一类具有苯甲酰胺母核结构的Grp94抑制剂及其用途的制作方法
本发明涉及药物化学领域,具体涉及一类具有Grp94抑制活性的苯甲酰胺类化合物及其在治疗由Grp94介导的疾病中的用途。
背景技术:
热休克蛋白Hsp90是细胞内高度保守的一类蛋白,其主要功能是辅助细胞内其他蛋白(客户蛋白)的成熟。Hsp90包括四个亚型,分别是细胞质中的Hsp90α/β,线粒体中的TRAP1和内质网中的Grp94。作为Hsp90的内质网亚型,Grp94的客户蛋白广泛参与炎症相关性疾病的发生发展中。
Grp94的客户蛋白Toll样受体和整合素Integrins在炎症的发生发展中有很重要的作用。Toll样受体属于病原相关分子模式识别受体,其广泛表达于T细胞、B细胞、DC细胞等各类免疫细胞,在天然免疫及获得性免疫中起重要作用。Toll样受体激活可以诱导TNF-α、IL-6、IL-8、IL-12、IFN等炎症因子的表达。(Leo A.B.Joosten等,Nat.Rev.Rheumatol.,2016,12(6):344)Integrins是细胞粘附分子的一类,广泛表达于细胞表面,介导细胞与细胞、细胞与细胞外基质、细胞与病原体的相互作用。Grp94可以通过调控Toll样受体、整合素来调节炎症的发生发展。在炎症相关性巨噬细胞中敲除Grp94,可以抑制DSS诱导的结肠炎发生,同时也可以减少AOM/DSS诱导的结肠炎向结肠癌的转化。(Crystal Morales等,Cancer Res.,2014,74(2):446–459.)因此,Grp94抑制剂在结肠炎、炎癌转化预防、哮喘、风湿、糖尿病等炎症相关性疾病中有很大的应用前景。
目前已经有多个Grp94选择性抑制剂被开发。如,PU-WS13对Grp94的抑制活性为0.22μM,对Hsp90α的抑制活性为27.3μM;(Hardik J.Patel等,J.Med.Chem.,2015,58(9):3922-43.)KUNG-94对Grp94的亲和力为0.44μM,对Hsp90α的亲和力超过100μM。(ACS Chem.Biol.2017,12(1):244-253.)这些抑制剂分子的Grp94抑制活性和选择性较弱,同时这些抑制剂在炎症相关性疾病中的治疗作用尚未研究。
技术实现要素:
本发明采用基于结构的药物设计方法,设计并合成得到一类具有强效、强选择性的Grp94抑制剂分子。其结构通式如(I)所示:
其中R代表芳香环或乙炔基,所述芳香环选自苯环、萘环、五元或六元含氮芳杂环,所述芳香环或乙炔基被一个或多个取代基取代,取代基选自卤素、氰基、硝基、C1-6烷基、C1-6卤代烷基、C1-6羟烷基、C3-6环烷基、C3-6含氮或含氧杂环基、苯环、五元或六元含氮芳杂环、-OR2、-C(O)R2、-C(O)OR2、-S(O)mR2、-NR3R4、-C(O)NR3R4、-C(O)NHR3、-NR3C(O)R4和-NR3S(O)mR4,其中所述取代基独立选自一个或多个,多个取代基也可以连接成环;
R1代表C1-6烷基、C1-6卤代烷基、C1-6羟烷基、C3-6环烷基、C3-6含氮或含氧杂环基、苯环、五元或六元含氮芳杂环,以上所述基因各自任选被选自C1-6烷基、C1-6卤代烷基、C1-6羟烷基、C3-6环烷基、C3-6含氮或含氧杂环基、苯环、五元或六元含氮芳杂环、-OR2、-C(O)R2、-C(O)OR2、-S(O)mR2、-NR3R4、-C(O)NR3R4、-C(O)NHR3、-NR3C(O)R4和-NR3S(O)mR4中的一个或多个取代基所取代;
R2、R3、R4各自独立地选自氢、C1-6烷基、C3-6环烷基、C3-6含氮或含氧杂环基,以上所述的取代基各自任选被选自C1-6烷基、卤素、羟基、C1-6羧酸酯基、氨基、硝基、氰基、C1-6烷氧基、C1-6羟烷基、C3-6环烷基、C3-6含氮或含氧杂环基中的一个或多个取代基所取代;
m为0、1或2。
R优选代表苯环、取代苯环、乙炔基或取代的乙炔基,取代基选自C1-6烷基、C3-6环烷基、C1-6烷氧基、C1-6烷基氨基或卤素。R更优选代表4-甲基苯基、4-乙基苯基、4-异丙基苯基、4-正丙基苯基、4-叔丁基苯基、4-氯苯基、4-甲氧基苯基、4-乙氧基苯基、2,3-二甲基苯基、3,4-二甲基苯基、2,3-二氯苯基、2,6-二氯苯基、3,3-二甲基-2-丁炔基、1-己炔基、5-甲基-1-己炔基。
R1优选代表其中R5代表C1-6烷基、C3-6环烷基、C3-6含氮或含氧杂环基,所述C1-6烷基、C3-6环烷基、C3-6含氮或含氧杂环基被一个或多个卤素、氨基或羟基取代。
R1更优选代表
本发明部分化合物可用下列方法1进行制备:
方法1:由原料1-1与不同取代的芳香卤代物在Pd(PPh3)4和CsCO3条件下偶联得到中间体1-2,再与各类胺、DIEA在DMSO中120℃下反应得到中间体1-3,进而在氢氧化钠溶液、30%H2O2和乙醇的混合溶液中发生氰基水解得到终产物。
本发明部分化合物可用下列方法2进行制备:
方法2:由原料2-1先与胺、DIEA在DMSO中120℃下反应得到中间体2-2。中间体2-2在氢氧化钠溶液、30%H2O2和乙醇的混合溶液中发生氰基水解得到中间体2-3,进而与取代的苯硼酸酯在Pd(PPh3)4和CsCO3条件下偶联得到终产物。
