一种由邻炔基苯酚合成苯并呋喃酮的方法
1.本发明涉及一种由邻炔基苯酚合成苯并呋喃酮的方法。
背景技术:
2.苯并呋喃-3(2h)-酮代表了合成某些具有广泛生物和药理活性的天然产物及其衍生物的关键结构。2,2-二取代苯并呋喃酮核心存在于多种药物中,常规用于治疗不同的疾病,如灰黄霉素(抗真菌剂)、墨沙酮(杀菌剂)、sch 202596(抗阿尔茨海默病)和松萝酸(抗生素)等(参见:firoozi,n.,roshan,z.,&mohammadizadeh,m.r.facile chemoselective synthesis of 2-(2-(methoxycarbonyl)-3-oxo-2,3-dihydrobenzofuran-2-yl)benzoic acids and 3h,3’h-spiro[benzofuran-2,1
′‑
isobenzofuran]-3,3
′‑
dione derivatives.applied organometallic chemistry.2017,32(1),e3963.)。目前合成苯并呋喃酮的方法主要有,n-杂环卡宾(nhc)和碱催化亲核取代或加氢酰化stetter重排级联反应构建苯并呋喃酮。(参见:a)he,j.,zheng,j.,liu,j.,she,x.,&pan,x.n-heterocyclic carbene catalyzed nucleophilic substitution reaction for construction of benzopyrones and benzofuranones.organic letters.2006,8(20),4637
–
4640.b)padmanaban,m.,biju,a.t.,&glorius,f.efficient synthesis of benzofuranones:n-heterocyclic carbene(nhc)/base-catalyzed hydroacylation
–
stetter
–
rearrangement cascade.organic letters.2011,13(20),5624
–
5627.)。可见光诱导和单线态氧介导的将苯并吡喃酮光化学转化为苯并呋喃酮的方法(参见:brahmachari,g.,&karmakar,i.visible light-induced and singlet oxygen-mediated photochemical conversion of 4-hydroxy-α-benzopyrones to 2-hydroxy-3-oxo-2,3-dihydrobenzofuran-2-carboxamides/carboxylates using rose bengal as a photosensitizer.the journal of organic chemistry.2020,85(14),8851
–
8864.)。而以稳定易得的邻炔基苯酚和甲醇为原料,采用条件温和的催化方式,为获得苯并呋喃酮打开一种新的思路与方法。
技术实现要素:
[0003]
要解决的技术问题
[0004]
本发明要解决的技术问题是提供一种由邻炔基苯酚制备苯并呋喃酮的方法及其应用。
[0005]
技术方案
[0006]
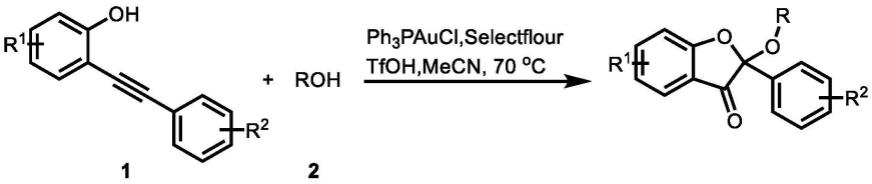
本发明的合成路线如下:
[0007]
[0008]
一种由邻炔基苯酚合成苯并呋喃酮的方法,它是以邻炔基苯酚和醇为原料,三苯基膦氯化金为金催化剂,selectfluor为氧化剂,三氟甲磺酸为助催化剂,在氩气气氛、70℃、乙腈溶液中,高效合成苯并呋喃酮化合物。
[0009]
上述的制备方法,所述的邻炔基苯酚中的r1基团是氢、甲基、酯基,r2基团是氢、甲基、叔丁基、氟、三氟甲基、甲氧基;roh中的r基团可以是甲基、乙基、丙基、异丙基、正丁基、异丁基等。
[0010]
上述的制备方法,所述的溶液乙腈是经过氢化钙重蒸处理的。
[0011]
上述的制备方法,所述的邻炔基苯酚与醇的摩尔比是1:5。
[0012]
上述的制备方法,所述的金催化剂用量是邻炔基苯酚摩尔数的5%的摩尔量。
[0013]
上述的制备方法,所述的氧化剂selectfluor用量是邻炔基苯酚摩尔数的200%的摩尔量。
[0014]
上述的制备方法,所述助催化剂三氟甲磺酸是邻炔基苯酚摩尔数的1-2倍,最优摩尔数为1.5。
[0015]
典型反应如下:
[0016][0017]
本发明的方法反应条件温和,从稳定易制备的邻炔基苯酚直接得到苯并呋喃酮化合物。
具体实施方式
[0018]
原料合成:
[0019]
原料邻炔基苯酚的合成
