s),31.48(s),25.80(s),23.23(s),22.76(s).hrms(esi):calcd for c
42h42n10
o7[m+h]
+
799.3311,found 799.3301。
[0094]
ⅰ‑
4:6-(4-(3-(4-氨基-3-(4-苯氧基苯基)-1h-吡唑并[3,4-d]嘧啶-1-基)丙酰基)哌嗪-1-基)-n-(2-(2,6-二氧杂哌啶-3-基)-1,3-二氧异吲哚-4-基)己酰胺
[0095]
白色固体,产率57.49%,mp:152-154℃。1h nmr(400mhz,cdcl3)δ9.86(s,1h),9.43(s,1h),8.82(d,j=8.4hz,1h),8.42(s,1h),7.71(t,j=8.1hz,1h),7.64(d,j=8.1hz,2h),7.55(d,j=7.2hz,1h),7.39(t,j=7.5hz,2h),7.20
–
7.12(m,4h),7.08(d,j=7.8hz,2h),4.98
–
4.90(m,1h),4.83
–
4.75(m,2h),3.60(m,2h),3.39(m,2h),3.10
–
2.98(m,2h),2.94
–
2.86(m,1h),2.78(t,j=11.3hz,2h),2.46(t,j=7.1hz,2h),2.37
–
2.26(m,6h),2.20
–
2.13(m,1h),1.80
–
1.73(m,2h),1.55
–
1.48(m,2h),1.43
–
1.37(m,2h);
13
c nmr(100mhz,cdcl3)δ172.18(s),171.74(s),169.28(s),168.70(s),168.45(s),166.73(s),158.52(s),157.89(s),156.35(s),155.80(s),154.42(s),144.00(s),137.81(s),136.45(s),131.13(s),129.97(s),129.91(s),127.71(s),125.26(s),124.05(s),119.53(s),119.12(s),118.48(s),115.33(s),98.45(s),58.06(s),53.16(s),52.64(s),49.33(s),45.39(s),43.61(s),41.55(s),37.85(s),32.69(s),31.47(s),26.89(s),26.18(s),25.06(s),22.78(s).hrms(esi):calcd for c
43h44n10
o7[m+h]
+
813.3467,found 813.3456。
[0096]
ⅰ‑
5:2-(4-(4-(4-氨基-3-(4-苯氧基苯基)-1h-吡唑并[3,4-d]嘧啶-1-基)丁酰基)哌嗪-1-基)-n-(2-(2,6-二氧杂哌啶-3-基)-1,3-二氧异吲哚-4-基)乙酰胺
[0097]
白色固体,产率28.6%,mp:164-166℃。1h nmr(400mhz,cdcl3)δ11.07(s,1h),8.84(d,j=8.5hz,1h),8.45(s,1h),7.73(t,j=7.8hz,1h),7.66(d,j=7.9hz,2h),7.58(d,j=7.3hz,1h),7.39(t,j=7.6hz,2h),7.21
–
7.13(m,3h),7.09(d,j=8.0hz,2h),5.04
–
4.91(m,1h),4.85
–
4.58(m,1h),4.54
–
4.36(m,1h),3.89(s,1h),3.50(s,1h),3.44
–
3.30(m,2h),3.18(dd,j=72.3,16.8hz,2h),2.99
–
2.82(m,1h),2.82
–
2.62(m,2h),2.62
–
2.44(m,4h),2.42
–
2.33(m,1h),2.33
–
2.28(m,2h),2.22
–
2.07(m,2h);
13
c nmr(100mhz,cdcl3)δ172.85(s),169.98(s),169.74(s),168.68(s),168.28(s),166.85(s),158.51(s),157.92(s),156.38(s),155.46(s),154.85(s),144.17(s),136.90(s),136.27(s),131.45(s),129.98(s),129.93(s),127.73(s),125.13(s),124.04(s),119.52(s),119.16(s),118.65(s),116.13(s),98.05(s),61.96(s),53.48(s),53.12(s),49.20(s),46.32(s),45.16(s),41.45(s),31.33(s),29.41(s),24.91(s),23.21(s).hrms(esi):calcd for c
40h38n10
o7[m+h]+771.2998,found 771.2994。
[0098]
ⅰ‑
6:4-(4-(4-(4-氨基-3-(4-苯氧基苯基)-1h-吡唑并[3,4-d]嘧啶-1-基)丁酰基)哌嗪-1-基)-n-(2-(2,6-二氧杂哌啶-3-基)-1,3-二氧异吲哚-4-基)丁酰胺
[0099]
白色固体,产率22.8%,mp:154-156℃。1h nmr(400mhz,cdcl3)δ10.94(s,1h),9.49(s,1h),8.81(d,j=8.5hz,1h),8.41(s,1h),7.71(t,j=7.8hz,1h),7.64(d,j=8.1hz,
2h),7.55(d,j=7.2hz,1h),7.39(t,j=7.4hz,2h),7.20
–
7.13(m,4h),7.08(d,j=7.7hz,2h),5.07
–
4.84(m,1h),4.57
–
4.50(m,2h),3.56(s,2h),3.28(s,2h),2.95
–
2.84(m,1h),2.84
–
2.67(m,2h),2.58
–
2.47(m,2h),2.42
–
2.35(m,4h),2.34
–
2.25(m,6h),2.14
–
2.06(m,1h),1.96
–
1.90(m,2h);
13
cnmr(100mhz,cdcl3)δ172.32(s),172.19(s),170.42(s),169.25(s),168.61(s),166.75(s),158.50(s),157.95(s),156.37(s),155.70(s),154.57(s),143.97(s),137.79(s),136.46(s),131.21(s),129.95(d,j=5.1hz),127.72(s),125.27(s),124.03(s),119.52(s),119.14(s),118.51(s),115.32(s),98.21(s),56.59(s),53.01(s),52.65(s),49.39(s),46.69(s),45.25(s),41.51(s),35.63(s),31.51(s),30.22(s),25.28(s),22.82(s),22.40(s).hrms(esi):calcd for c
42h42n10
o7[m+h]
+
799.3311,found 799.3279。
[0100]
ⅰ‑
7:5-(4-(4-(4-氨基-3-(4-苯氧基苯基)-1h-吡唑并[3,4-d]嘧啶-1-基)丁酰基)哌嗪-1-基)-n-(2-(2,6-二氧杂哌啶-3-基)-1,3-二氧异吲哚-4-基)戊酰胺