本发明中部分R代表乙炔基或者取代乙炔基的化合物可用下列方法3进行制备:
方法3:由原料3-1先与炔原料在CuI和(PPh3)2Cl2Pd催化下在三乙胺中反应得到中间体3-2,再与各类胺、DIEA在DMSO中120℃下反应得到中间体3-3。中间体3-3在氢氧化钠溶液、30%H2O2和乙醇的混合溶液中发生氰基水解得到目标产物。
本发明所述化合物具有强效的Grp94选择性抑制活性,对Hsp90细胞质亚型Hsp90α没有任何抑制作用。在DSS诱导的结肠炎模型中,代表性化合物30可以显著抑制结肠炎的发展。此类Grp94抑制剂分子可以用于治疗/预防Grp94介导的炎症相关性疾病,如结肠炎、炎癌转化、哮喘、风湿、糖尿病、青光眼、自身免疫性疾病等。
本发明还公开了一种药物组合物,通式(I)的化合物、药学上可接受的盐或溶剂化物可以添加药学上可接受的载体制成常见的药用制剂,如片剂、胶囊、粉剂、糖浆、液剂、悬浮剂、针剂,可以加入香料、甜味剂、液体或固体填充料或稀释剂等常用的药物辅料。
本发明所述化合物和药学上可接受的载体在临床上可以采用口服、静脉注射等给药方式。在临床上可以用于单药治疗,也可以和其他临床上使用的药物组合使用。本发明的化合物临床所用剂量为0.01mg~1000mg/天,也可根据病情的轻重或剂型的不同偏离此范围。
下面是本发明所述化合物的部分药理学实验及结果:
一、化合物对Grp94和Hsp90α抑制活性采用荧光偏正的方法测试(FP实验)
通过测试化合物与荧光基团标记的格尔德霉素(GM-FITC)竞争性结合重组Grp94和Hsp90α蛋白的能力,反应化合物对两个蛋白的抑制作用。测试中使用384孔黑板(型号为Corning#3575),测试终体积选择60μL,所测试化合物和GM-FITC溶解于DMSO中备用。将化合物用assay buffer倍比稀释12个浓度梯度后每个孔板加入20μL稀释好的化合物,每个孔板再加入20μL用assay buffer稀释好的6nM/L(终浓度)的GM-FITC,再加入20μL用assay buffer稀释好的15nM/L(终浓度)蛋白。每个化合物浓度设定两个附孔,每次实验设置空白对照(20μL GM-FITC+40μL assay buffer)和阴性对照(20μL GM-FITC+20μL蛋白+20μL assay buffer)。之后在4℃摇床上震摇孵育2h,用Synergy读板器扫板,激发波长设置为485nm,发射波长设置为535nm,测试结果Graphpad Prism 6分析。代表性化合物FP测试结果见表1。
表1化合物对Grp94蛋白的抑制活性(FP实验)
测试结果显示大部分化合物具有较好的Grp94抑制活性,部分化合物表现出很强的Grp94抑制活性,尤其是化合物30,对Grp94表现出2nM/L的强抑制活性。所有化合物在100μM/L浓度下对Hsp90α没有任何抑制作用,表现出很好的Grp94选择性抑制作用。
二、化合物对DSS诱导结肠炎的抑制作用
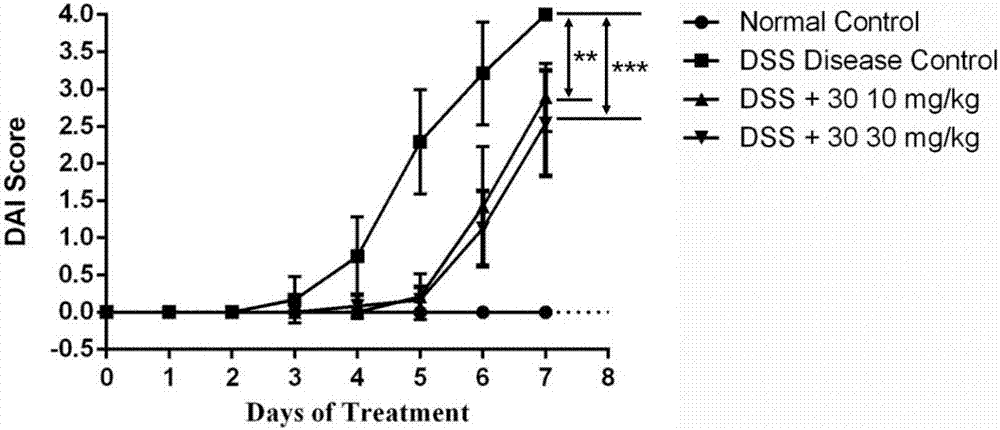
雄性C57BL/6(18-22g)小鼠随机分成4组,每组8只。DSS(MP Biomedicals,Morgan Irvine,CA)溶于饮用水中制成3%的水溶液。空白组小鼠给予正常饮用水,模型组和治疗组小鼠饮用7天3%DSS溶液诱发小鼠结肠炎模型。治疗组小鼠(2组)造模第一天开始每天腹腔注射10mg/kg和30mg/kg化合物30,空白组和模型组腹腔注射空白溶剂。每天观察记录小鼠体重、大便、便血症状,并依据以上症状轻重进行DAI评分,DAI评分高低可以反映出结肠炎的严重程度,DAI评分低则结肠炎症状轻,反之炎症较重。第8天眼球取血并处死小鼠,收取血清和结肠组织。通过Elisa方法测试血清和结肠组织中炎症因子TNFα和IL-6的含量。
实验结果显示,给药组(10mg/kg和30mg/kg)小鼠体重减轻程度(见图1)和DAI评分(见图2)低于模型组。Elisa法测试结果(见图3)显示,给药组血清和结肠组织中炎症因子TNFα和IL-6的含量显著低于模型组。以上结果均表明化合物表现出较好的抗结肠炎效果。
附图说明
图1是给药期间小鼠体重变化
图2是给药期间小鼠DAI评分变化
图3是Elisa法测试小鼠血清和结肠组织中炎症因子TNFα和IL-6的含量
具体实施方式
实施例1
4-(((1r,4r)-4-羟基环己基)氨基)-[1,1'-联苯]-3-甲酰胺(1)的制备
本品采用方法1制备,具体操作如下。
(1)4-氟-[1,1'-联苯]-3-苯甲腈的制备
将5-氰基-2-氟苯硼酸频哪醇酯(247mg,1.0mmol)和碘苯(306mg,1.5mmol)溶于二氧六环中,加入Pd(PPh3)4(116mg,0.1mmol)和CsCO3(652mg,2.0mmol),氮气条件下90℃反应过夜。反应液硅藻土抽滤,滤液旋干后分散于20ml乙酸乙酯中,用20ml水和氯化钠溶液分别洗两次,无水硫酸钠干燥。石油醚柱层析得无色油状物168mg,产率:85.3%。1H NMR(300MHz,Chloroform-d)δ7.86–7.81(m,2H),7.61–7.46(m,5H),7.32(m,1H).