[0020]
通用步骤:
[0021][0022]
根据文献(alonso-maranon,l.;martinez,m.m.;sarandeses,l.a.;gomez-bengoa,e.;perez sestelo,j.indium(iii)-catalyzed synthesis of benzo[b]furans by intramolecular hydroalkoxylation of ortho-alkynylphenols:scope and mechanistic insights.j.org.chem.2018,83,7970-7980.)进行合成,具体操作如下,首先取一个250ml的圆底烧瓶,称取10mmol的2-碘苯酚,0.2mmol的双三苯基磷二氯化钯,0.4mmol的碘化亚铜,然后加入100ml四氢呋喃、25ml三乙胺溶解,搅拌均匀,再加入20mmol的末端炔烃。将装置置于室温搅拌条件下,反应过夜。反应结束后,使用氯化铵水溶液和二
氯甲烷对反应液进行萃取处理,然后将有机相收集并用无水硫酸钠干燥、过滤、减压蒸馏得到粗产物,最后使用正己烷与乙酸乙酯洗脱液进行柱层析提纯粗产物,得到所需的邻炔基苯酚。
[0023]
利用下述实施例将有助于理解本发明,但并不限制本发明的内容。
[0024]
实施例1
[0025]
依次称取(38.8mg,0.2mmol),金催化剂ph3paucl(5.0mg,0.01mmol),氟试剂selectfluor(141.7mg,0.4mmol),三氟甲磺酸(45mg,0.3mmol),甲醇(32mg,1.0mmol)和乙腈(2.0ml)于10ml反应瓶中,将反应瓶移出手套箱,于70℃反应3小时。干法上样,柱层析(200-300目层析硅胶)得到产物36.5mg,(洗脱液:正己烷-乙酸乙酯,50:1,下同),产率76%,1h nmr(600mhz,cdcl3)δ7.69(t,j=7.8hz,1h),7.65(d,j=5.4hz,3h),7.38(d,j=5.7hz,3h),7.24(d,j=8.4hz,1h),7.11(t,j=7.5hz,1h),3.44(s,3h).
13
c nmr(151mhz,cdcl3)δ196.7,171.0,139.0,133.9,129.6,128.6,126.4,125.4,122.7,119.4,113.1,106.9,52.6.hrms(esi)calc.for c
15h12o3+
[m+h]
+
:241.0859,found 241.0855.
[0026]
实施例2
[0027]
依次称取(41.6mg,0.2mmol),金催化剂ph3paucl(5.0mg,0.01mmol),氟试剂selectfluor(141.7mg,0.4mmol),三氟甲磺酸(45mg,0.3mmol),甲醇(32mg,1.0mmol)和乙腈(2.0ml)于10ml反应瓶中,将反应瓶移出手套箱,于70℃反应3小时。干法上样,柱层析(200-300目层析硅胶)得到产物34.6mg,产率68%,1h nmr(600mhz,cdcl3)δ7.68(t,j=7.8hz,1h),7.64(d,j=7.6hz,1h),7.53(d,j=8.1hz,2h),7.23(d,j=8.3hz,1h),7.19(d,j=8.0hz,2h),7.10(t,j=7.4hz,1h),3.43(s,3h),2.34(s,3h).
13
c nmr(151mhz,cdcl3)δ196.8,171.0,139.6,139.0,130.9,129.3,126.4,125.4,122.6,119.5,113.1,107.0,52.6,21.3.hrms(esi)calc.for c
16h14o3+
[m+h]
+
:255.1016,found 255.1014.
[0028]
实施例3
[0029]
依次称取(50.0mg,0.2mmol),金催化剂ph3paucl(5.0mg,0.01mmol),氟试剂selectfluor(141.7mg,0.4mmol),三氟甲磺酸(45mg,0.3mmol),甲醇(32mg,1.0mmol)和乙腈(2.0ml)于10ml反应瓶中,将反应瓶移出手套箱,于70℃反应3小时。干法上样,柱层析(200-300目层析硅胶)得到产物40.3mg,产率68%,1h nmr(600mhz,cdcl3)δ7.68(t,j=8.5hz,1h),7.64(d,j=8.5hz,1h),7.57(d,j=8.6hz,2h),7.40(d,j=8.6hz,2h),7.23(d,j=8.4hz,1h),7.10(t,j=7.5hz,1h),3.44(s,3h),1.30(s,9h).