[0101]
白色固体,产率29.3%,mp:144-146℃。1h nmr(400mhz,cdcl3)δ11.06(s,1h),9.48(s,1h),8.82(d,j=8.5hz,1h),8.42(s,1h),7.72(t,j=7.8hz,1h),7.65(d,j=7.9hz,2h),7.56(d,j=7.4hz,1h),7.39(t,j=7.5hz,2h),7.16(t,j=9.8hz,3h),7.08(d,j=7.9hz,2h),5.02
–
4.90(m,1h),4.62
–
4.42(m,2h),3.72
–
3.49(m,2h),3.29(s,2h),2.95
–
2.85(m,1h),2.85
–
2.71(m,2h),2.47(dd,j=13.2,7.0hz,2h),2.36(s,6h),2.28(s,4h),2.21
–
2.14(m,1h),1.86
–
1.73(m,2h),1.59
–
1.52(m,2h);
13
c nmr(100mhz,cdcl3)δ172.41(s),172.15(s),170.33(s),169.32(s),168.71(s),166.76(s),158.48(s),157.94(s),156.38(s),155.61(s),154.62(s),143.98(s),137.79(s),136.44(s),131.14(s),129.97(s),129.92(s),127.77(s),125.28(s),124.02(s),119.50(s),119.15(s),118.52(s),115.40(s),98.14(s),57.63(s),53.12(s),52.89(s),49.36(s),46.60(s),45.28(s),41.54(s),37.86(s),31.52(s),29.81(s),25.96(s),25.14(s),23.30(s),22.83(s).hrms(esi):calcd for c
43h44n10
o7[m+h]
+
813.3467,found 813.3442。
[0102]
ⅰ‑
8:6-(4-(4-(4-氨基-3-(4-苯氧基苯基)-1h-吡唑并[3,4-d]嘧啶-1-基)丁酰基)哌嗪-1-基)-n-(2-(2,6-二氧杂哌啶-3-基)-1,3-二氧异吲哚-4-基)己酰胺
[0103]
白色固体,产率60.5%,mp:146-148℃。1h nmr(400mhz,cdcl3)δ11.52(s,1h),9.45(s,1h),8.82(d,j=8.4hz,1h),8.42(s,1h),7.72(t,j=7.8hz,1h),7.64(d,j=7.8hz,2h),7.56(d,j=7.1hz,1h),7.39(t,j=7.4hz,2h),7.20
–
7.13(m,3h),7.08(d,j=7.7hz,2h),5.08
–
4.90(m,1h),4.60
–
4.44(m,2h),3.66
–
3.53(m,2h),3.30(s,2h),2.96
–
2.85(m,1h),2.85
–
2.68(m,2h),2.53
–
2.42(m,2h),2.37(s,2h),2.30(s,8h),2.18(s,1h),1.76(td,j=13.8,7.0hz,2h),1.56
–
1.48(m,2h),1.44
–
1.36(m,2h);
13
c nmr(100mhz,cdcl3)δ172.63(s),172.20(s),170.26(s),169.33(s),168.77(s),166.75(s),158.49(s),157.98(s),156.38(s),155.60(s),154.65(s),144.00(s),137.84(s),136.48(s),131.14(s),129.98(s),129.92(s),127.75(s),125.26(s),124.03(s),119.51(s),119.16(s),118.51(s),115.32(s),98.13(s),58.00(s),53.00(s),49.34(s),46.60(s),45.31(s),41.57(s),37.82(s),31.53(s),29.97(s),26.66(s),26.27(s),25.27(s),25.13(s),22.87(s).hrms(esi):calcd for c
44h46n10
o7[m+h]
+
827.3624,found 827.3615。
[0104]
ⅰ‑
9:2-(4-(5-(4-氨基-3-(4-苯氧基苯基)-1h-吡唑并[3,4-d]嘧啶-1-基)戊酰
基)哌嗪-1-基)-n-(2-(2,6-二氧杂哌啶-3-基)-1,3-二氧异吲哚-4-基)乙酰胺
[0105]
白色固体,产率41.2%,mp:160-162℃。1h nmr(400mhz,cdcl3)δ11.21(s,1h),8.93(d,j=8.5hz,1h),8.46(s,1h),7.77
–
7.70(m,1h),7.66
–
7.58(m,3h),7.40(t,j=7.9hz,2h),7.20
–
7.13(m,3h),7.09(d,j=7.8hz,2h),5.05
–
4.93(m,1h),4.80
–
4.62(m,1h),4.41
–
4.32(m,1h),4.33
–
4.16(m,1h),3.88
–
3.64(m,1h),3.44
–
3.33(m,1h),3.32
–
3.09(m,3h),2.97
–
2.87(m,1h),2.88
–
2.56(m,6h),2.52
–
2.43(m,1h),2.40
–
2.29(m,2h),2.20
–
2.13(m,1h),2.12
–
2.02(m,1h),1.92
–
1.72(m,2h);
13
c nmr(100mhz,cdcl3)δ173.08(s),171.20(s),169.89(s),168.72(s),168.36(s),166.99(s),158.54(s),158.06(s),156.37(s),155.25(s),154.66(s),144.49(s),136.90(s),136.24(s),131.44(s),129.98(s),129.91(s),127.63(s),125.08(s),124.03(s),119.53(s),119.17(s),118.67(s),116.13(s),98.09(s),61.80(s),53.62(s),53.38(s),49.34(s),44.97(s),44.93(s),41.51(s),31.56(s),31.16(s),28.20(s),23.13(s),20.93(s).hrms(esi):calcd for c
41h40n10
o7[m+h]
+
785.3154,found 785.3146。
[0106]
ⅰ‑
10:4-(4-(5-(4-氨基-3-(4-苯氧基苯基)-1h-吡唑并[3,4-d]嘧啶-1-基)戊酰基)哌嗪-1-基)-n-(2-(2,6-二氧杂哌啶-3-基)-1,3-二氧异吲哚-4-基)丁酰胺
[0107]
白色固体,产率28.6%,mp:138-140℃。1h nmr(400mhz,cdcl3)δ9.50(s,1h),8.81(d,j=8.2hz,1h),8.43(s,1h),7.72(t,j=7.9hz,1h),7.64(d,j=8.1hz,2h),7.56(d,j=7.3hz,1h),7.39(t,j=7.6hz,2h),7.19