(2)标题化合物的制备
将上述所得白色固体(168mg,0.85mmol)溶于5ml DMSO中,加入反式-4-氨基环己醇(441mg,3.83mmol)和DIEA(1.34ml,7.7mmol),120℃下(冷凝管)加热反应24h。反应液倒入20ml水中,用20ml乙酸乙酯萃取两次,合并乙酸乙酯层,无水硫酸钠干燥。旋干后溶于10ml乙醇中,加入2ml 1M/L NaOH溶液和2ml 30%H2O2,30℃下反应过夜。将反应液中的乙醇旋干后分散于20ml乙酸乙酯中,20ml水洗两次,20ml饱和氯化钠溶液洗1次,无水硫酸钠干燥。旋干后用二氯甲烷打浆得到白色粉末78mg。总产率25.1%。1H NMR(300MHz,DMSO-d6)δ8.17(d,J=7.7Hz,1H),8.02(s,1H),7.90(s,1H),7.64(d,J=7.7Hz,2H),7.58(d,J=8.7Hz,1H),7.39(t,J=7.6Hz,2H),7.23(t,J=7.3Hz,1H),7.17(s,1H),6.79(d,J=8.8Hz,1H),4.57(s,1H),3.52–3.43(m,1H),3.39–3.33(m,1H),2.01–1.96(m,2H),1.85–1.81(m,2H),1.38–1.14(m,4H).HRMS(ESI):calcd for C19H23N2O2[M+H]+311.1681,found 311.1757.13C NMR(75MHz,DMSO-d6)δ171.65,148.37,139.92,130.61,128.65,127.29,125.91,125.57,125.37,113.93,112.15,68.03,49.43,33.45,30.25.
实施例2
4-(((1r,4r)-4-羟基环己基)氨基)-2'-甲基-[1,1'-联苯]-3-甲酰胺(2)的制备
本品制备方法同实施例1,将碘苯更换为2-碘甲苯(327mg,1.5mmol)。得白色固体85mg,总产率26.2%。1H NMR(300MHz,DMSO-d6)δ8.19(d,J=7.6Hz,1H),7.86(s,1H),7.58(s,1H),7.26–7.21(m,5H),7.12(s,1H),6.75(d,J=8.7Hz,1H),4.60(s,1H),3.52–3.35(m,1H),3.32–3.27(m,1H),2.26(s,3H),2.01–1.98(m,2H),1.85–1.81(m,2H),1.38–1.30(m,2H),1.26–1.15(m,2H).HRMS(ESI):calcd for C20H25N2O2[M+H]+325.1838,found 325.1915.
实施例3
4-(((1r,4r)-4-羟基环己基)氨基)-3'-甲基-[1,1'-联苯]-3-甲酰胺(3)的制备
本品制备方法同实施例1,将碘苯更换为3-碘甲苯(327mg,1.5mmol)。得白色固体52mg,总产率16.0%。1H NMR(300MHz,DMSO-d6)δ8.16(d,J=7.7Hz,1H),8.02(s,1H),7.87(d,J=1.9Hz,1H),7.56(dd,J=8.7,1.7Hz,1H),7.47(s,1H),7.42(d,J=8.2Hz,1H),7.27(t,J=7.5Hz,1H),7.19(s,1H),7.05(d,J=7.4Hz,1H),6.78(d,J=8.9Hz,1H),4.58(d,J=4.1Hz,1H),3.52–3.44(m,1H),3.40–3.34(m,1H),2.35(s,3H),2.00–1.96(m,2H),1.85–1.80(m,2H),1.39–1.29(m,2H),1.27–1.14(m,2H).HRMS(ESI):calcd for C20H25N2O2[M+H]+325.1838,found 325.1916.
实施例4
4-(((1r,4r)-4-羟基环己基)氨基)-4'-甲基-[1,1'-联苯]-3-甲酰胺(4)的制备
本品制备方法同实施例1,将碘苯更换为4-碘甲苯(327mg,1.5mmol)。得白色固体34mg,总产率10.5%。1H NMR(300MHz,DMSO-d6)δ8.13(d,J=7.7Hz,1H),8.01(s,1H),7.86(d,J=2.0Hz,1H),7.54(d,J=8.0Hz,3H),7.20(d,J=8.0Hz,3H),6.77(d,J=8.9Hz,1H),4.58(d,J=4.1Hz,1H),3.51–3.42(m,1H),3.34–3.26(m,1H),2.30(s,3H),2.00–1.95(m,2H),1.84–1.81(m,2H),1.38–1.30(m,2H),1.29–1.16(m,2H).HRMS(ESI):calcd for C20H25N2O2[M+H]+325.1838,found 325.1913.13C NMR(75MHz,DMSO-d6)δ171.67,148.15,137.03,134.97,130.38,129.23,126.95,125.39,125.35,113.90,112.12,68.04,49.41,33.46,30.26,20.59.
实施例5
2'-氯-4-(((1r,4r)-4-羟基环己基)氨基)-[1,1'-联苯]-3-甲酰胺(5)的制备
本品制备方法同实施例1,将碘苯更换为1-氯-2-碘苯(358mg,1.5mmol)。得白色固体33mg,总产率9.6%。1H NMR(300MHz,DMSO-d6)δ8.22(d,J=7.7Hz,1H),7.87(s,1H),7.66(d,J=2.1Hz,1H),7.51(dd,J=7.8,1.2Hz,1H),7.45–7.28(m,4H),7.14(s,1H),6.77(d,J=8.9Hz,1H),4.58(d,J=4.2Hz,1H),3.51–3.42(m,1H),3.39–3.35(m,1H),2.01–1.96(m,2H),1.85–1.80(m,2H),1.39–1.31(m,2H),1.26–1.14(m,2H).HRMS(ESI):calcd for C19H22ClN2O2[M+H]+345.1292,found 345.1367.
实施例6
3'-氯-4-(((1r,4r)-4-羟基环己基)氨基)-[1,1'-联苯]-3-甲酰胺(6)的制备
本品制备方法同实施例1,将碘苯更换为1-氯-3-碘苯(358mg,1.5mmol)。得白色固体36mg,总产率10.5%。1H NMR(300MHz,DMSO-d6)δ8.29(d,J=7.7Hz,1H),8.08(s,1H),7.92(d,J=2.3Hz,1H),7.76(t,J=1.7Hz,1H),7.61(dd,J=8.7,1.7Hz,2H),7.41(t,J=7.9Hz,1H),7.29–7.26(m,1H),7.22(s,1H),6.79(d,J=8.9Hz,1H),4.59(d,J=4.1Hz,1H),3.52–3.44(m,1H),3.42–3.37(m,1H),2.00–1.95(m,2H),1.85–1.79(m,2H),1.39–1.14(m,4H).HRMS(ESI):calcd for C19H22ClN2O2[M+H]+345.1292,found 345.1368.
