一种手性氮取代四氢喹喔啉的制备方法
1.本发明属于有机合成方向氮杂环类化合物制备方法技术领域,具体涉及手性氮取代四氢喹喔啉类化合物的制备方法。
背景技术:
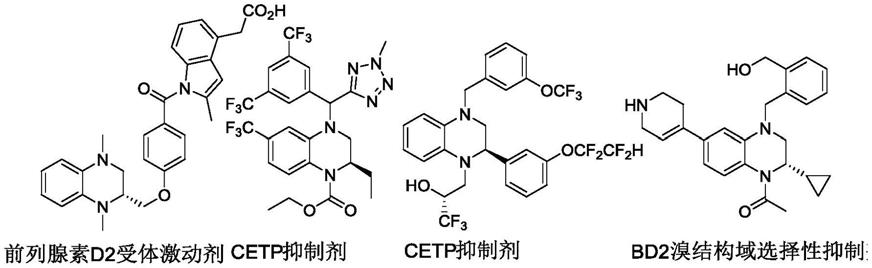
2.1,2,3,4-四氢喹喔啉及其衍生物是一类重要的含氮杂环化合物,具有广泛的生理和药理活性,广泛应用于医药、化工等领域。特别是氮取代手性四氢喹喔啉类化合物,具有较为广泛的生物学活性,如前列腺d2受体激动剂(torisu,k.;kobayashi,k.;iwahashi,m.;nakai,y.;onoda,t.;nagase,t.;sugimoto,i.;okada,y.;matsumoto,r.;nanbu,f.;ohuchida,s.;nakai,h.;toda,m.,bioorg.med.chem.2004,12,5361-5378),胆固醇酯转移蛋白(cetp)抑制剂(sikorski,j.a.,j.med.chem.2006,49,1-22),溴结构域(bd2)选择性抑制剂(law,r.p.;atkinson,s.j.;bamborough,p.;chung,c.-w.;demont,e.h.;gordon,l.j.;lindon,m.;prinjha,r.k.;watson,a.j.b.;hirst,d.j.,j.med.chem.2018,61,4317-4334)。下面是部分氮取代手性四氢喹喔啉类生物学活性化合物实例:
[0003][0004]
目前,该类化合物的合成方法已有诸多报道,但是基本上都是先合成相应的喹喔啉类分子后,再通过还原得到相应的四氢喹喔啉,最后再烷基化得到氮取代四氢喹喔啉产物。该类方法的缺点是合成路线长,反应选择性差,特别是最后给氮选择性上取代基较难控制。另外,欲获得光学纯的氮取代四氢喹喔啉类,通常需要经过消旋体的拆分,过程繁琐且不经济环保。因此,开发简单、易得的合成手性氮取代四氢喹喔啉的制备方法是非常有必要的。
技术实现要素:
[0005]
本发明的目的是提供一类手性氮取代四氢喹喔啉的制备方法,通过简单、易得的原料经过选择性环化、不对称转移氢化,一锅反应实现手性氮取代四氢喹喔啉类化合物的制备,从而克服现有技术的上述缺陷。
[0006]
本发明的手性氮取代四氢喹喔啉类化合物的制备方法,其特征在于:采取一锅法,在氮气保护环境下,以氮取代邻苯二胺类化合物即式i类化合物作为底物,与α-羟基酮类化合物即式ii类化合物按摩尔比1:1~1:2,以及按式i类化合物0.05~0.15倍当量的酸催化剂,加入到有机溶剂中,在50~130℃进行反应2~24小时,薄层色谱点板确定反应终点;
[0007]
再在原反应体系中加入按式i类化合物1~2倍当量的式iii类还原剂,50~90℃反应12~24小时,薄层色谱点板确定反应终点,然后经过柱层析即得到相应的式iv类手性化合物,或其对映体、消旋体、非对映异构体:
[0008][0009]
式中的手性氮取代四氢喹喔啉类化合物iv的取代基r1、r6、r7各自独立地选择各种烷基、芳基,r2、r3、r4、r5各自独立地选择各种烷基、烷氧基、羟基、杂环、苯基、烯烃、取代苯基、卤素、氢。
[0010]
所述有机溶剂为苯、甲苯、二甲苯、氯苯、氟苯、1,2-二氯乙烷。
[0011]
所述酸催化剂为醋酸、三氟乙酸、对甲苯磺酸、手性磷酸。
[0012]
所述式iii类还原剂的取代基r8为各种烷基、芳基、烯丙基。
[0013]
本发明的采用上述方法制备的氮取代四氢喹喔啉类化合物,其特征在于是具有如下化学结构式的化合物或其对映体、消旋体、非对映异构体的相应产物:
[0014][0015]
式中的手性氮取代四氢喹喔啉类化合物iv的取代基r1、r6、r7各自独立地选择各种烷基、芳基,r2、r3、r4、r5各自独立地选择各种烷基、烷氧基、羟基、杂环、苯基、烯烃、取代苯基、卤素、氢。
[0016]
上述本发明手性氮取代四氢喹喔啉类化合物的合成方法,采取了一锅法。首先以氮取代邻苯二胺衍生物与α-羟基酮作反应底物,在酸催化下,通过选择性的环化反应得到相应的二氢喹喔啉产物。该过程选择性高,而传统合成方法对于非对称的邻苯二胺类底物不能选择性得到相应的喹喔啉产物。本发明通过向上述得到的二氢喹喔啉溶液中加入还原剂1,4-二氢吡啶,通过对映选择性的氢化反应得到相应的氮取代四氢喹喔啉产物。整个反应过程一锅内进行,中间不需要纯化分离,只需要加入一次催化剂,一次加入反应溶剂,通过高区域选择性与立体选择性的反应得到相应的氮取得四氢喹喔啉产物。本发明简化了传统繁琐的合成步骤,简便易行,易于操作,且底物适用性非常广,能耐受多种官能团。并且本发明采取的是无金属催化体系,既经济环保,又避免了参与一些药物分子合成过程中的金属残留。通过本发明能以简单易得的起始原料,高产率、高光学纯度(ee值》99%)的获得多种氮取代手性四氢喹喔啉类化合物,并且可通过进一步的衍生获得多种活性分子,具有经济实用性和工业应用前景。
具体实施方式
[0017]
实施例1:(s)-1-甲基-3-苯基-1,2,3,4-四氢喹喔啉的制备(3a):
[0018][0019]
在氮气环境下,在反应管中加入1a(0.2mmol),2a(0.2mmol),cpa1(0.3mmol),(2ml)甲苯作为反应溶剂,在反应管上加上橡胶塞后,放入预先升温至110℃的油浴锅中搅拌2h。薄层色谱(tlc)点板确定反应终点,当tlc板显示反应原料1a完全消失后,将反应管从油浴锅中提起,直接在反应管中加入heh(0.3mmol),再次氮气保护后,放入预先升温至65℃的油浴锅中搅拌16h,反应结束后,待反应管冷却至室温,直接经柱层析分离得到目标产物3a。该类发明简化了传统繁琐的合成步骤,简便易行,易于操作。
[0020]