–
7.13(m,3h),7.08(d,j=7.9hz,2h),5.06
–
4.86(m,1h),4.56
–
4.40(m,2h),3.71
–
3.54(m,1h),3.48
–
3.29(m,3h),3.00
–
2.83(m,1h),2.88
–
2.71(m,2h),2.58
–
2.46(m,2h),2.46
–
2.35(m,5h),2.34
–
2.23(m,3h),2.22
–
2.16(m,1h),2.14
–
2.07(m,1h),2.04
–
1.97(m,1h),1.97
–
1.88(m,2h),1.57
–
1.47(m,2h);
13
c nmr(100mhz,cdcl3)δ172.28(s),172.26(s),171.19(s),169.24(s),168.91(s),166.78(s),158.44(s),157.99(s),156.37(s),155.56(s),154.26(s),143.93(s),137.75(s),136.45(s),131.19(s),129.97(s),129.94(s),127.73(s),125.28(s),124.00(s),119.48(s),119.16(s),118.51(s),115.33(s),98.17(s),56.86(s),53.28(s),52.57(s),50.66(s),49.37(s),46.32(s),45.44(s),35.62(s),32.37(s),31.50(s),29.09(s),22.78(s),22.17(s),22.04(s).hrms(esi):calcd for c
43h44n10
o7[m+h]
+
813.3467,found 813.3461。
[0108]
ⅰ‑
11:5-(4-(5-(4-氨基-3-(4-苯氧基苯基)-1h-吡唑并[3,4-d]嘧啶-1-基)戊酰基)哌嗪-1-基)-n-(2-(2,6-二氧杂哌啶-3-基)-1,3-二氧异吲哚-4-基)戊酰胺
[0109]
白色固体,产率45.2%,mp:140-421℃。1h nmr(400mhz,cdcl3)δ9.48(s,1h),8.81(d,j=8.3hz,1h),8.40(s,1h),7.72(t,j=7.7hz,1h),7.64(d,j=8.0hz,2h),7.56(d,j=7.1hz,1h),7.39(t,j=7.4hz,2h),7.19
–
7.12(m,3h),7.08(d,j=7.8hz,2h),5.03
–
4.87(m,1h),4.56
–
4.40(m,2h),3.69
–
3.45(m,2h),3.47
–
3.26(m,2h),2.98
–
2.82(m,1h),2.86
–
2.63(m,2h),2.52
–
2.45(m,2h),2.45
–
2.24(m,8h),2.18(s,1h),2.10
–
2.00(m,2h),1.84
–
1.76(m,2h),1.64
–
1.51(m,4h);
13
c nmr(100mhz,cdcl3)δ172.08(s),172.01(s),171.24(s),169.31(s),168.68(s),166.73(s),158.46(s),157.94(s),156.40(s),155.57(s),154.38(s),143.87(s),137.79(s),136.45(s),131.14(s),129.97(s),129.94(s),127.80(s),125.26(s),124.00(s),119.49(s),119.17(s),118.52(s),115.38(s),98.21(s),57.73(s),53.48(s),52.70(s),49.34(s),46.38(s),45.52(s),41.48(s),37.72(s),32.57
(s),31.48(s),29.20(s),25.92(s),23.23(s),22.83(s),22.24(s).hrms(esi):calcd for c
44h46n10
o7[m+h]
+
827.3624,found 827.3611。
[0110]
ⅰ‑
12:6-(4-(5-(4-氨基-3-(4-苯氧基苯基)-1h-吡唑并[3,4-d]嘧啶-1-基)戊酰基)哌嗪-1-基)-n-(2-(2,6-二氧杂哌啶-3-基)-1,3-二氧异吲哚-4-基)己酰胺
[0111]
白色固体,产率50.3%,mp:134-136℃。1h nmr(400mhz,cdcl3)δ11.21
–
10.66(m,1h),9.47(s,1h),8.82(d,j=8.4hz,1h),8.41(s,1h),7.74
–
7.69(m,1h),7.64(d,j=8.6hz,2h),7.56(d,j=7.3hz,1h),7.38(t,j=7.9hz,2h),7.18
–
7.12(m,3h),7.08(d,j=7.8hz,2h),5.04
–
4.90(m,1h),4.48(dt,j=12.2,6.8hz,2h),3.63
–
3.49(m,2h),3.41
–
3.32(m,2h),3.05
–
2.86(m,1h),2.87
–
2.60(m,2h),2.49
–
2.43(m,2h),2.43
–
2.36(m,2h),2.36
–
2.27(m,6h),2.21
–
2.15(m,1h),2.12
–
2.02(m,2h),1.81
–
1.73(m,2h),1.59
–
1.48(m,4h),1.42(dd,j=18.3,3.7hz,2h);
13
c nmr(100mhz,cdcl3)δ172.21(s),172.18(s),171.15(s),169.29(s),168.87(s),166.74(s),158.46(s),157.98(s),156.41(s),155.53(s),154.41(s),143.91(s),137.81(s),136.43(s),131.15(s),129.96(s),129.94(s),127.80(s),125.26(s),124.00(s),119.48(s),119.17(s),118.48(s),115.36(s),98.18(s),57.98(s),53.48(s),52.60(s),49.35(s),46.25(s),45.50(s),41.47(s),37.92(s),32.47(s),31.51(s),29.05(s),26.78(s),26.18(s),25.15(s),22.79(s),22.13(s).hrms(esi):calcd for c
45h48n10
o7[m+h]
+
841.3780,found 841.3773。
[0112]
ⅰ‑
13:2-(4-(6-(4-氨基-3-(4-苯氧基苯基)-1h-吡唑并[3,4-d]嘧啶-1-基)己酰基)哌嗪-1-基)-n-(2-(2,6-二氧杂哌啶-3-基)-1,3-二氧异吲哚-4-基)乙酰胺
[0113]
白色固体,产率50.3%,mp:160-162℃。1h nmr(400mhz,cdcl3)δ11.16(s,1h),8.88(d,j=8.4hz,1h),8.42(s,1h),7.73(t,j=7.7hz,1h),7.65(d,j=7.9hz,2h),7.58(d,j=7.2hz,1h),7.39(t,j=7.6hz,2h),7.20