实施例7
4'-氯-4-(((1r,4r)-4-羟基环己基)氨基)-[1,1'-联苯]-3-甲酰胺(7)的制备
本品制备方法同实施例1,将碘苯更换为1-氯-4-碘苯(358mg,1.5mmol)。得白色固体150mg,总产率43.6%。1H NMR(300MHz,DMSO-d6)δ8.23(d,J=7.7Hz,1H),8.04(s,1H),7.90(d,J=2.2Hz,1H),7.68(d,J=8.6Hz,2H),7.58(dd,J=8.8,2.2Hz,1H),7.44(d,J=8.6Hz,2H),7.21(s,1H),6.79(d,J=8.9Hz,1H),4.59(d,J=4.1Hz,1H),3.51–3.44(m,1H),3.42–3.35(m,1H),2.00–1.96(m,2H),1.85–1.80(m,2H),1.39–1.14(m,4H).HRMS(ESI):calcd for C19H22ClN2O2[M+H]+345.1292,found 345.1371.13C NMR(75MHz,DMSO-d6)δ171.55,148.61,138.72,130.53,128.55,127.26,127.16,123.84,113.83,112.19,68.00,49.39,33.42,30.21.
实施例8
4-(((1r,4r)-4-羟基环己基)氨基)-2'-甲氧基-[1,1'-联苯]-3-甲酰胺(8)的制备
本品制备方法同实施例1,将碘苯更换为2-碘苯甲醚(351mg,1.5mmol)。得白色固体97mg,总产率28.5%。1H NMR(300MHz,DMSO-d6)δ8.06(d,J=7.7Hz,1H),7.83(s,1H),7.66(d,J=2.1Hz,1H),7.42(dd,J=8.7,1.7Hz,1H),7.32–7.23(m,2H),7.06(s,1H),7.03–6.95(m,2H),6.72(d,J=8.8Hz,1H),4.57(d,J=4.1Hz,1H),3.74(s,3H),3.52–3.43(m,1H),3.31–3.26(m,1H),2.01–1.97(m,2H),1.84–1.81(m,2H),1.38–1.27(m,2H),1.24–1.15(m,2H).HRMS(ESI):calcd for C20H25N2O3[M+H]+341.1787,found 341.1865.
实施例9
4-(((1r,4r)-4-羟基环己基)氨基)-3'-甲氧基-[1,1'-联苯]-3-甲酰胺(9)的制备
本品制备方法同实施例1,将碘苯更换为3-碘苯甲醚(351mg,1.5mmol)。得淡黄色固体91mg,总产率26.8%。1H NMR(300MHz,DMSO-d6)δ8.10(d,J=7.7Hz,1H),7.94(s,1H),7.79(d,J=2.3Hz,1H),7.49(dd,J=8.8,2.1Hz,1H),7.21(t,J=7.8Hz,1H),7.13–7.10(m,3H),6.74–6.67(m,2H),4.47(d,J=4.2Hz,1H),3.72(s,3H),3.43–3.35(m,1H),3.32–3.24(m,1H),1.92–1.88(m,2H),1.75–1.71(m,2H),1.30–1.06(m,4H).HRMS(ESI):calcd for C20H25N2O3[M+H]+341.1787,found 341.1857.
实施例10
4-(((1r,4r)-4-羟基环己基)氨基)-4'-甲氧基-[1,1'-联苯]-3-甲酰胺(10)的制备
本品制备方法同实施例1,将碘苯更换为4-碘苯甲醚(351mg,1.5mmol)。得淡黄色固体60mg,总产率17.6%。1H NMR(300MHz,DMSO-d6)δ7.99(d,J=7.7Hz,1H),7.90(s,1H),7.73(d,J=2.3Hz,1H),7.51–7.45(d,J=8.8,2H),7.42(dd,J=8.7,2.2Hz,1H),7.05(s,1H),6.87(d,J=8.9,2H),6.67(d,J=8.9Hz,1H),4.47(d,J=4.2Hz,1H),3.68(s,3H),3.43–3.37(m,1H),3.30–3.24(m,1H),1.91–1.88(m,2H),1.76–1.71(m,2H),1.30–1.03(m,4H).HRMS(ESI):calcd for C20H25N2O3[M+H]+341.1787,found 341.1855.13C NMR(75MHz,DMSO-d6)δ171.69,157.82,147.85,132.49,130.24,126.74,126.66,125.32,114.08,113.95,112.15,68.05,55.07,49.43,33.46,30.27.
实施例11
2'-氰基-4-(((1r,4r)-4-羟基环己基)氨基)-[1,1'-联苯]-3-甲酰胺(11)的制备
本品采用方法2制备,具体操作如下。
(1)2-(((1r,4r)-4-羟基环己基)氨基)-5-碘苯甲腈的制备
2-氟-5-碘苯甲腈(2.47g,10mmol)溶于10ml DMSO中,加入反式-4-氨基环己醇(1.73g,15mmol)和DIEA(5.2ml,30mmol),120℃下(冷凝管)加热反应7h。反应液倒入100ml水中,用乙酸乙酯萃取(200ml×2),合并乙酸乙酯层,水洗有机层(150ml×3),无水硫酸钠干燥。旋干后柱层析(石油醚:乙酸乙酯=1:1)得到白色固体3.15g。将所得固体溶于50ml乙醇中,加入5ml 1M/L NaOH溶液和5ml 30%H2O2,30℃下反应过夜。将反应液中的乙醇旋干后分散于50ml乙酸乙酯中,50ml水洗两次,50ml饱和氯化钠溶液洗1次,无水硫酸钠干燥,旋干得白色固体3.2g,两步反应总产率88.9%。1H NMR(300MHz,DMSO-d6)δ8.04(d,J=7.7Hz,1H),7.80(s,1H),7.74(d,J=2.2Hz,1H),7.37(dd,J=8.8,2.1Hz,1H),7.10(s,1H),6.47(d,J=9.0Hz,1H),4.47(d,J=4.3Hz,1H),3.41–3.31(m,1H),3.24–3.11(m,1H),1.86–1.82(m,2H),1.73–1.68(m,2H),1.26–1.00(m,4H).
(2)标题化合物的制备
将上述所得中间体2-(((1r,4r)-4-羟基环己基)氨基)-5-碘苯甲腈(180mg,0.5mmol)和2-氰基苯硼酸频哪醇酯(172mg,0.75mmol)溶于二氧六环中,加入Pd(PPh3)4(58mg,0.1mmol)和CsCO3(652mg,2.0mmol),氮气条件下90℃反应过夜。反应液硅藻土抽滤,滤液旋干后分散于20ml乙酸乙酯中,用20ml水和氯化钠溶液分别洗两次,无水硫酸钠干燥。柱层析(石油醚:乙酸乙酯=1:1)得白色固体100mg,产率:59.5%。1H NMR(300MHz,DMSO-d6)δ8.23(d,J=7.7Hz,1H),7.84(s,1H),7.79–7.74(m,2H),7.67–7.62(m,1H),7.55(d,J=7.8Hz,1H),7.45–7.35(m,2H),7.12(s,1H),6.76(d,J=9.0Hz,1H),4.48(d,J=4.2Hz,1H),3.44–3.36(m,1H),3.34–3.25(m,1H),1.93–1.88(m,2H),1.76–1.72(m,2H),1.31–1.23(m,2H),1.16–1.08(m,2H).HRMS(ESI):calcd for C20H22N3O2[M+H]+336.1634,found 336.1700.