产率92%;氮黄色油状物;80%ee;1h nmr(400mhz,cdcl3)δ7.46
–
7.31(m,5h),6.84
–
6.51(m,4h),4.64(dd,j=8.2,3.1hz,1h),3.98(s,1h),3.38
–
3.18(m,2h),2.91(s,3h);
13
c nmr(101mhz,cdcl3)δ141.76,135.48,134.64,128.65,127.95,127.02,118.89,118.32,113.43,111.76,57.63,54.70,39.10hrms(esi):c
15h16n2 neutral mass:224.1313,observed([m+h])
+
:225.1384;[α]d
25
=+22
°
(c 0.01,ch2cl2);hplc:diacel chiralcel ad-h column 95:5hexanes/2-propanol,1ml/min,254nm;t
r1
=9.98min(major),t
r2
=12.27min(minor)。
[0021]
实施例2:(s)-1-异丙基-3-苯基-1,2,3,4-四氢喹喔啉的制备(3b):
[0022][0023]
邻苯二胺1b与α-羟基酮2a作为反应的起始底物制备目标产物3b,制备方法与实施例1相同。
[0024]
产率95%;氮黄色油状物;94%ee;1h nmr(400mhz,cdcl3)δ7.46
–
7.37(m,4h),7.37
–
7.32(m,1h),6.75(dd,j=3.3,1.6hz,2h),6.61(q,j=4.3hz,2h),4.47(dd,j=8.3,3.1hz,1h),4.12(dt,j=13.2,6.6hz,1h),3.97(s,1h),3.40(dd,j=11.3,3.2hz,1h),3.05(dd,j=11.3,8.3hz,1h),1.24(d,j=6.6hz,3h),1.10(d,j=6.6hz,3h);
13
c nmr(101mhz,cdcl3)δ142.26,134.79,134.23,128.59,127.86,127.03,118.94,117.26,113.98,111.40,55.00,47.00,46.75,19.35,17.94;hrms(esi):c
17h20n2 neutral mass:252.1626,observed([m+h])
+
:253.1678;[α]d
25
=+33
°
(c 0.01,ch2cl2);hplc:diacel chiralcel ad-h column 95:5hexanes/2-propanol,1ml/min,254nm;t
r1
=7.55min(major);t
r2
=8.48min(minor)。
[0025]
实施例3:(s)-1-丙基-3-苯基-1,2,3,4-四氢喹喔啉的制备(3c):
[0026][0027]
邻苯二胺1c与α-羟基酮2a作为反应的起始底物制备目标产物3c,制备方法与实施
例1相同。
[0028]
产率92%;氮黄色油状物;90%ee;1h nmr(400mhz,cdcl3)δ7.49
–
7.40(m,4h),7.40
–
7.33(m,1h),6.77(dd,j=9.9,4.1hz,1h),6.70
–
6.59(m,3h),4.52(t,j=5.7hz,1h),3.96(s,1h),3.38(d,j=5.7hz,3h),3.25
–
3.09(m,1h),1.77
–
1.57(m,2h),0.98(t,j=7.4hz,3h);
13
c nmr(101mhz,cdcl3)δ141.78,134.32,128.66,127.94,127.11,119.21,117.16,114.02,111.32,55.86,54.19,53.36,19.37,11.66;hrms(esi):c
17h20n2 neutral mass:252.1626,observed ([m+h])
+
:253.1693;[α]d
25
=+72
°
(c 0.01,ch2cl2);hplc:diacel chiralcel ad-h column 95:5 hexanes/2-propanol,1ml/min,254nm;t
r1
=6.37min(major);t
r2
=7.12min(minor)。
[0029]
实施例4:(s)-1-己基-3-苯基-1,2,3,4-四氢喹喔啉的制备(3d):
[0030][0031]
邻苯二胺1d与α-羟基酮2a作为反应的起始底物制备目标产物3d,制备方法与实施例1相同。
[0032]
产率94%;氮黄色油状物;90%ee;1h nmr(400mhz,cdcl3)δ8.34(dd,j=6.5,2.9hz,2h),7.99(d,j=7.9hz,1h),7.60(t,j=7.4hz,1h),7.54
–
7.48(m,3h),7.42
–
7.34(m,2h),4.42
–
4.27(m,2h),1.83(dt,j=15.6,7.7hz,2h),1.52(dd,j=14.6,7.1hz,2h),1.48
–
1.33(m,5h),0.93(t,j=6.9hz,4h);
13
c nmr(101mhz,cdcl3)δ141.82,134.33,128.65,127.93,127.11,119.21,117.15,114.00,111.30,55.77,54.23,51.56,31.78,26.99,26.01,22.71,14.12;hrms(esi):c
20h26n2 neutral mass:294.2096,observed([m+h])
+
:295.2140;[α]d
25
=+48
°