–
7.12(m,3h),7.08(d,j=7.7hz,2h),5.15
–
4.76(m,1h),4.55
–
4.36(m,2h),3.88(s,1h),3.80
–
3.44(m,3h),3.21(q,j=17.0hz,2h),2.98
–
2.85(m,1h),2.85
–
2.70(m,2h),2.68
–
2.46(m,4h),2.36
–
2.21(m,2h),2.16(s,1h),2.05
–
1.93(m,2h),1.66
–
1.59(m,2h),1.44
–
1.32(m,2h);
13
c nmr(100mhz,cdcl3)δ172.23(s),171.45(s),169.80(s),168.86(s),168.38(s),166.92(s),158.43(s),157.99(s),156.39(s),155.49(s),154.29(s),143.83(s),136.87(s),136.28(s),131.42(s),129.96(s),129.89(s),127.79(s),125.12(s),124.00(s),119.47(s),119.16(s),118.66(s),116.14(s),98.24(s),61.79(s),53.58(s),53.24(s),50.71(s),49.32(s),47.06(s),45.42(s),32.98(s),31.52(s),29.36(s),26.40(s),24.66(s),22.75(s).hrms(esi):calcd for c
42h42n10
o7[m+h]
+
799.3311,found 799.3297。
[0114]
ⅰ‑
14:4-(4-(6-(4-氨基-3-(4-苯氧基苯基)-1h-吡唑并[3,4-d]嘧啶-1-基)己酰基)哌嗪-1-基)-n-(2-(2,6-二氧杂哌啶-3-基)-1,3-二氧异吲哚-4-基)丁酰胺
[0115]
白色固体,产率48.5%,mp:126-128℃。1h nmr(400mhz,cdcl3)δ10.50(s,1h),9.47(s,1h),8.82(d,j=8.4hz,1h),8.40(s,1h),7.72(t,j=7.8hz,1h),7.65(d,j=8.0hz,2h),7.56(d,j=7.2hz,1h),7.39(t,j=7.5hz,2h),7.20
–
7.11(m,3h),7.08(d,j=7.8hz,2h),5.06
–
4.85(m,1h),4.52
–
4.33(m,2h),3.65
–
3.45(m,2h),3.38(s,2h),3.01
–
2.84(m,1h),2.88
–
2.66(m,2h),2.51(t,j=6.5hz,2h),2.49
–
2.30(m,6h),2.31
–
2.22(m,2h),2.21
–
2.13(m,1h),2.01
–
1.90(m,4h),1.71
–
1.62(m,2h),1.39
–
1.29(m,2h);
13
c nmr(100mhz,
cdcl3)δ172.24(s),172.06(s),171.39(s),169.26(s),168.72(s),166.76(s),158.43(s),157.94(s),156.42(s),155.54(s),154.31(s),143.79(s),137.80(s),136.47(s),131.19(s),129.96(s),129.90(s),127.84(s),125.26(s),123.99(s),119.47(s),119.18(s),118.50(s),115.30(s),98.22(s),56.98(s),53.24(s),52.72(s),49.38(s),46.95(s),45.50(s),41.45(s),35.75(s),33.04(s),31.51(s),29.41(s),26.37(s),24.74(s),22.77(s),22.19(s).hrms(esi):calcd for c
44h46n10
o7[m+h]
+
827.3624,found827.3608。
[0116]
ⅰ‑
15:5-(4-(6-(4-氨基-3-(4-苯氧基苯基)-1h-吡唑并[3,4-d]嘧啶-1-基)己酰基)哌嗪-1-基)-n-(2-(2,6-二氧杂哌啶-3-基)-1,3-二氧异吲哚-4-基)戊酰胺
[0117]
白色固体,产率45.6%,mp:130-132℃。1h nmr(400mhz,cdcl3)δ10.59(s,1h),9.46(s,1h),8.82(d,j=8.5hz,1h),8.40(s,1h),7.72(t,j=7.8hz,1h),7.65(d,j=7.9hz,2h),7.56(d,j=7.0hz,1h),7.39(t,j=7.7hz,2h),7.20
–
7.12(m,3h),7.08(d,j=7.8hz,2h),5.10
–
4.81(m,1h),4.53
–
4.37(m,2h),3.60(d,j=17.5hz,2h),3.42(s,2h),2.97
–
2.83(m,1h),2.87
–
2.66(m,2h),2.57
–
2.43(m,2h),2.45
–
2.31(m,6h),2.27(t,j=7.4hz,2h),2.21
–
2.13(m,1h),2.02
–
1.93(m,2h),1.88
–
1.74(m,2h),1.71
–
1.65(m,2h),1.59
–
1.55(m,3h),1.38
–
1.31(m,2h);
13
c nmr(100mhz,cdcl3)δ172.14(s),172.09(s),171.44(s),169.31(s),168.71(s),166.74(s),158.43(s),157.94(s),156.43(s),155.53(s),154.33(s),143.76(s),137.79(s),136.44(s),131.14(s),129.96(s),129.91(s),127.87(s),125.26(s),123.99(s),119.47(s),119.18(s),118.51(s),115.38(s),98.22(s),57.70(s),53.35(s),52.81(s),49.36(s),46.93(s),45.56(s),41.48(s),37.73(s),33.04(s),31.50(s),29.40(s),26.37(s),25.90(s),24.81(s),23.17(s),22.80(s).hrms(esi):calcd for c
45h48n10
o7[m+h]
+
841.3780,found 841.3770。
[0118]
ⅰ‑
16:6-(4-(6-(4-氨基-3-(4-苯氧基苯基)-1h-吡唑并[3,4-d]嘧啶-1-基)己酰基)哌嗪-1-基)-n-(2-(2,6-二氧杂哌啶-3-基)-1,3-二氧异吲哚-4-基)己酰胺
[0119]
白色固体,产率30.6%,mp:134-136℃。1h nmr(400mhz,cdcl3)δ9.43(s,1h),8.83(d,j=8.2hz,1h),8.42(s,1h),7.72(t,j=7.7hz,1h),7.65(d,j=7.7hz,2h),7.56(d,j=7.2hz,1h),7.39(t,j=7.4hz,2h),7.19