13
c nmr(151mhz,cdcl3)δ196.9,171.0,152.7,139.0,130.9,126.1,125.6,125.4,122.5,119.5,113.1,107.1,52.6,34.7,31.2.hrms(esi)calc.for c
19h20o3+
[m+h]
+
:297.1485,found 297.1485.
[0030]
实施例4
[0031]
依次称取(52.8mg,0.2mmol),金催化剂ph3paucl(5.0mg,0.01mmol),氟试剂selectfluor(141.7mg,0.4mmol),三氟甲磺酸(45mg,0.3mmol),甲醇(32mg,1.0mmol)和乙腈(2.0ml)于10ml反应瓶中,将反应瓶移出手套箱,于70℃反应3小时。干法上样,柱层析(200-300目层析硅胶)得到产物38.5mg,产率62%,1h nmr(600mhz,cdcl3)δ7.55(d,j=8.6hz,2h),7.50(d,j=6.6hz,1h),7.42(s,1h),7.39(d,j=8.6hz,2h),7.13(d,j=8.4hz,1h),3.42(s,3h),2.34(s,3h),1.29(s,9h).
13
c nmr(151mhz,cdcl3)δ197.1,169.4,152.6,140.1,132.2,131.1,126.1,125.6,124.7,119.4,112.7,107.3,52.6,34.7,31.2,20.6.hrms(esi)calc.for c
20h22o3+
[m+h]
+
:311.1642,found 311.1640.
[0032]
实施例5
[0033]
依次称取(50.5mg,0.2mmol),金催化剂ph3paucl(5.0mg,0.01mmol),氟试剂selectfluor(141.7mg,0.4mmol),三氟甲磺酸(45mg,0.3mmol),甲醇(32mg,1.0mmol)和乙腈(2.0ml)于10ml反应瓶中,将反应瓶移出手套箱,于70℃反应3小时。干法上样,柱层析(200-300目层析硅胶)得到产物34.1mg,产率57%,1h nmr(600mhz,cdcl3)δ8.40(d,j=10.6hz,1h),8.35(s,1h),7.62(dd,j=9.8hz,2h),7.40(m,j=6.2hz,3h),7.29(d,j=9.2hz,1h),3.92(s,3h),3.43(s,3h).
13
c nmr(151mhz,cdcl3)δ195.5,173.4,165.6,140.2,133.2,129.9,128.8,127.8,126.4,125.1,119.5,113.2,108.2,52.8,52.4.hrms(esi)calc.for c
17h14o5+
[m+h]
+
:299.0914,found 299.0914.
[0034]
实施例6
[0035]
依次称取(42.4mg,0.2mmol),金催化剂ph3paucl(5.0mg,0.01mmol),氟试剂selectfluor(141.7mg,0.4mmol),三氟甲磺酸(45mg,0.3mmol),甲醇(32mg,1.0mmol)和乙腈(2.0ml)于10ml反应瓶中,将反应瓶移出手套箱,于70℃反应3小时。干法上样,柱层析(200-300目层析硅胶)得到产物37.7mg,产率73%,1h nmr(600mhz,cdcl3)δ87.70(t,j=7.8hz,1h),7.67-7.61(m,3h),7.24(d,j=8.4hz,1h),7.12(t,j=7.5hz,1h),7.06(t,j=8.7hz,2h),3.41(s,3h).
13
c nmr(151mhz,cdcl3)δ196.5,170.9,164.4,162.8,139.2,129.9(d,j
c-f
=3.0hz),128.5(d,j
c-f
=9.1hz),125.4,122.8,119.3,115.6,(d,j
c-f
=22.7hz),113.1,106.4,52.6.
19
f nmr(565mhz,cdcl3)δ-111.79.hrms(esi)calc.for c
15h11
fo
3+
[m+h]
+
:259.0765,found 259.0763.