(c 0.01,ch2cl2);hplc:diacel chiralcel ad-h column 95:5hexanes/2-propanol,1ml/min,254nm;t
r1
=5.96min(major);t
r2
=6.58min(minor)。
[0033]
实施例5:(s)-1-烯丙基-3-苯基-1,2,3,4-四氢喹喔啉的制备(3e):
[0034][0035]
邻苯二胺1e与α-羟基酮2a作为反应的起始底物制备目标产物3e,制备方法与实施例1相同。
[0036]
产率95%;氮黄色油状物;82%ee;1h nmr(400mhz,cdcl3)δ7.52
–
7.32(m,5h),6.82
–
6.57(m,4h),5.91(ddd,j=22.5,10.5,5.4hz,1h),5.23(ddd,j=13.7,11.7,1.5hz,2h),4.54(dd,j=7.7,4.0hz,1h),4.00(dd,j=16.5,5.2hz,2h),3.83(dd,j=16.5,5.5hz,1h),3.45
–
3.25(m,2h);
13
c nmr(101mhz,cdcl3)δ141.69,134.49,134.19,133.52,128.66,127.96,127.09,119.09,117.83,116.86,113.97,112.01,55.38,54.36,54.05;hrms(esi):c
17h18n2 neutral mass:250.1470,observed([m+h])
+
:251.1491;[α]d
25
=+21
°
(c 0.01,ch2cl2);hplc:diacel chiralcel ad-h column 95:5hexanes/2-propanol,1ml/min,
254nm;t
r1
=8.25min(major);t
r2
=10.23min(minor)。
[0037]
实施例6:(s)-1-环己基-3-苯基-1,2,3,4-四氢喹喔啉的制备(3f):
[0038][0039]
邻苯二胺1f与α-羟基酮2a作为反应的起始底物制备目标产物3f,制备方法与实施例1相同。
[0040]
产率97%;氮黄色油状物;98%ee;1h nmr(400mhz,cdcl3)δ7.43(dd,j=8.2,5.6hz,4h),7.40
–
7.33(m,1h),6.76(d,j=6.6hz,2h),6.63(s,2h),4.46(d,j=5.3hz,1h),3.97(s,1h),3.64(t,j=9.6hz,1h),3.48(d,j=10.3hz,1h),3.19
–
3.07(m,1h),1.89(dd,j=24.8,11.8hz,4h),1.75(d,j=13.1hz,1h),1.60
–
1.26(m,5h);
13
c nmr(101mhz,cdcl3)δ142.26,134.76,134.23,128.60,127.86,127.07,119.00,117.11,114.12,111.37,56.17,55.05,48.62,29.88,28.86,26.30,26.16,26.09;hrms(esi):c
20h24n2 neutral mass:292.1939,observed([m+h])
+
:293.2033;[α]d
25
=+78
°
(c 0.01,ch2cl2);hplc:diacel chiralcel ad-h column 95:5hexanes/2-propanol,1ml/min,254nm;t
r1
=10.01min(major);t
r2
=11.91min(minor)。
[0041]
实施例7:(s)-1-苯基-3-苯基-1,2,3,4-四氢喹喔啉的制备(3g):
[0042][0043]
邻苯二胺1g与α-羟基酮2a作为反应的起始底物制备目标产物3g,制备方法与实施例1相同。
[0044]
产率96%;氮黄色油状物;92%ee;1h nmr(400mhz,cdcl3)δ7.53
–
7.32(m,7h),7.25(d,j=7.6hz,2h),7.15
–
6.92(m,2h),6.75(dd,j=32.9,26.2hz,3h),4.56(s,1h),4.20(s,1h),3.88(s,1h),3.60(s,1h);
13
c nmr(101mhz,cdcl3)δ147.98,141.08,135.78,130.95,129.29,128.75,128.07,127.06,122.91,122.62,120.80,118.10,117.47,114.86,55.99,54.04;hrms(esi):c
20h18n2 neutral mass:286.1470,observed([m+h])
+
:287.1546;[α]d
25
=+53
°
(c 0.01,ch2cl2);hplc:diacel chiralcel ad-h column 95:5hexanes/2-propanol,1ml/min,254nm;t
r1
=11.70min(major);t
r2
=13.62min(minor)。
[0045]
实施例8:(s)-6,7-二氟-1-异丙基-3-苯基-1,2,3,4-四氢喹喔啉的制备(3h):
[0046][0047]
邻苯二胺1h与α-羟基酮2a作为反应的起始底物制备目标产物3h,制备方法与实施
例1相同。
[0048]
产率93%;氮黄色油状物;84%ee;1h nmr(400mhz,cdcl3)δ7.52
–
7.32(m,5h),6.50(dd,j=13.5,7.6hz,1h),6.38(dd,j=11.4,7.9hz,1h),4.41(dd,j=8.2,2.9hz,1h),3.93(dt,j=19.5,6.4hz,2h),3.35(dd,j=11.4,3.0hz,1h),2.99(dd,j=11.3,8.4hz,1h),1.22(d,j=6.6hz,3h),1.08(d,j=6.5hz,3h);