–
7.13(m,3h),7.08(d,j=7.9hz,2h),5.04
–
4.92(m,1h),4.53
–
4.37(m,2h),3.59(s,2h),3.42(s,2h),2.96
–
2.86(m,1h),2.86
–
2.69(m,2h),2.46(t,j=7.2hz,2h),2.42
–
2.30(m,6h),2.27(t,j=7.6hz,2h),2.22
–
2.14(m,1h),2.01
–
1.93(m,2h),1.84
–
1.73(m,2h),1.69
–
1.63(m,2h),1.54
–
1.49(m,2h),1.45
–
1.40(m,2h),1.38
–
1.32(m,2h);
13
c nmr(100mhz,cdcl3)δ172.16(s),172.06(s),171.32(s),169.29(s),168.77(s),166.73(s),158.44(s),157.94(s),156.44(s),155.55(s),154.34(s),143.75(s),137.84(s),136.44(s),131.15(s),129.95(s),129.86(s),127.87(s),125.25(s),123.99(s),119.47(s),119.18(s),118.47(s),115.32(s),98.25(s),58.04(s),53.25(s),52.89(s),49.36(s),47.04(s),45.56(s),41.48(s),37.78(s),33.07(s),31.52(s),29.44(s),26.81(s),26.46(s),26.12(s),25.02(s),24.85(s),22.81(s).hrms(esi):calcd for c
46h50n10
o7[m+h]
+
855.3937,found 855.3910。
[0120]
ⅰ‑
17:5-(4-(4-(3-(4-氨基-3-(4-苯氧基苯基)-1h-吡唑并[3,4-d]嘧啶-1-基)吡咯烷-1-基)丁酰基)哌嗪-1-基)-n-(2-(2,6-二氧杂哌啶-3-基)-1,3-二氧异吲哚-4-基)戊酰胺
[0121]
白色固体,产率33%,mp:128-130℃。1h nmr(400mhz,cdcl3)δ9.46(s,1h),8.82(d,j=8.4hz,1h),8.40(s,1h),7.72(t,j=7.8hz,1h),7.65(d,j=7.8hz,2h),7.56(d,j=7.2hz,1h),7.39(t,j=7.4hz,2h),7.22
–
7.12(m,3h),7.08(d,j=7.6hz,2h),5.61
–
5.54(m,1h),5.06
–
4.85(m,1h),3.60(s,2h),3.47(s,2h),2.99(s,1h),2.93
–
2.87(m,1h),2.87
–
2.72(m,3h),2.54
–
2.44(m,5h),2.44
–
2.34(m,7h),2.20
–
2.13(m,1h),1.92
–
1.88(m,1h),1.86
–
1.75(m,3h),1.68
–
1.63(m,2h),1.63
–
1.55(m,4h);
13
c nmr(100mhz,cdcl3)δ172.10(s),171.79(s),171.05(s),169.29(s),168.61(s),166.74(s),158.59(s),157.92(s),156.31(s),155.21(s),154.24(s),144.16(s),137.77(s),136.43(s),131.16(s),129.98(s),129.90(s),127.68(s),125.26(s),124.07(s),119.56(s),119.08(s),118.49(s),115.38(s),98.58(s),58.05(s),57.60(s),55.15(s),55.03(s),53.24(s),53.20(s),52.78(s),49.38(s),45.41(s),41.49(s),37.68(s),31.51(s),30.73(s),25.71(s),23.43(s),23.36(s),23.19(s),22.74(s).hrms(esi):calcd for c
47h51n11
o7[m+h]
+
882.4046,found 882.4037。
[0122]
ⅰ‑
18:2-(4-(5-(3-(4-氨基-3-(4-苯氧基苯基)-1h-吡唑并[3,4-d]嘧啶-1-基)吡咯烷-1-基)戊酰基)哌嗪-1-基)-n-(2-(2,6-二氧杂哌啶-3-基)-1,3-二氧异吲哚-4-基)乙酰胺
[0123]
白色固体,产率18%,mp:146-148℃。1h nmr(400mhz,cdcl3)δ11.40(d,j=10.8hz,1h),8.85(d,j=8.4hz,1h),8.36(s,1h),7.72(t,j=7.9hz,1h),7.65(d,j=7.8hz,2h),7.57(d,j=5.6hz,1h),7.39(t,j=7.5hz,2h),7.21
–
7.13(m,3h),7.08(d,j=7.9hz,2h),5.55
–
5.51(m,2h),4.98
–
4.86(m,1h),4.25
–
3.96(m,1h),3.90
–
3.53(m,3h),3.23(q,j=17.1hz,2h),3.00(s,2h),2.95
–
2.89(m,1h),2.89
–
2.76(m,2h),2.77
–
2.66(m,4h),2.64
–
2.49(m,4h),2.49
–
2.38(m,3h),2.37
–
2.30(m,1h),2.16
–
2.06(m,1h),1.76
–
1.66(m,4h);
13
c nmr(100mhz,cdcl3)δ171.81(s),171.78(s),169.83(s),168.79(s),168.46(s),166.87(s),158.62(s),157.89(s),156.30(s),155.19(s),154.23(s),144.14(s),136.87(s),136.28(s),131.47(s),130.07(s),129.99(s),127.67(s),124.98(s),124.09(s),119.58(s),119.10(s),118.64(s),116.17(s),98.58(s),61.60(s),58.14(s),55.71(s),54.96(s),53.30(s),53.15(s),53.01(s),49.36(s),45.46(s),41.55(s),32.94(s),31.53(s),30.74(s),27.50(s),22.79(s),21.70(s).hrms(esi):calcd for c
45h47n11
o7[m+h]
+
854.3733,found 854.3725。
[0124]
ⅰ‑
19:2-(4-(4-(3-(4-氨基-3-(4-苯氧基苯基)-1h-吡唑并[3,4-d]嘧啶-1-基)吡咯烷-1-基)丁酰基)哌嗪-1-基)-n-(2-(2,6-二氧杂哌啶-3-基)-1,3-二氧异吲哚-4-基)乙酰胺
[0125]
白色固体,产率25.1%,mp:164-166℃。1h nmr(400mhz,cdcl3)δ11.23(s,1h),8.95
–