实施例12
2'-乙基-4-(((1r,4r)-4-羟基环己基)氨基)-[1,1'-联苯]-3-甲酰胺(12)的制备
本品制备方法同实施例1,将碘苯更换为1-乙基-2-碘苯(348mg,1.5mmol)。得白色固体125mg,总产率37.0%。1H NMR(300MHz,DMSO-d6)δ8.17(d,J=7.6Hz,1H),7.83(s,1H),7.55(d,J=2.1Hz,1H),7.30–7.14(m,5H),7.08(s,1H),6.75(d,J=8.7Hz,1H),4.57(d,J=4.2Hz,1H),3.52–3.43(m,1H),3.31–3.23(m,1H),2.58(q,J=7.5Hz,2H),2.01–1.97(m,2H),1.85–1.81(m,2H),1.39–1.14(m,4H),1.05(t,J=7.5Hz,3H).HRMS(ESI):calcd for C21H27N2O2[M+H]+339.1994,found 339.2069.
实施例13
4'-乙基-4-(((1r,4r)-4-羟基环己基)氨基)-[1,1'-联苯]-3-甲酰胺(13)的制备
本品制备方法同实施例1,将碘苯更换为1-乙基-4-碘苯(348mg,1.5mmol)。得白色固体55mg,总产率16.3%。1H NMR(300MHz,DMSO-d6)δ8.12(d,J=7.8Hz,1H),7.98(s,1H),7.86(d,J=2.2Hz,1H),7.55(d,J=8.2Hz,3H),7.22(d,J=7.9Hz,2H),7.12(s,1H),6.77(d,J=8.9Hz,1H),4.54(d,J=4.2Hz,1H),3.52–3.43(m,1H),3.39–3.30(m,1H),2.61(q,J=7.6Hz,2H),2.01–1.96(m,2H),1.85–1.80(m,2H),1.39–1.14(m,7H).HRMS(ESI):calcd for C21H27N2O2[M+H]+339.1994,found 339.2063.13C NMR(75MHz,DMSO-d6)δ171.68,148.16,141.43,137.37,130.45,128.05,127.07,125.53,125.46,113.92,112.12,68.04,49.43,33.46,30.26,27.75,15.74.
实施例14
4'-乙酰基-4-(((1r,4r)-4-羟基环己基)氨基)-[1,1'-联苯]-3-甲酰胺(14)的制备
本品制备方法同实施例1,将碘苯更换为4-碘代苯乙酮(369mg,1.5mmol)。得亮黄色固体100mg,总产率28.4%。1H NMR(300MHz,DMSO-d6)δ8.33(d,J=7.6Hz,1H),8.07(s,1H),8.01–7.99(m,3H),7.83–7.80(m,2H),7.69(dd,J=8.8,2.2Hz,1H),7.22(s,1H),6.83(d,J=9.0Hz,1H),4.56(d,J=4.3Hz,1H),3.53–3.43(m,1H),3.41–3.34(m,1H),2.58(s,3H),2.01–1.96(m,2H),1.85–1.81(m,2H),1.39–1.15(m,4H).HRMS(ESI):calcd for C21H25N2O3[M+H]+353.1787,found 353.1856.
实施例15
4-(((1r,4r)-4-羟基环己基)氨基)-4'-丙基-[1,1'-联苯]-3-甲酰胺(15)的制备
本品制备方法同实施例1,将碘苯更换为4-丙基溴苯(299mg,1.5mmol)。得白色固体80mg,总产率22.7%。1H NMR(300MHz,DMSO-d6)δ8.13(d,J=7.7Hz,1H),7.99(s,1H),7.87(d,J=2.3Hz,1H),7.56(d,J=8.2,3H),7.21(d,J=7.9Hz,2H),7.14(s,1H),6.78(d,J=8.9Hz,1H),4.55(d,J=4.2Hz,1H),3.53–3.43(m,1H),3.39–3.33(m,1H),2.56(t,J=7.4Hz,2H),2.00–1.97(m,2H),1.85–1.80(m,2H),1.67–1.54(m,2H),1.31–1.14(m,4H),0.91(t,J=7.3Hz,3H).HRMS(ESI):calcd for C22H29N2O2[M+H]+353.2151,found 353.2218.
实施例16
4'-叔丁基-4-(((1r,4r)-4-羟基环己基)氨基)-[1,1'-联苯]-3-甲酰胺(16)的制备
本品制备方法同实施例1,将碘苯更换为4-叔丁基碘苯(390mg,1.5mmol)。得淡黄色固体48mg,总产率13.1%。1H NMR(300MHz,DMSO-d6)δ8.12(d,J=7.7Hz,1H),7.97(s,1H),7.86(d,J=2.2Hz,1H),7.55(d,J=8.3Hz,3H),7.40(d,J=8.4Hz,2H),7.12(s,1H),6.78(d,J=8.9Hz,1H),4.54(d,J=4.2Hz,1H),3.52–3.43(m,1H),3.39–3.31(m,1H),2.01–1.97(m,2H),1.85–1.81(m,2H),1.38–1.15(m,13H).HRMS(ESI):calcd for C23H31N2O2[M+H]+367.2307,found 367.2380.13C NMR(75MHz,DMSO-d6)δ171.67,148.27,148.16,137.17,130.49,127.19,125.46,125.37,125.33,113.92,112.11,68.03,49.43,34.08,33.45,31.13,30.26.
实施例17
4'-乙氧基-4-(((1r,4r)-4-羟基环己基)氨基)-[1,1'-联苯]-3-甲酰胺(17)的制备
本品制备方法同实施例1,将碘苯更换为4-碘苯乙醚(372mg,1.5mmol)。得白色固体66mg,总产率18.6%。1H NMR(300MHz,DMSO-d6)δ8.08(d,J=7.7Hz,1H),7.98(s,1H),7.82(s,1H),7.57–7.50(m,3H),7.12(s,1H),6.94(d,J=8.4Hz,2H),6.76(d,J=8.8Hz,1H),4.54(d,J=4.1Hz,1H),4.04(q,J=6.8Hz,2H),3.52–3.44(m,1H),3.40–3.30(m,1H),2.00–1.96(m,2H),1.85–1.80(m,2H),1.33(t,J=6.9Hz,3H),1.26–1.13(m,4H).HRMS(ESI):calcd for C21H27N2O3[M+H]+355.1943,found 355.2009.