13
c nmr(101mhz,cdcl3)δ141.59,140.77,140.64,130.65(dd,j=8.08hz,2.02hz),130.42(dd,j=8.08hz,2.02hz),128.69,128.08,126.94,101.43(d,j=179.78hz),101.22 130.65(d,j=180.79hz),54.96,47.53,46.62,19.24,17.68;
19
f{1h}nmr(376mhz,cdcl3)δ-150.95(d,j=22.6),-133.36(d,j=22.6);hrms(esi):c
17h18
f2n
2 neutral mass:288.1438,observed([m+h])
+
:289.1456;[α]d
25
=+48
°
(c 0.01,ch2cl2);hplc:diacel chiralcel ad-h column 95:5hexanes/2-propanol,1ml/min,254nm;t
r1
=8.75min(major);t
r2
=9.47min(minor)。
[0049]
实施例9:(s)-6,7-二氯-1-异丙基-3-苯基-1,2,3,4-四氢喹喔啉的制备(3i):
[0050][0051]
邻苯二胺1i与α-羟基酮2a作为反应的起始底物制备目标产物3i,制备方法与实施例1相同。
[0052]
产率95%;氮黄色油状物;94%ee;1h nmr(400mhz,cdcl3)δ7.45
–
7.32(m,5h),6.71(s,1h),6.61(s,1h),4.42(dd,j=7.7,2.6hz,1h),3.98(dt,j=13.0,6.5hz,2h),3.36(dd,j=11.3,2.8hz,1h),3.01(dd,j=11.3,8.1hz,1h),1.22(d,j=6.6hz,3h),1.07(d,j=6.6hz,3h);
13
c nmr(101mhz,cdcl3)δ141.40,134.49,133.99,128.72,128.15,126.88,120.84,119.00,114.06,112.09,54.69,47.17,46.30,19.11,17.89;hrms(esi):c
17h18
cl2n
2 neutral mass:320.0847,observed([m+h])
+
:321.0919;[α]d
25
=+61
°
(c 0.01,ch2cl2);hplc:diacel chiralcel ad-hcolumn 95:5hexanes/2-propanol,1ml/min,254nm;t
r1
=7.55min(major);t
r2
=8.48min(minor)。
[0053]
实施例10:(s)-6,7-二甲基-1-异丙基-3-苯基-1,2,3,4-四氢喹喔啉的制备(3j):
[0054][0055]
邻苯二胺1j与α-羟基酮2a作为反应的起始底物制备目标产物3j,制备方法与实施例1相同。
[0056]
产率91%;氮黄色油状物;94%ee;1h nmr(400mhz,cdcl3)δ7.51
–
7.32(m,5h),6.56(s,1h),6.43(s,1h),4.43(dd,j=8.0,2.4hz,1h),4.16
–
4.03(m,2h),3.83(s,1h),3.34(d,j=13.7hz,1h),3.00(dd,j=10.9,8.5hz,1h),2.21(s,3h),2.15(s,3h),1.22(d,j=6.6hz,3h),1.09(d,j=6.5hz,3h);
13
c nmr(101mhz,cdcl3)δ142.56,132.66,132.11,128.54,127.76,127.05,126.28,124.87,115.83,113.39,55.25,47.27,46.86,19.45,
18.74,17.87;hrms(esi):c
19h24
n2neutralmass:280.1939,observed([m+na])
+
:303.1851;[α]d
25
=+43
°
(c0.01,ch2cl2);hplc:diacelchiralcelad-hcolumn95:5hexanes/2-propanol,1ml/min,254nm;t
r1
=10.44min(minor);t
r2
=11.22min(major)。
[0057]
实施例11:(s)-6-氟-1-异丙基-3-苯基-1,2,3,4-四氢喹喔啉的制备(3k):
[0058][0059]
邻苯二胺1k与α-羟基酮2a作为反应的起始底物制备目标产物3k,制备方法与实施例1相同。
[0060]
产率85%;氮黄色油状物;86%ee;1hnmr(400mhz,cdcl3)δ7.48
–
7.31(m,5h),6.62(dd,j=8.7,5.3hz,1h),6.46
–
6.30(m,2h),4.51(d,j=5.5hz,1h),4.03(dt,j=13.0,6.1hz,2h),3.36(d,j=11.0hz,1h),2.97(d,j=19.4hz,1h),1.24(d,j=6.6hz,3h),1.07(d,j=6.5hz,3h);
13
cnmr(101mhz,cdcl3)δ156.02(d,j=234.32hz),141.92,136.02(d,j=10.10hz),130.29(d,j=3.03hz),128.65,127.98,126.93,112.06(d,j=9.09hz),103.75(d,j=22.22hz),100.76(d,j=26.26hz),55.29,47.29,46.73,19.43,17.57;