[0036]
实施例7
[0037]
依次称取(42.4mg,0.2mmol),金催化剂ph3paucl(5.0mg,0.01mmol),氟试剂selectfluor(141.7mg,0.4mmol),三氟甲磺酸(45mg,0.3mmol),甲醇(32mg,1.0mmol)和乙腈(2.0ml)于10ml反应瓶中,将反应瓶移出手套箱,于70℃反应3小时。干法上样,柱层析(200-300目层析硅胶)得到产物40.8mg,产率79%,1h nmr(600mhz,cdcl3)δ7.71(t,j=7.1hz,1h),7.66(d,j=7.7hz,1h),7.42(d,j=8.3hz,1h),7.38-7.33(m,2h),7.25(d,j=8.4hz,1h),7.13(t,j=7.8hz,1h),7.07(t,j=9.7hz,1h),3.43(s,3h).
13
c nmr(151mhz,cdcl3)δ196.1,170.9,163.7,162.0,139.2,136.5(d,j
c-f
=7.6hz),130.3(d,j
c-f
=7.6hz),125.5,122.9,122.1(d,j
c-f
=3.0hz),119.2,116.6(d,j
c-f
=21.1hz),113.8(d,j
c-f
=24.2hz),113.1,106.1,52.7.
19
f nmr(565mhz,cdcl3)δ-112.06.hrms(esi)calc.for c
15h11
fo
3+
[m+h]
+
:259.0765,found 259.0763.
[0038]
实施例8
[0039]
依次称取(52.4mg,0.2mmol),金催化剂ph3paucl(5.0mg,0.01mmol),氟试剂selectfluor(141.7mg,0.4mmol),三氟甲磺酸(45mg,0.3mmol),甲醇(32mg,1.0mmol)和乙腈(2.0ml)于10ml反应瓶中,将反应瓶移出手套箱,于70℃反应3小时。干法上样,柱层析(200-300目层析硅胶)得到产物24.7mg,产率40%,1h nmr(600mhz,cdcl3)δ7.77(d,j=8.0hz,2h),7.73(t,j=7.8hz,1h),7.65(t,j=8.8hz,3h),7.27(d,j=9.4hz,1h),7.15(t,j=7.4hz,1h),3.43(s,3h).
13
c nmr(151mhz,cdcl3)δ196.0,171.0,139.4,138.0,131.7(q,j
c-f
=96.6hz),127.0,125.6(q,j
c-f
=10.6hz),125.5,123.0,119.1,113.1,106.1,52.8.
19
f nmr(565mhz,cdcl3)δ-62.84.hrms(esi)calc.for c
16h11
f3o
3+
[m+h]
+
:309.0733,found 309.0736.
[0040]
实施例9
[0041]
依次称取(44.8mg,0.2mmol),金催化剂ph3paucl(5.0mg,0.01mmol),氟试剂selectfluor(141.7mg,0.4mmol),三氟甲磺酸(45mg,0.3mmol),甲醇(32mg,1.0mmol)和乙腈(2.0ml)于10ml反应瓶中,将反应瓶移出手套箱,于70℃反应3小时。干法上样,柱层析(200-300目层析硅胶)得到产物33.5mg,产率62%,1h nmr(600mhz,cdcl3)δ7.70-7.67(m,1h),7.65(d,j=8.5hz,1h),7.29(t,j=8.2hz,1h),7.23(d,j=8.4hz,1h),7.21(s,2h),7.11(t,j=7.8hz,1h),6.92(d,j=11.7hz,1h),3.81(s,3h),3.44(s,3h).
13
c nmr(151mhz,cdcl3)δ196.5,171.0,159.8,139.0,135.4,129.7,125.4,122.7,119.4,118.7,115.4,113.1,111.9,106.7,55.4,52.7.hrms(esi)calc.for c
16h14o4+
[m+h]
+
:271.0965,found 271.0959.
[0042]
实施例10
[0043]
依次称取(44.8mg,0.2mmol),金催化剂ph3paucl(5.0mg,0.01mmol),氟试剂selectfluor(141.7mg,0.4mmol),三氟甲磺酸(45mg,0.3mmol),甲醇(32mg,1.0mmol)和乙腈(2.0ml)于10ml反应瓶中,将反应瓶移出手套箱,于70℃反应3小时。干法上样,柱层析(200-300目层析硅胶)得到产物22.2mg,产率41%,1h nmr(600mhz,cdcl3)δ7.68(t,j=7.8hz,1h),7.64(d,j=7.6hz,1h),7.57(d,j=8.8hz,2h),7.22(d,j=8.3hz,1h),7.10(t,j=7.4hz,1h),6.90(d,j=8.8hz,2h),3.79(s,3h),3.41(s,3h).