实施例18
4-(((1r,4r)-4-羟基环己基)氨基)-2',3'-二甲基-[1,1'-联苯]-3-甲酰胺(18)的制备
本品制备方法同实施例1,将碘苯更换为1,2-二甲基-3-碘苯(348mg,1.5mmol)。得白色固体47mg,总产率13.9%。1H NMR(300MHz,DMSO-d6)δ8.16(d,J=7.6Hz,1H),7.82(s,1H),7.53(d,J=2.1Hz,1H),7.18(dd,J=8.5,2.1Hz,1H),7.09(d,J=5.1Hz,2H),7.06–7.02(m,1H),6.74(d,J=8.7Hz,1H),4.58(d,J=4.2Hz,1H),3.52–3.43(m,1H),3.32–3.25(m,1H),2.27(s,3H),2.12(s,3H),2.01–1.97(m,2H),1.85–1.81(m,2H),1.38–1.14(m,4H).HRMS(ESI):calcd for C21H27N2O2[M+H]+339.1994,found 339.2069.13C NMR(75MHz,DMSO-d6)δ171.62,147.79,141.35,136.60,133.49,133.41,129.77,128.01,127.56,127.03,125.14,113.16,111.17,68.07,49.47,33.50,30.33,20.41,16.77.
实施例19
4-(((1r,4r)-4-羟基环己基)氨基)-2',4'-二甲基-[1,1'-联苯]-3-甲酰胺(19)的制备
本品制备方法同实施例1,将碘苯更换为2,4-二甲基碘苯(348mg,1.5mmol)。得白色固体67mg,总产率19.8%。1H NMR(300MHz,DMSO-d6)δ8.14(d,J=7.7Hz,1H),7.83(s,1H),7.54(d,J=2.0Hz,1H),7.21(dd,J=8.5,2.1Hz,1H),7.10–7.00(m,4H),6.73(d,J=8.8Hz,1H),4.58(d,J=4.2Hz,1H),3.53–3.42(m,1H),3.32–3.26(m,1H),2.28(s,3H),2.21(s,3H),2.00–1.97(m,2H),1.85–1.80(m,2H),1.38–1.14(m,4H).HRMS(ESI):calcd for C21H27N2O2[M+H]+339.1994,found 339.2070.
实施例20
4-(((1r,4r)-4-羟基环己基)氨基)-2',6'-二甲基-[1,1'-联苯]-3-甲酰胺(20)的制备
本品制备方法同实施例1,将碘苯更换为1,3-二甲基-2-碘苯(348mg,1.5mmol)。得淡黄色固体86mg,总产率25.4%。1H NMR(300MHz,DMSO-d6)δ8.25(d,J=7.5Hz,1H),7.77(s,1H),7.39(d,J=2.1Hz,1H),7.08(s,3H),7.01(d,J=8.4Hz,2H),6.76(d,J=8.6Hz,1H),4.57(d,J=4.2Hz,1H),3.52–3.44(m,1H),3.31–3.26(m,1H),2.01(s,6H),1.99–1.94(m,2H),1.86–1.82(m,2H),1.39–1.30(m,2H),1.26–1.15(m,2H).HRMS(ESI):calcd for C21H27N2O2[M+H]+339.1994,found 339.2068.
实施例21
4-(((1r,4r)-4-羟基环己基)氨基)-3',4'-二甲基-[1,1'-联苯]-3-甲酰胺(21)的制备
本品制备方法同实施例1,将碘苯更换为3,4-二甲基碘苯(348mg,1.5mmol)。得白色固体38mg,总产率11.2%。1H NMR(300MHz,DMSO-d6)δ8.03(d,J=7.7Hz,1H),7.90(s,1H),7.76(d,J=2.2Hz,1H),7.45(d,J=8.9,1H),7.35(s,1H),7.26(d,J=7.8Hz,1H),7.06–7.03(m,2H),6.67(d,J=8.8Hz,1H),4.47(d,J=4.1Hz,1H),3.43–3.33(m,2H),2.18(s,3H),2.13(s,3H),1.92–1.88(m,2H),1.76–1.72(m,2H),1.30–1.04(m,4H).HRMS(ESI):calcd for C21H27N2O2[M+H]+339.1994,found 339.2060.
实施例22
4-(((1r,4r)-4-羟基环己基)氨基)-3',5'-二甲基-[1,1'-联苯]-3-甲酰胺(22)的制备
本品制备方法同实施例1,将碘苯更换为3,5-二甲基碘苯(348mg,1.5mmol)。得白色固体118mg,总产率34.9%。1H NMR(300MHz,DMSO-d6)δ8.15(d,J=7.7Hz,1H),8.01(s,1H),7.85(d,J=2.3Hz,1H),7.54(d,J=8.4,1H),7.25(s,2H),7.19(s,1H),6.87(s,1H),6.76(d,J=8.7Hz,1H),4.59(d,J=4.1Hz,1H),3.53–3.42(m,1H),3.32–3.25(m,1H),2.30(s,6H),2.00–1.96(m,2H),1.85–1.80(m,2H),1.38–1.30(m,2H),1.27–1.13(m,2H).HRMS(ESI):calcd for C21H27N2O2[M+H]+339.1994,found 339.2068.
实施例23
4-(((1r,4r)-4-羟基环己基)氨基)-2',4',6'-三甲基-[1,1'-联苯]-3-甲酰胺(23)的制备
本品制备方法同实施例1,将碘苯更换为2,4,6-三甲基碘苯(369mg,1.5mmol)。得白色固体126mg,总产率35.8%。1H NMR(300MHz,DMSO-d6)δ8.21(d,J=7.6Hz,1H),7.73(s,1H),7.36(d,J=2.1Hz,1H),6.98(dd,J=8.5,2.0Hz,2H),6.89(s,2H),6.75(d,J=8.7Hz,1H),4.54(d,J=4.2Hz,1H),3.52–3.43(m,1H),3.36–3.31(m,1H),2.24(s,3H),2.03–1.98(m,2H),1.97(s,6H),1.86–1.82(m,2H),1.39–1.15(m,4H).HRMS(ESI):calcd for C22H29N2O2[M+H]+353.2151,found 353.2220.13C NMR(75MHz,DMSO-d6)δ171.61,147.77,138.26,135.85,135.36,133.39,129.51,127.78,125.28,113.08,111.54,68.12,49.53,33.55,30.44,20.68,20.58.