8.80(m,1h),8.38(d,j=7.0hz,1h),7.73(t,j=8.4hz,1h),7.64(d,j=8.6hz,2h),7.61
–
7.55(m,1h),7.39(t,j=7.9hz,2h),7.20
–
7.13(m,3h),7.08(d,j=7.7hz,2h),5.58
–
5.53(m,1h),5.01
–
4.83(m,1h),4.19
–
3.86(m,1h),3.75
–
3.50(m,3h),3.31
–
3.26(m,1h),3.21
–
3.13(m,1h),3.12
–
2.97(m,2h),2.96
–
2.83(m,2h),2.83
–
2.57(m,7h),2.58
–
2.31(m,6h),2.19
–
2.08(m,1h),1.93
–
1.83(m,2h);
13
c nmr(100mhz,cdcl3)δ171.81(s),171.78(s),169.83(s),168.47(s),166.87(s),158.62(s),157.89(s),156.30(s),155.19(s),154.23
(s),144.14(s),136.87(s),136.28(s),131.47(s),129.99(s),129.92(s),127.72(s),124.96(s),124.09(s),119.58(s),119.12(s),119.09(s),118.64(s),116.19(s),98.59(s),61.56(s),58.05(s),55.76(s),54.92(s),53.30(s),53.18(s),53.01(s),49.36(s),45.52(s),41.55(s),32.96(s),31.47(s),30.78(s),23.48(s),21.70(s).hrms(esi):calcd for c
44h45n11
o7[m+h]
+
840.3576,found 840.3568。
[0126]
ⅰ‑
20:2-(4-(5-(4-(4-氨基-3-(4-苯氧基苯基)-1h-吡唑并[3,4-d]嘧啶-1-基)哌啶-1-基)戊酰基)哌嗪-1-基)-n-(2-(2,6-二氧杂哌啶-3-基)-1,3-二氧异吲哚-4-基)乙酰胺
[0127]
白色固体,产率25.1%,mp:162-164℃。1h nmr(400mhz,cdcl3)δ11.34(s,1h),8.86(d,j=8.5hz,1h),8.37(s,1h),7.71(t,j=7.9hz,1h),7.64(d,j=8.5hz,2h),7.56(d,j=7.2hz,1h),7.39(t,j=7.9hz,2h),7.20
–
7.13(m,3h),7.09(d,j=8.0hz,2h),5.03
–
4.92(m,1h),4.90
–
4.66(m,1h),4.15
–
3.85(m,1h),3.79
–
3.61(m,3h),3.30
–
3.21(m,2h),3.21
–
3.13(m,2h),2.95
–
2.85(m,1h),2.83
–
2.74(m,2h),2.73
–
2.58(m,4h),2.51(s,2h),2.45
–
2.34(m,4h),2.26
–
2.16(m,2h),2.15(d,j=10.0hz,1h),2.01(d,j=9.3hz,2h),1.59
–
1.50(m,4h);
13
c nmr(100mhz,cdcl3)δ172.08(s),171.83(s),169.81(s),168.77(s),168.42(s),166.92(s),158.44(s),157.88(s),156.38(s),155.46(s),153.86(s),143.53(s),136.86(s),136.25(s),131.49(s),129.96(s),129.91(s),127.92(s),124.96(s),124.01(s),119.54(s),119.09(s),118.61(s),116.19(s),98.60(s),61.63(s),57.51(s),54.05(s),53.38(s),53.15(s),52.58(s),52.27(s),49.37(s),45.51(s),41.58(s),32.73(s),31.51(s),30.62(s),30.58(s),26.14(s),23.02(s),22.70(s).hrms(esi):calcd for c
46h49n11
o7[m+h]
+
868.3889,found 868.3878。
[0128]
ⅰ‑
21:4-(4-(5-(4-(4-氨基-3-(4-苯氧基苯基)-1h-吡唑并[3,4-d]嘧啶-1-基)哌啶-1-基)戊酰基)哌嗪-1-基)-n-(2-(2,6-二氧杂哌啶-3-基)-1,3-二氧异吲哚-4-基)丁酰胺
[0129]
白色固体,产率26.3%,mp:142-144℃。1h nmr(400mhz,cdcl3)δ9.51(s,1h),8.82(d,j=8.5hz,1h),8.38(s,1h),7.74
–
7.69(m,1h),7.65(d,j=8.6hz,2h),7.55(d,j=7.3hz,1h),7.41
–
7.36(m,2h),7.19
–
7.12(m,3h),7.08(d,j=7.7hz,2h),4.99
–
4.91(m,1h),4.82
–
4.72(m,1h),3.64
–
3.54(m,2h),3.49
–
3.40(m,2h),3.16
–
3.05(m,2h),2.95
–
2.85(m,1h),2.84
–
2.70(m,2h),2.54
–
2.49(m,2h),2.45
–
2.39(m,8h),2.38
–
2.33(m,3h),2.21
–
2.13(m,3h),2.06
–
1.97(m,3h),1.96
–
1.92(m,2h),1.61
–
1.55(m,4h);
13
c nmr(100mhz,cdcl3)δ172.24(s),171.62(s),171.56(s),169.23(s),168.64(s),166.74(s),158.34(s),157.87(s),156.44(s),155.32(s),153.84(s),143.34(s),137.79(s),136.43(s),131.21(s),129.98(s),129.94(s),128.11(s),125.24(s),123.96(s),119.48(s),119.10(s),118.46(s),115.30(s),98.58(s),57.79(s),56.84(s),54.84(s),53.40(s),52.78(s),52.73(s),52.63(s),49.42(s),45.58(s),41.48(s),35.61(s),33.12(s),31.53(s),31.13(s),26.92(s),26.75(s),23.31(s),22.70(s),22.14(s).hrms(esi):calcd for c
48h53n11
o7[m+h]
+
896.4202,found 896.4212。
[0130]
ⅰ‑
22:2-(4-(4-(4-(4-氨基-3-(4-苯氧基苯基)-1h-吡唑并[3,4-d]嘧啶-1-基)哌啶-1-基)丁酰基)哌嗪-1-基)-n-(2-(2,6-二氧杂哌啶-3-基)-1,3-二氧代异吲哚-4-基)乙
酰胺
[0131]