13
c nmr(151mhz,cdcl3)δ196.9,170.9,160.7,139.0,127.9,125.9,125.4,122.6,119.5,114.0,113.1,106.9,55.3,52.5.hrms(esi)calc.for c
16h14o4+
[m+h]
+
:271.0965,found 271.0959.
[0044]
实施例11
[0045]
依次称取(47.6mg,0.2mmol),金催化剂ph3paucl
(5.0mg,0.01mmol),氟试剂selectfluor(141.7mg,0.4mmol),三氟甲磺酸(45mg,0.3mmol),甲醇(32mg,1.0mmol)和乙腈(2.0ml)于10ml反应瓶中,将反应瓶移出手套箱,于70℃反应3小时。干法上样,柱层析(200-300目层析硅胶)得到产物20.1mg,产率35%,1h nmr(600mhz,cdcl3)δ7.55(d,j=8.9hz,2h),7.49(d,j=8.4hz,1h),7.42(s,1h),7.12(d,j=8.4hz,1h),6.89(d,j=8.9hz,2h),3.79(s,3h),3.40(s,3h),2.34(s,3h).
13
c nmr(151mhz,cdcl3)δ197.1,169.3,160.6,140.1,132.2,127.8,126.2,124.8,119.4,114.0,112.6,107.1,55.3,52.5,20.6.hrms(esi)calc.for c
17h16o4+
[m+h]
+
:285.1121,found 285.1122.
[0046]
实施例12
[0047]
依次称取(38.8mg,0.2mmol),金催化剂ph3paucl(5.0mg,0.01mmol),氟试剂selectfluor(141.7mg,0.4mmol),三氟甲磺酸(45mg,0.3mmol),乙醇(46mg,1.0mmol)和乙腈(2.0ml)于10ml反应瓶中,将反应瓶移出手套箱,于70℃反应3小时。干法上样,柱层析(200-300目层析硅胶)得到产物36.1mg,产率71%,1h nmr(600mhz,cdcl3)δ7.67(dt,j=21.0,8.0hz,4h),7.40-7.36(m,3h),7.23(d,j=8.4hz,1h),7.10(t,j=7.5hz,1h),3.67(q,j=7.4hz,2h),1.28(t,j=7.0hz,3h).
13
c nmr(151mhz,cdcl3)δ196.9,171.0,139.0,134.5,129.5,128.6,126.4,125.4,122.6,119.5,113.1,107.0,61.2,15.3.hrms(esi)calc.for c
16h14o3+
[m+h]
+
:255.1016,found 255.1014.
[0048]
实施例13
[0049]
依次称取(38.8mg,0.2mmol),金催化剂ph3paucl(5.0mg,0.01mmol),氟试剂selectfluor(141.7mg,0.4mmol),三氟甲磺酸(45mg,0.3mmol),丙醇(60mg,1.0mmol)和乙腈(2.0ml)于10ml反应瓶中,将反应瓶移出手套箱,于70℃反应3小时。
干法上样,柱层析(200-300目层析硅胶)得到产物37.1mg,产率69%,1h nmr(600mhz,cdcl3)δ7.69(t,j=7.8hz,1h),7.66-7.64(m,j=4.2hz,3h),7.37(dd,3h),7.23(d,j=8.4hz,1h),7.10(t,j=7.4hz,1h),3.57(t,j=6.7hz,2h),1.68(h,j=7.4hz,2h),0.95(t,j=7.4hz,3h).
13
c nmr(151mhz,cdcl3)δ197.0,171.0,139.0,134.6,129.5,128.6,126.4,125.4,122.5,119.5,113.1,106.9,67.0,23.0,10.5.hrms(esi)calc.for c
17h16o3+
[m+h]
+
:269.1172,found 269.1172.