实施例24
2',3'-二氯-4-(((1r,4r)-4-羟基环己基)氨基)-[1,1'-联苯]-3-甲酰胺(24)的制备
本品制备方法同实施例1,将碘苯更换为2,3-二氯碘苯(409mg,1.5mmol)。得白色固体31mg,总产率8.2%。1H NMR(300MHz,DMSO-d6)δ8.29(d,J=7.6Hz,1H),7.86(s,1H),7.67(d,J=2.2Hz,1H),7.58(t,J=4.8Hz,1H),7.40–7.35(m,3H),7.16(s,1H),6.78(d,J=9.0Hz,1H),4.59(d,J=4.2Hz,1H),3.52–3.44(m,1H),3.40–3.36(m,1H),2.00–1.97(m,2H),1.85–1.80(m,2H),1.39–1.29(m,2H),1.25–1.14(m,2H).HRMS(ESI):calcd for C19H21Cl2N2O2[M+H]+379.0902,found 379.0981.
实施例25
2',6'-二氯-4-(((1r,4r)-4-羟基环己基)氨基)-[1,1'-联苯]-3-甲酰胺(25)的制备
本品制备方法同实施例1,将碘苯更换为2,6-二氯碘苯(409mg,1.5mmol)。得淡黄色固体37mg,总产率9.8%。1H NMR(300MHz,DMSO-d6)δ8.46(d,J=7.5Hz,1H),7.78(s,1H),7.56–7.54(m,3H),7.39–7.34(m,1H),7.18–7.07(m,2H),6.78(d,J=8.9Hz,1H),4.58(d,J=4.2Hz,1H),3.52–3.45(m,1H),3.32–3.27(m,1H),2.01–1.97(m,2H),1.85–1.82(m,2H),1.38–1.27(m,2H),1.23–1.15(m,2H).HRMS(ESI):calcd for C19H21Cl2N2O2[M+H]+379.0902,found 379.0978.13C NMR(75MHz,DMSO-d6)δ171.30,148.84,138.62,134.71,133.74,130.40,129.52,128.33,121.21,112.48,111.18,68.02,49.42,33.44,30.29.
实施例26
3',4'-二氯-4-(((1r,4r)-4-羟基环己基)氨基)-[1,1'-联苯]-3-甲酰胺(26)的制备
本品制备方法同实施例1,将碘苯更换为3,4-二氯碘苯(409mg,1.5mmol)。得淡黄色固体42mg,总产率11.1%。1H NMR(300MHz,DMSO-d6)δ8.34(d,J=7.7Hz,1H),8.05(s,1H),7.95(dd,J=8.5,2.1Hz,2H),7.68–7.60(m,3H),7.22(s,1H),6.80(d,J=8.9Hz,1H),4.54(d,J=4.2Hz,1H),3.52–3.44(m,1H),3.39–3.33(m,1H),2.00–1.96(m,2H),1.85–1.79(m,2H),1.40–1.20(m,4H).HRMS(ESI):calcd for C19H21Cl2N2O2[M+H]+379.0902,found 379.0970.
实施例27
3',5'-二氯-4-(((1r,4r)-4-羟基环己基)氨基)-[1,1'-联苯]-3-甲酰胺(27)的制备
本品制备方法同实施例1,将碘苯更换为3,5-二氯碘苯(409mg,1.5mmol)。得白色固体340mg,总产率89.9%。1H NMR(300MHz,DMSO-d6)δ8.41(d,J=7.7Hz,1H),8.09(s,1H),7.95(d,J=2.3Hz,1H),7.75(d,J=1.8Hz,2H),7.67(dd,J=8.8,2.2Hz,1H),7.41(t,J=1.9Hz,1H),7.26(s,1H),6.79(d,J=8.9Hz,1H),4.58(d,J=4.2Hz,1H),3.52–3.38(m,2H),2.00–1.96(m,2H),1.85–1.81(m,2H),1.40–1.15(m,4H).HRMS(ESI):calcd for C19H21Cl2N2O2[M+H]+379.0902,found 379.0981.
实施例28
2-(((1r,4r)-4-羟基环己基)氨基)-5-(萘-2-基)苯甲酰胺(28)的制备
本品制备方法同实施例1,将碘苯更换为2-碘萘(381mg,1.5mmol)。得淡黄色固体48mg,总产率:13.3%。1H NMR(300MHz,DMSO-d6)δ8.24(d,J=7.6Hz,1H),8.16(s,1H),8.10(s,1H),8.06(d,J=2.2Hz,1H),7.96–7.87(m,4H),7.75(dd,J=8.8,2.2Hz,1H),7.53–7.42(m,2H),7.24(s,1H),6.84(d,J=9.0Hz,1H),4.59(d,J=3.8Hz,1H),3.52–3.45(m,1H),3.42–3.37(m,1H),2.02–1.98(m,2H),1.86–1.82(m,2H),1.40–1.15(m,4H).HRMS(ESI):calcd for C23H25N2O2[M+H]+361.1838,found 361.1905.
实施例29
2-(((1r,4r)-4-羟基环己基)氨基)-5-(吡啶-2-基)苯甲酰胺(29)的制备
本品制备方法同实施例1,将碘苯更换为2-碘吡啶(308mg,1.5mmol)。得白色固体92mg,总产率:29.6%。1H NMR(300MHz,DMSO-d6)δ8.59–8.52(d,J=7.6Hz,1H),8.35(d,J=7.7Hz,1H),8.26(d,J=2.2Hz,1H),8.04(dd,J=8.9,2.1Hz,2H),7.92–7.89(m,1H),7.81–7.75(m,1H),7.21–7.17(m,2H),6.81(d,J=9.0Hz,1H),4.56(d,J=4.3Hz,1H),3.52–3.44(m,1H),3.41–3.34(m,1H),2.01–1.97(m,2H),1.85–1.81(m,2H),1.40–1.15(m,4H).HRMS(ESI):calcd for C18H22N3O2[M+H]+312.1634,found 312.1705.
实施例30
4-(((1r,4r)-4-羟基环己基)氨基)-4'-异丙基-[1,1'-联苯]-3-甲酰胺(30)的制备
采用方法1制备,具体操作如下。
(1)4-氟-4'-异丙基-[1,1'-联苯]-3-苯甲腈的制备
将5-氰基-2-氟苯硼酸频哪醇酯(1.98g,8.0mmol)和4-溴异丙苯(2.39g,12mmol)溶于20ml二氧六环中,加入Pd(PPh3)4(924mg,0.8mmol)和CsCO3(5.2g,16.0mmol),氮气条件下90℃反应过夜。反应液硅藻土抽滤,滤液旋干后石油醚柱层析得无色油状液体1.35g,产率:70.6%。1H NMR(300MHz,DMSO-d6)δ8.19(dd,J=6.2,2.5Hz,1H),8.07–8.02(m,1H),7.64–7.55(m,3H),7.34(d,J=8.2Hz,2H),2.98–2.86(m,1H),1.23(d,J=6.9Hz,6H).