白色固体,产率28.6%,mp:156-158℃。1h nmr(400mhz,cdcl3)δ11.23(s,1h),8.87(d,j=8.4hz,1h),8.32(s,1h),7.72(t,j=7.9hz,1h),7.62(d,j=8.5hz,2h),7.57(d,j=7.2hz,1h),7.39(t,j=7.9hz,2h),7.22
–
7.11(m,3h),7.08(d,j=7.8hz,2h),5.03
–
4.93(m,1h),4.89
–
4.78(m,1h),3.92
–
3.71(m,4h),3.31
–
3.19(m,4h),2.95
–
2.85(m,1h),2.85
–
2.74(m,2h),2.73
–
2.59(m,6h),2.52
–
2.40(m,6h),2.18
–
2.07(m,3h),1.98
–
1.89(m,2h);
13
c nmr(100mhz,cdcl3)δ176.29(s),171.69(s),171.39(s),169.83(s),168.67(s),168.45(s),166.90(s),158.54(s),157.96(s),156.31(s),154.78(s),153.65(s),143.88(s),136.86(s),136.32(s),131.44(s),129.99(s),129.97(s),127.66(s),125.09(s),124.07(s),119.57(s),119.08(s),118.69(s),116.15(s),98.47(s),61.69(s),57.09(s),53.40(s),53.15(s),51.98(s),50.70(s),49.33(s),45.32(s),41.57(s),31.51(s),30.89(s),30.23(s),22.71(s),21.88(s),21.70(s).hrms(esi):calcd for c
45h47n11
o7[m+h]
+
854.3733,found 854.3743。
[0132]
ⅰ‑
23:5-(4-(3-(4-(4-氨基-3-(4-苯氧基苯基)-1h-吡唑并[3,4-d]嘧啶-1-基)哌啶-1-基)丙酰基)哌嗪-1-基)-n-(2-(2,6-二氧杂哌啶-3-基)-1,3-二氧异吲哚-4-基)戊酰胺
[0133]
白色固体,产率18.9%,mp:134-136℃。1h nmr(400mhz,cdcl3)δ9.45(s,1h),8.84(d,j=8.5hz,1h),8.37(s,1h),7.73
–
7.69(m,1h),7.65(d,j=8.6hz,2h),7.55(d,j=7.2hz,1h),7.39(t,j=7.9hz,2h),7.18
–
7.13(m,3h),7.09(d,j=7.8hz,2h),5.03
–
4.91(m,1h),4.79(t,j=10.8hz,1h),3.78
–
3.61(m,2h),3.54
–
3.45(m,2h),3.17(s,2h),2.93
–
2.86(m,1h),2.80
–
2.74(m,2h),2.61
–
2.57(m,2h),2.55
–
2.50(m,2h),2.49
–
2.46(m,1h),2.45
–
2.40(m,4h),2.39
–
2.37(m,1h),2.37
–
2.26(m,4h),2.17
–
2.12(m,1h),2.05
–
1.98(m,2h),1.93
–
1.83(m,2h),1.81
–
1.76(m,2h),1.61
–
1.57(m,2h);
13
c nmr(100mhz,cdcl3)δ172.23(s),171.79(s),170.27(s),169.32(s),168.87(s),166.78(s),158.40(s),157.87(s),156.38(s),155.34(s),153.82(s),143.44(s),137.75(s),136.44(s),131.15(s),129.99(s),129.97(s),127.98(s),125.26(s),124.01(s),119.53(s),119.08(s),118.52(s),115.36(s),98.58(s),72.38(s),61.73(s),57.72(s),53.81(s),53.07(s),52.99(s),52.88(s),52.64(s),49.44(s),45.49(s),41.51(s),37.65(s),31.59(s),30.96(s),30.91(s),25.54(s),23.30(s),22.72(s).hrms(esi):calcd for c
47h51n11
o7[m+h]
+
882.4046,found 882.4037。
[0134]
ⅰ‑
24:4-(4-(4-(4-(4-氨基-3-(4-苯氧基苯基)-1h-吡唑并[3,4-d]嘧啶-1-基)哌啶-1-基)丁酰基)哌嗪-1-基)-n-(2-(2,6-二氧杂哌啶-3-基)-1,3-二氧异吲哚-4-基)丁酰胺
[0135]
白色固体,产率23.9%,mp:140-142℃。1h nmr(400mhz,cdcl3)δ9.53(s,1h),8.84(d,j=8.6hz,1h),8.37(s,1h),7.74
–
7.69(m,1h),7.65(d,j=8.5hz,2h),7.55(d,j=7.1hz,1h),7.39(t,j=7.9hz,2h),7.16(dd,j=12.5,8.0hz,3h),7.09(d,j=7.9hz,2h),5.07
–
4.91(m,1h),4.89
–
4.67(m,1h),3.68(s,1h),3.60
–
3.28(m,3h),3.30
–
2.95(m,2h),2.95
–
2.86(m,1h),2.85
–
2.72(m,2h),2.58
–
2.47(m,4h),2.48
–
2.32(m,9h),2.33
–
2.19(m,3h),2.17
–
2.12(m,1h),2.07
–
2.00(m,2h),1.96
–
1.92(m,2h),1.87
–
1.84(m,2h);
13
c nmr
(100mhz,cdcl3)δ172.37(s),171.65(s),171.40(s),169.23(s),168.72(s),166.76(s),158.39(s),157.87(s),156.43(s),155.35(s),153.85(s),143.44(s),137.77(s),136.43(s),131.23(s),129.99(s),129.96(s),128.02(s),125.26(s),123.98(s),119.49(s),119.12(s),118.49(s),115.30(s),98.59(s),57.36(s),56.66(s),53.49(s),52.65(s),52.57(s),52.53(s),52.48(s),49.43(s),45.53(s),41.52(s),35.59(s),31.57(s),30.98(s),30.86(s),30.78(s),22.68(s),22.49(s),22.28(s).hrms(esi):calcd for c
47h51n11
o7[m+h]
+
882.4046,found 882.4057。
[0136]
实验例13:目标化合物对不同肿瘤细胞的生长抑制活性测定实验
[0137]