19
f{1h}nmr(376mhz,cdcl3)δ-127.28;hrms(esi):c
17h19
fn2neutralmass:270.1532,observed([m+h])
+
:271.1605;[α]d
25
=+44
°
(c0.01,ch2cl2);hplc:diacelchiralcelad-hcolumn95:5hexanes/2-propanol,1ml/min,254nm;t
r1
=7.84min(major);t
r2
=8.67min(minor)。
[0061]
实施例12:(s)-7-氯-1-异丙基-3-苯基-1,2,3,4-四氢喹喔啉的制备(3l):
[0062][0063]
邻苯二胺1l与α-羟基酮2a作为反应的起始底物制备目标产物3l,制备方法与实施例1相同。
[0064]
产率92%;氮黄色油状物;94%ee;1hnmr(400mhz,cdcl3)δ7.47
–
7.33(m,5h),6.69(s,1h),6.56(d,j=7.1hz,1h),6.49(d,j=8.1hz,1h),4.41(d,j=5.6hz,1h),4.05(dt,j=13.2,6.5hz,1h),3.98(s,1h),3.40(d,j=13.3hz,1h),3.13
–
2.98(m,1h),1.24(d,j=6.5hz,3h),1.11(d,j=6.5hz,3h);
13
cnmr(101mhz,cdcl3)δ141.80,135.27,133.25,128.68,128.04,127.01,123.74,116.37,114.31,111.03,54.72,46.97,46.68,19.18,18.07;hrms(esi):c
17h19
cln2neutralmass:286.1237,observed([m+h])
+
:287.1296;[α]d
25
=+52
°
(c0.01,ch2cl2);hplc:diacelchiralcelad-hcolumn95:5hexanes/2-propanol,1ml/min,254nm;t
r1
=9.09min(major);t
r2
=10.42min(minor)。
[0065]
实施例13:(s)-6-溴-1-异丙基-3-苯基-1,2,3,4-四氢喹喔啉的制备(3m):
[0066][0067]
邻苯二胺1m与α-羟基酮2a作为反应的起始底物制备目标产物3m,制备方法与实施例1相同。
[0068]
产率87%;氮黄色油状物;92%ee;1h nmr(400mhz,cdcl3)δ7.47
–
7.32(m,5h),6.81(dd,j=8.6,2.1hz,1h),6.69(d,j=2.2hz,1h),6.58(d,j=8.7hz,1h),4.47(dd,j=7.9,3.0hz,1h),4.04(dt,j=13.1,6.5hz,2h),3.37(dd,j=11.4,3.0hz,1h),3.01(dd,j=11.3,8.1hz,1h),1.23(d,j=6.6hz,3h),1.08(d,j=6.6hz,3h);
13
c nmr(101mhz,cdcl3)δ141.79,136.27,133.33,128.69,128.04,126.95,121.03,116.02,112.61,108.97,54.90,46.97,46.59,19.25,17.8;hrms(esi):c
17h19
brn
2 neutral mass:330.0732,observed([m+h])
+
:331.0791;[α]d
25
=+42
°
(c 0.01,ch2cl2);hplc:diacel chiralcel ad-h column 95:5hexanes/2-propanol,1ml/min,254nm;t
r1
=11.20min(major);t
r2
=14.07min(minor)。
[0069]
实施例14:(s)-6-碘-1-异丙基-3-苯基-1,2,3,4-四氢喹喔啉的制备(3n):
[0070][0071]
邻苯二胺1n与α-羟基酮2a作为反应的起始底物制备目标产物3n,制备方法与实施例1相同。
[0072]
产率90%;氮黄色油状物;88%ee;1h nmr(400mhz,cdcl3)δ7.45
–
7.32(m,5h),6.99(d,j=8.4hz,1h),6.85(s,1h),6.48(d,j=8.5hz,1h),4.45(dd,j=7.7,2.3hz,1h),4.08
–
3.96(m,2h),3.38(dd,j=11.4,2.6hz,1h),3.01(dd,j=11.2,8.2hz,1h),1.22(d,j=6.6hz,3h),1.07(d,j=6.5hz,3h);
13
c nmr(101mhz,cdcl3)δ141.74,136.60,134.05,128.67,128.05,127.31,126.95,126.46,121.64,113.18,54.77,46.88,46.59,38.80,19.23,17.94;hrms(esi):c
17h19
in
2 neutral mass:378.0593,observed([m+h])
+
:379.0656;[α]d
25
=+28
°
(c 0.01,ch2cl2);hplc:diacel chiralcel ad-h column 95:5hexanes/2-propanol,1ml/min,254nm;t
r1
=11.43min(major);t
r2
=13.91min(minor)。
[0073]
实施例15:(s)-6-碘-1-异丙基-3-苯基-1,2,3,4-四氢喹喔啉的制备(3o):