[0050]
实施例14
[0051]
依次称取(38.8mg,0.2mmol),金催化剂ph3paucl(5.0mg,0.01mmol),氟试剂selectfluor(141.7mg,0.4mmol),三氟甲磺酸(45mg,0.3mmol),丁醇(74mg,1.0mmol)和乙腈(2.0ml)于10ml反应瓶中,将反应瓶移出手套箱,于70℃反应3小时。干法上样,柱层析(200-300目层析硅胶)得到产物41.8mg,产率74%,1h nmr(600mhz,cdcl3)δ7.69(t,j=7.8hz,1h),7.66-7.62(m,3h),7.40-7.36(d,3h),7.23(d,j=8.4hz,1h),7.12-7.09(t,1h),3.60(t,2h),1.66-1.60(m,2h),1.44-1.38(m,j=7.5hz,2h),0.90(t,j=7.4hz,3h).
13
c nmr(151mhz,cdcl3)δ197.0,171.0,139.0,134.6,129.5,128.6,126.4,125.4,122.5,119.5,113.0,106.9,65.1,31.7,19.1,13.8.hrms(esi)calc.for c
18h18o3+
[m+h]
+
:283.1329,found 283.1328.
[0052]
实施例15
[0053]
依次称取(38.8mg,0.2mmol),金催化剂ph3paucl(5.0mg,0.01mmol),氟试剂selectfluor(141.7mg,0.4mmol),三氟甲磺酸(45mg,0.3mmol),异丙醇(60mg,1.0mmol)和乙腈(2.0ml)于10ml反应瓶中,将反应瓶移出手套箱,于70℃反应3小时。
干法上样,柱层析(200-300目层析硅胶)得到产物27.9mg,产率52%,1h nmr(600mhz,cdcl3)δ7.70-7.63(m,4h),7.37(dd,j=5.2,1.9hz,3h),7.22(d,j=8.4hz,1h),7.09(t,j=7.1hz,1h),3.94(dt,j=12.5,6.3hz,1h),1.30(d,j=6.1hz,3h),1.23(d,j=6.2hz,3h).
13
c nmr(151mhz,cdcl3)δ197.1,170.7,138.9,135.0,129.4,128.5,126.5,125.4,122.4,119.6,113.1,107.7,70.3,24.2,23.9.hrms(esi)calc.for c
17h16o3+
[m+h]
+
:269.1172,found 269.1171.
[0054]
实施例16
[0055]
依次称取(38.8mg,0.2mmol),金催化剂ph3paucl(5.0mg,0.01mmol),氟试剂selectfluor(141.7mg,0.4mmol),三氟甲磺酸(45mg,0.3mmol),异丁醇(74mg,1.0mmol)和乙腈(2.0ml)于10ml反应瓶中,将反应瓶移出手套箱,于70℃反应3小时。干法上样,柱层析(200-300目层析硅胶)得到产物39.5mg,产率70%,1h nmr(600mhz,cdcl3)δ7.69(t,j=7.8hz,1h),7.66-7.63(m,3h),7.39-7.36(m,3h),7.23(d,j=8.4hz,1h),7.11(t,j=7.6hz,1h),3.40-3.35(m,j=6.6,3.0hz,2h),1.95(dt,j=13.4,6.7hz,1h),0.94(dd,j=8.8,6.7hz,6h).
13
c nmr(151mhz,cdcl3)δ197.1,171.0,139.0,134.7,129.5,128.5,126.4,125.4,122.5,119.6,113.1,106.9,71.6,28.6,19.2.hrms(esi)calc.for c
18h18o3+
[m+h]
+
:283.1329,found 283.1328.
[0056]
实施例17
[0057]
依次称取(38.8mg,0.2mmol),金催化剂ph3paucl(5.0mg,0.01mmol),氟试剂selectfluor(141.7mg,0.4mmol),乙酸(60mg,1.0mmol)和乙腈(2.0ml)于10ml反应瓶中,将反应瓶移出手套箱,于70℃反应3小时。干法上样,柱层析(200-300目层
析硅胶)得到产物22.0mg,产率41%,1h nmr(600mhz,cdcl3)δ7.72-7.65(m,4h),7.43(d,j=5.2hz,3h),7.24(d,j=8.6hz,1h),7.18(t,j=7.4hz,1h),2.23(s,3h).
13
c nmr(151mhz,cdcl3)δ194.5,169.2,168.7,138.1,132.8,129.9,128.7,125.8,125.2,123.0,119.8,112.4,101.8,20.6.hrms(esi)calc.for c
16h12o4+
[m+na]
+
:291.0628,found 291.0628.
相关技术
网友询问留言
已有0条留言
- 还没有人留言评论。精彩留言会获得点赞!
1