实验材料和仪器:淋巴瘤细胞株jeko-1、k562、hel(中国科学院细胞库),1640培养基购买自以色列biological industries公司、pbs磷酸盐缓冲剂(粉剂)购买自北京鼎国昌盛生物技术有限责任公司,青霉素-链霉素混合液(100
×
双抗)购买自美国genview公司,泊洛沙姆(f-127)购买自amresco,细胞增殖及毒性检测试剂盒(cell counting kit-8,cck8)购买自大连美仑生物技术有限公司,胎牛血清(fbs)购买自美国gibco公司,冷冻离心机购买自德国eppendorf公司,infinite f50吸收光酶标仪购买自奥地利tecan公司,细胞培养箱购买自日本panasonic公司(型号:mco-170aicuvl-pc)。
[0138]
实验方法:取对数生长期细胞,1000rpm转速离心5min后,弃去上层培养基,用含20%fbs的1640培养基将离心后的细胞稀释吹打成均匀的细胞悬液,根据细胞数4
×
104/孔计算出所需的细胞数,按照100μl/孔将细胞悬液接种于96孔培养板中进行接种,同时设立100%细胞对照加入100μl细胞悬液和100μl空白培养基,空白对照加入200μl空白培养基。随后不同浓度化合物的培养基(f127≤1%)分别加入对应的96孔板中,每个浓度设置4个复孔,完毕后,将96孔板放置37℃,5%co2恒温培养箱中孵育48h。孵育48h之后,避光下向每孔加入20μl的cck-8溶液,锡纸避光,置于培养箱内培养0.5~4h,2h后用infinite f50吸收光酶标仪在波长为450nm条件下测定化合物的吸光度值。使用graphpad prism 6.01软件,以抑制率为纵坐标,化合物的浓度对数为横坐标,拟合曲线,计算化合物的ic
50
值。
[0139][0140]
实验结果:目标化合物的细胞生长抑制活性结果如表1所示。
[0141]
表1.目标化合物对不同肿瘤细胞的生长抑制活性结果
[0142][0143][0144]
ibn:依鲁替尼(ibrutinib)
[0145]
由表1可知,大部分化合物对三株细胞系具有一定的生长抑制作用,有的化合物对肿瘤细胞的生长抑制作用优于阳性对照药ibn,其中化合物
ⅰ‑
7、
ⅰ‑
8、
ⅰ‑
10、
ⅰ‑
16、
ⅰ‑
17、
ⅰ‑
18、
ⅰ‑
21、
ⅰ‑
23和
ⅰ‑
24对jeko-1细胞株的抑制率均在75%以上,ibn的抑制率为64%;进一步
测定化合物对肿瘤细胞的半数生长抑制浓度(ic
50
值),结果显示化合物
ⅰ‑
7、
ⅰ‑
21、
ⅰ‑
23和
ⅰ‑
24抑制jeko-1细胞的ic
50
值分别为4.6μm、4.1μm、3.6μm和4.2μm,阳性对照药ibn的ic
50
值为4.7μm;化合物
ⅰ‑
21和
ⅰ‑
23对k562细胞的半数生长抑制浓度(ic
50
值)分别为8μm和7μm,阳性对照药ibn的ic
50
值为10μm;化合物
ⅰ‑
21和
ⅰ‑
23对hel细胞的生长抑制作用是阳性对照药ibn两倍(ic
50
值分别为16μm、15μm和31μm)。
[0146]
实验例14:目标化合物对b细胞淋巴瘤细胞株jeko-1的蛋白质印迹法实验
[0147]
实验材料和仪器:蛋白裂解液-pierce ripa buffer、蛋白酶抑制剂-protease&phosohatease inhibitor cocktail、bca蛋白浓度测定试剂盒、mini-protean tgxtm gels、10
×
电泳缓冲液-10
×
tris/glycine/sds buffer、10
×
转膜缓冲液-10
×
transfer buffer、牛血清白蛋白-bovine serum albumin,bsa、20
×
tbst、吐温20-tween-20、脱脂奶粉-nonfat milk、硝酸纤维素杂交膜-nitrocellulose membrane;nc membrane、预染蛋白marker-rageruler prestained protein ladder、hrp标记山羊抗小鼠igg、hrp标记山羊抗兔igg、显影液、定影液、室温摇shaker、低温离心机、其他电泳和转膜相关装置。
[0148]
实验方法:(1)处理细胞:取对数生长期jeko-1细胞,接种于t25培养瓶,细胞数为4
×
106/瓶,加入不同浓度的化合物(dmso≤1
‰
),将培养瓶置于37℃(5%co2)的恒温培养箱中培养一定时间。(2)提取蛋白:收集jeko-1细胞,pbs洗涤,离心后加入蛋白裂解液,使细胞与裂解液均匀混合,反应40min。裂解反应完毕后,吸取上清液即所需蛋白样品,使用bsa蛋白定量法测定蛋白浓度,-80℃低温保存。(3)sds-page电泳:取梯度分离胶,每组取20μg蛋白样品进行上样,同时取蛋白marker上样。(4)转膜:将定性滤纸和pvdf膜一起浸泡在电转液中;分开取出电泳好的玻璃板,后将sds-page胶移至电转液;将凝胶和pvdf夹在滤纸中间,浸泡在转移装置的电转液中。(5)封闭:将含目标蛋白的膜放置5%脱脂奶粉溶液中以封闭非特异结合。(6)抗体孵育:加入一抗4℃过夜;tbs-t洗涤一抗孵育后的pvdf膜;加入hrp标记的二抗以结合一抗;tbs-t洗涤二抗孵育后的pvdf膜。(7)蛋白质印迹法结果:使用化学发光法检测,经x胶片曝光显影,保存电脑图片,并用image j软件将灰度值定量化。(8)结果分析:以内参β-actin灰度值作对照,以校正误差,计算目标蛋白的相对含量。
[0149]
实验结果:ⅰ系列目标终产物中,部分化合物可有效降解jeko-1细胞的btk蛋白,化合物
ⅰ‑
6、
ⅰ‑
7、
ⅰ‑
8、
ⅰ‑
10、
ⅰ‑
11、
ⅰ‑
14、
ⅰ‑
15和
ⅰ‑
16在15μm浓度时btk蛋白表达明显下降,且在化合物浓度为5μm条件下,化合物
ⅰ‑
7、
ⅰ‑
8和
ⅰ‑
10也能显著下调btk蛋白;此外,化合物
ⅰ‑
17、
ⅰ‑
18、
ⅰ‑
21、
ⅰ‑
23和
ⅰ‑
24在浓度为5μm时明显下调btk蛋白,在化合物浓度为0.5μm时,化合物
ⅰ‑
21和
ⅰ‑
23即能显著下调btk蛋白(图1)。进一步测定化合物
ⅰ‑
7、
ⅰ‑
21和
ⅰ‑
23在不同浓度和不同时间下对多珠b细胞淋巴瘤细胞btk蛋白的降解作用,结果表明:化合物
ⅰ‑
7、
ⅰ‑
21和
ⅰ‑
23对b细胞淋巴瘤细胞珠jeko-1显示浓度依赖性和时间依赖性地显著降解btk蛋白。在化合物浓度为5μm时处理jeko-1细胞24h,化合物
ⅰ‑
7对btk蛋白最大降解量(d
max
)为72.84%,化合物
ⅰ‑
21对btk蛋白最大降解量(d
max
)为91.86%,化合物
ⅰ‑
23对btk蛋白最大降解量(d
max
)为94.44%;化合物
ⅰ‑
7、化合物
ⅰ‑
21和化合物
ⅰ‑
23对btk的半数降解浓度(dc
50
)分别为0.45μm、0.25μm和0.10μm。在相同实验条件下,对照药依鲁替尼和泊马度胺均未显示出对btk蛋白的降解作用。
[0150]
以上所述仅为本发明的优选实施例而已,并不用于限制本发明,尽管参照前述实施例对本发明进行了详细的说明,对于本领域的技术人员来说,其依然可以对前述各实施
例所记载的技术方案进行修改或替换。凡在本发明的精神和原则之内,所作的任何修改、等同替换、改进等,均应包含在本发明的保护范围之内。
相关技术
网友询问留言
已有0条留言
- 还没有人留言评论。精彩留言会获得点赞!
1