[0074][0075]
邻苯二胺1o与α-羟基酮2a作为反应的起始底物制备目标产物3o,制备方法与实施例1相同。
[0076]
产率85%;氮黄色油状物;92%ee;1h nmr(400mhz,cdcl3)δ7.40(dd,j=8.7,5.2hz,4h),7.37
–
7.31(m,1h),6.66(d,j=8.7hz,1h),6.32(dd,j=8.7,2.3hz,1h),6.25
(d,j=2.7hz,1h),4.51(dd,j=8.1,2.7hz,1h),4.02(dt,j=13.0,6.5hz,2h),3.77(s,3h),3.34(dd,j=11.3,2.7hz,1h),2.96(dd,j=11.1,8.4hz,1h),1.23(d,j=6.6hz,3h),1.08(d,j=6.5hz,3h);
13
c nmr(101mhz,cdcl3)δ152.36,142.30,136.15,128.51,127.84,126.98,112.74,102.90,100.82,55.53,47.23,19.55,17.53;hrms(esi):c
18h22
n2o neutral mass:282.1732,observed([m+na])
+
:305.1650;[α]d
25
=+46
°
(c 0.01,ch2cl2);hplc:diacel chiralcel ad-h column 95:5hexanes/2-propanol,1ml/min,254nm;t
r1
=16.93min(major);t
r2
=23.36min(minor)。
[0077]
实施例16:(s)-7-溴-6-氯-1-异丙基-3-苯基-1,2,3,4-四氢喹喔啉的制备(3p):
[0078][0079]
邻苯二胺1p与α-羟基酮2a作为反应的起始底物制备目标产物3p,制备方法与实施例1相同。
[0080]
产率95%;氮黄色油状物;92%ee;1h nmr(400mhz,cdcl3)δ7.45
–
7.32(m,5h),6.85(s,1h),6.63(s,1h),4.43(dd,j=7.8,3.0hz,1h),4.06(s,1h),3.97(dq,j=13.2,6.6hz,1h),3.36(dd,j=11.5,3.1hz,1h),3.00(dd,j=11.4,7.9hz,1h),1.22(d,j=6.6hz,3h),1.07(d,j=6.6hz,3h);
13
c nmr(101mhz,cdcl3)δ141.38,135.12,134.16,128.72,128.15,126.87,121.09,115.02,113.98,109.65,54.67,47.15,46.24,19.10,17.90;hrms(esi):c
17h18
brcln
2 neutral mass:364.0342,observed([m+h])
+
:365.0395;[α]d
25
=+56
°
(c 0.01,ch2cl2);hplc:diacel chiralcel ad-h column 95:5hexanes/2-propanol,1ml/min,254nm;t
r1
=10.58min(major);t
r2
=11.66min(minor)。
[0081]
实施例17:(s)-3-(2-呋喃基)-1-异丙基-1,2,3,4-四氢喹喔啉的制备(3q):
[0082][0083]
邻苯二胺1b与α-羟基酮2b作为反应的起始底物制备目标产物3q,制备方法与实施例1相同。
[0084]
产率98%;氮黄色油状物;90%ee;1h nmr(400mhz,cdcl3)δ7.41(d,j=1.0hz,1h),6.79
–
6.69(m,2h),6.66
–
6.57(m,2h),6.38(dd,j=3.1,1.8hz,1h),6.28(d,j=3.2hz,1h),4.64(dd,j=7.2,3.0hz,1h),4.11(dt,j=13.2,6.6hz,1h),4.02(s,1h),3.49(dd,j=11.2,3.1hz,1h),3.28(dd,j=11.2,7.3hz,1h),1.24(d,j=6.6hz,3h),1.14(d,j=6.6hz,3h);
13
c nmr(101mhz,cdcl3)δ155.26,141.70,134.40,133.57,119.29,117.39,114.42,111.47,110.38,105.91,49.22,46.72,43.50,19.07,18.27;hrms(esi):c
15h18
n2o neutral mass:242.1419,observed([m+h])
+
:243.1428;[α]d
25
=+38
°
(c 0.01,ch2cl2);hplc:diacel chiralcel ad-hcolumn 95:5hexanes/2-propanol,1ml/min,254nm;t
r1
=7.99min(major);t
r2
=9.40min(minor)。
[0085]
实施例18:(s)-3-(2-噻吩基)-1-异丙基-1,2,3,4-四氢喹喔啉的制备(3r):
[0086][0087]
邻苯二胺1b与α-羟基酮2c作为反应的起始底物制备目标产物3r,制备方法与实施例1相同。
[0088]
产率96%;氮黄色油状物;92%ee;1h nmr(400mhz,cdcl3)δ7.31(dd,j=5.0,0.9hz,1h),7.10(d,j=3.0hz,1h),7.04(dd,j=5.0,3.5hz,1h),6.80
–
6.73(m,2h),6.66
–
6.56(m,2h),4.85(dd,j=7.6,3.1hz,1h),4.20
–
4.04(m,2h),3.48(dd,j=11.3,3.1hz,1h),3.19(dd,j=11.3,7.6hz,1h),1.25(d,j=6.6hz,3h),1.17(d,j=6.6hz,3h);
13
c nmr(101mhz,cdcl3)δ146.18,134.29,133.83,126.50,125.00,124.05,119.39,117.46,114.35,111.50,50.91,47.15,46.70,19.21,18.14;hrms(esi):c
15h18
n2s neutral mass:258.1191,observed([m+h])
+
:259.1261;[α]d
25
=+56
°
(c 0.01,ch2cl2);hplc:diacel chiralcel ad-h column 95:5hexanes/2-propanol,1ml/min,254nm;t
r1
=12.39min(major);t
r2
=16.95min(minor)。
[0089]
实施例19:(s)-3-(4-氟苯基)-1-异丙基-1,2,3,4-四氢喹喔啉的制备(3s):
[0090][0091]
邻苯二胺1a与α-羟基酮2d作为反应的起始底物制备目标产物3s,制备方法与实施例1相同。
[0092]
产率89%;氮黄色油状物;96%ee;1h nmr(400mhz,cdcl3)δ7.38(dd,j=8.5,5.5hz,2h),7.08(t,j=8.6hz,2h),6.75(d,j=2.7hz,2h),6.61(s,2h),4.46(d,j=5.7hz,1h),4.11(dt,j=12.8,6.4hz,1h),3.95(s,1h),3.35(d,j=11.0hz,1h),3.07
–
2.93(m,1h),1.23(d,j=6.6hz,3h),1.09(d,j=6.6hz,3h);
13
c nmr(101mhz,cdcl3)δ162.40(d,j=246.44hz),138.12,134.58,134.18,128.59(d,j=8.08hz),119.06,117.36,115.41(d,j=21.21hz),114.01,111.43,54.31,46.91,46.68,19.27,17.99;
19
f{1h}nmr(376mhz,cdcl3)δ-114.75;hrms(esi):c
17h19
fn
2 neutral mass:270.1532,observed([m+h])
+
:271.1601;[α]d
25
=+72
°
(c 0.01,ch2cl2);hplc:diacel chiralcel ad-h column 95:5hexanes/2-propanol,1ml/min,254nm;t
r1
=8.47min(major);t
r2
=11.17min(minor)。
[0093]
实施例20:(s)-3-(4-氯苯基)-1-异丙基-1,2,3,4-四氢喹喔啉的制备(3t):
[0094][0095]
邻苯二胺1b与α-羟基酮2e作为反应的起始底物制备目标产物3t,制备方法与实施
例1相同。
[0096]
产率93%;氮黄色油状物;98%ee;1h nmr(400mhz,cdcl3)δ7.40
–
7.31(m,4h),6.89
–
6.43(m,4h),4.47(d,j=4.9hz,1h),4.19
–
3.82(m,2h),3.35(dd,j=11.2,2.5hz,1h),3.01(dd,j=11.1,8.0hz,1h),1.29(s,1h),1.22(d,j=6.6hz,3h),1.08(d,j=6.6hz,3h);
13
c nmr(101mhz,cdcl3)δ140.98,134.44,134.19,133.48,128.71,128.37,119.08,117.44,114.04,111.50,54.41,46.69,29.73,19.18,18.08;hrms(esi):c
17h19
cln
2 neutral mass:286.1237,observed([m+h])
+
:287.1245;[α]d
25
=+82
°
(c 0.01,ch2cl2);hplc:diacel chiralcel ad-h column 95:5hexanes/2-propanol,1ml/min,254nm;t
r1
=7.07min(major);t
r2
=9.23min(minor)。
[0097]
实施例21:(s)-3-(4-溴苯基)-1-异丙基-1,2,3,4-四氢喹喔啉的制备(3u):
[0098][0099]
邻苯二胺1b与α-羟基酮2f作为反应的起始底物制备目标产物3u,制备方法与实施例1相同。
[0100]
产率88%;氮黄色油状物;99%ee;1h nmr(400mhz,cdcl3)δ7.51(d,j=8.4hz,2h),7.29(d,j=3.2hz,2h),6.75(dd,j=4.4,2.0hz,2h),6.62(qd,j=7.3,2.0hz,2h),4.45(dd,j=7.8,3.0hz,1h),4.09(dq,j=13.2,6.6hz,1h),3.95(s,1h),3.34(dd,j=11.4,3.1hz,1h),3.01(dd,j=11.3,7.8hz,1h),1.22(d,j=6.6hz,3h),1.08(d,j=6.6hz,3h);
13
c nmr(101mhz,cdcl3)δ141.51,134.41,134.19,131.66,128.72,121.59,119.08,117.44,114.03,111.50,54.47,46.66,19.17,18.09;hrms(esi):c
17h19
brn
2 neutral mass:330.0732,observed([m+h])
+
:331.0795;[α]d
25
=+80
°
(c 0.01,ch2cl2);hplc:diacel chiralcel ad-h column 95:5hexanes/2-propanol,1ml/min,254nm;t
r1
=9.72min(major);t
r2
=13.09min(minor)。
[0101]
实施例22:(s)-3-(4-甲氧基苯基)-1-异丙基-1,2,3,4-四氢喹喔啉的制备(3v):
[0102][0103]
邻苯二胺1b与α-羟基酮2g作为反应的起始底物制备目标产物3v,制备方法与实施例1相同。
[0104]
产率93%;氮黄色油状物;98%ee;1h nmr(400mhz,cdcl3)δ7.33(d,j=8.6hz,2h),6.93(d,j=8.6hz,2h),6.74(d,j=2.4hz,2h),6.67
–
6.54(m,2h),4.40(dd,j=8.4,3.0hz,1h),4.12(dp,j=13.4,6.7hz,1h),3.93(s,1h),3.85(s,3h),3.36(dd,j=11.3,3.1hz,1h),3.02(dd,j=11.2,8.5hz,1h),1.23(d,j=6.6hz,3h),1.11(d,j=6.6hz,3h);
13
c nmr(101mhz,cdcl3)δ155.25,141.70,134.40,133.56,128.16,119.29,117.38,114.42,113.98,111.47,110.38,105.91,55.36,49.21,46.72,43.49,19.07,18.26;hrms(esi):c18h22
n2o neutral mass:282.1732,observed([m+na])
+
:305.1744;[α]d
25
=+76
°
(c 0.01,ch2cl2);hplc:diacel chiralcel ad-h column 95:5hexanes/2-propanol,1ml/min,254nm;t
r1
=10.76min(major);t
r2
=16.28min(minor)。
[0105]
实施例23:(s)-3-(4-甲基)-1-异丙基-1,2,3,4-四氢喹喔啉的制备(3w):
[0106][0107]
邻苯二胺1b与α-羟基酮2h作为反应的起始底物制备目标产物3w,制备方法与实施例1相同。
[0108]
产率80%;氮黄色油状物;64%ee;1h nmr(400mhz,cdcl3)δ6.68(d,j=5.4hz,2h),6.60
–
6.49(m,2h),4.16
–
4.04(m,1h),3.50(s,1h),3.29
–
3.20(m,1h),2.84
–
2.72(m,1h),1.22(d,j=1.9hz,3h),1.21(d,j=1.3hz,3h),1.18(d,j=6.6hz,3h);
13
c nmr(101mhz,cdcl3)δ134.49,134.34,118.86,117.03,113.99,111.06,46.38,45.85,20.32,19.29,17.88;hrms(esi):c
12h18n2 neutral mass:190.1470,observed([m+h])
+
:191.1479;[α]d
25
=+34
°
(c 0.01,ch2cl2);hplc:diacel chiralcel ad-h column 95:5hexanes/2-propanol,1ml/min,254nm;t
r1
=10.37min(major);t
r2
=16.31min(minor)。
[0109]
实施例24:(r)-3-(3-三氟甲氧基)-1-异丙基-1,2,3,4-四氢喹喔啉的制备(3x):
[0110][0111]
邻苯二胺1b与α-羟基酮2i作为反应的起始底物制备目标产物3x,制备方法与实施例1相同,催化剂用(s)-cpa1。
[0112]
产率96%;氮黄色油状物;64%ee;1h nmr(400mhz,cdcl3)δ7.41(dd,j=7.0,4.7hz,4h),7.36(dd,j=5.1,3.5hz,1h),7.35
–
7.31(m,1h),7.22(d,j=7.7hz,1h),7.17
–
7.09(m,2h),6.73
–
6.63(m,3h),6.59(dd,j=7.6,4.4hz,1h),4.58(t,j=5.6hz,1h),4.54
–
4.42(m,2h),4.07(s,1h),3.40(d,j=5.7hz,2h);
13
c nmr(101mhz,cdcl3)δ149.62(d,j=2.02),141.40(d,j=15.15),134.32(d,j=21.21),129.93,128.68,128.02,127.00,125.22,123.02(t,j=258.56)121.74,119.48,119.32,119.13,118.37,114.07,112.04,56.05,55.15,54.33;hrms(esi):c
22h19
f3n2o neutral mass:384.1449,observed([m+h])
+
:385.1522;
19
f{1h}nmr(376mhz,cdcl3)δ-57.66;[α]d
25
=+34
°
(c 0.01,ch2cl2);hplc:diacel chiralcel ad-h column 95:5hexanes/2-propanol,1ml/min,254nm;t
r1
=10.37min(major);t
r2
=16.31min(minor)。
相关技术
网友询问留言
已有0条留言
- 还没有人留言评论。精彩留言会获得点赞!
1