(2)标题化合物的制备
将4-氟-4'-异丙基-[1,1'-联苯]-3-苯甲腈(200mg,0.84mmol)溶于5ml DMSO中,加入反式-4-氨基环己醇(434mg,3.76mmol)和DIEA(1.32ml,7.6mmol),120℃下(冷凝管)加热反应24h。反应液倒入20ml水中,用20ml乙酸乙酯萃取两次,合并乙酸乙酯层,无水硫酸钠干燥。旋干后溶于10ml乙醇中,加入2ml 1M/L NaOH溶液和2ml 30%H2O2,30℃下反应过夜。将反应液中的乙醇旋干后分散于20ml乙酸乙酯中,20ml水洗两次,20ml饱和氯化钠溶液洗1次,无水硫酸钠干燥。柱层析(石油醚:乙酸乙酯=1:1)得白色固体122mg,产率:41.2%。1H NMR(300MHz,DMSO-d6)δ8.14(d,J=7.7Hz,1H),8.01(s,1H),7.86(d,J=2.3Hz,1H),7.55(d,J=8.5Hz,3H),7.26(d,J=7.9Hz,2H),7.18(s,1H),6.78(d,J=8.8Hz,1H),4.60(d,J=4.1Hz,1H),3.52–3.44(m,1H),3.33–3.26(m,1H),2.94–2.85(m,1H),2.00–1.97(m,2H),1.85–1.81(m,2H),1.39–1.28(m,2H),1.22(d,J=6.9Hz,6H),1.17–1.14(m,2H).HRMS(ESI):calcd for C22H29N2O2[M+H]+353.2151,found 353.2227.13C NMR(75MHz,DMSO-d6)δ171.69,148.16,146.05,137.59,130.48,127.16,126.54,125.59,113.91,112.10,68.04,49.44,33.46,33.01,30.27,23.91.
实施例31
4-异丁氨基-4'-异丙基-[1,1'-联苯]-3-甲酰胺(31)的制备
本品制备方法同实施例30。由4-氟-4'-异丙基-[1,1'-联苯]-3-苯甲腈(150mg,0.63mmol)和异丁胺(461mg,6.3mmol)反应制备,得淡黄色固体107mg,产率:54.8%。1H NMR(300MHz,Chloroform-d)δ8.10(s,1H),7.60–7.56(m,2H),7.46(d,J=8.2Hz,2H),7.30(s,1H),6.81(d,J=8.6Hz,1H),5.74(s,2H),3.05(d,J=6.8Hz,2H),3.01–2.91(m,1H),2.08–1.95(m,1H),1.30(d,J=7.0Hz,6H),1.05(d,J=6.6Hz,6H).HRMS(ESI):calcd for C20H27N2O[M+H]+311.2045,found 311.2116.
实施例32
4-环戊氨基-4'-异丙基-[1,1'-联苯]-3-甲酰胺(32)的制备
本品制备方法同实施例30。由4-氟-4'-异丙基-[1,1'-联苯]-3-苯甲腈(150mg,0.63mmol)和环戊胺(240mg,2.82mmol)反应制备,得白色固体108mg,产率:53.2%。1H NMR(300MHz,Chloroform-d)δ7.88(s,1H),7.50–7.47(m,2H),7.36(d,J=8.2Hz,2H),7.21(s,1H),6.75(d,J=8.6Hz,1H),5.62(s,2H),3.83–3.75(m,1H),2.91–2.82(m,1H),2.02–1.92(m,2H),1.76–1.66(m,2H),1.60–1.48(m,4H),1.21(d,J=6.9Hz,6H).HRMS(ESI):calcd for C21H27N2O[M+H]+323.2045,found 323.2122.
实施例33
4-环己氨基-4'-异丙基-[1,1'-联苯]-3-甲酰胺(33)的制备
本品制备方法同实施例30。由4-氟-4'-异丙基-[1,1'-联苯]-3-苯甲腈(150mg,0.63mmol)和环己胺(279mg,2.82mmol)反应制备,得淡黄色固体75mg,产率:35.4%。1HNMR(300MHz,Chloroform-d)δ7.78(d,J=7.6Hz,1H),7.49–7.44(m,2H),7.36(d,J=8.2Hz,2H),7.21(s,1H),6.71(d,J=8.7Hz,1H),5.58(s,2H),3.38–3.28(m,1H),2.93–2.80(m,1H),1.98–1.94(m,2H),1.74–1.69(m,2H),1.57–1.49(m,2H),1.39–1.26(m,4H),1.21(d,J=6.9Hz,6H).HRMS(ESI):calcd for C22H29N2O[M+H]+337.2202,found 337.2279.
实施例34
(S)-4'-异丙基-4-(四氢呋喃-3-氨基)-[1,1'-联苯]-3-甲酰胺(34)的制备
本品制备方法同实施例30。由4-氟-4'-异丙基-[1,1'-联苯]-3-苯甲腈(150mg,0.63mmol)和(S)-3-氨基四氢呋喃盐酸盐(348mg,2.82mmol)反应制备,得白色固体10mg,产率:4.9%。1H NMR(300MHz,DMSO-d6)δ8.23(d,J=6.6Hz,1H),7.96(s,1H),7.80(d,J=2.2Hz,1H),7.50–7.47(m,3H),7.19–7.14(m,3H),6.68(d,J=8.7Hz,1H),4.08–4.00(m,1H),3.85–3.80(m,1H),3.77–3.62(m,2H),3.43(dd,J=8.8,3.2Hz,1H),2.85–2.76(m,1H),2.23–2.11(m,1H),1.69–1.59(m,1H),1.13(d,J=6.9Hz,6H).HRMS(ESI):calcd for C20H25N2O2[M+H]+325.1838,found 325.1915.
实施例35
4'-异丙基-4-(四氢-2H-吡喃-4-氨基)-[1,1'-联苯]-3-甲酰胺(35)的制备
本品制备方法同实施例30。由4-氟-4'-异丙基-[1,1'-联苯]-3-苯甲腈(150mg,0.63mmol)和4-氨基四氢吡喃(285mg,2.82mmol)反应制备,得白色固体83mg,产率:40.0%。1H NMR(300MHz,DMSO-d6)δ8.16(d,J=7.6Hz,1H),7.94(s,1H),7.79(d,J=2.2Hz,1H),7.47(d,J=7.8Hz,3H),7.17(d,J=8.3Hz,2H),7.11(s,1H),6.75(d,J=8.8Hz,1H),3.77–3.73(m,2H),3.58–3.49(m,1H),3.38(t,J=10.3Hz,2H),2.85–2.76(m,1H),1.87–1.83(m,2H),1.35–1.22(m,2H),1.13(d,J=6.9Hz,6H).HRMS(ESI):calcd for C21H27N2O2[M+H]+339.1994,found 339.2073.13C NMR(75MHz,DMSO-d6)δ171.66,147.73,146.15,137.52,130.47,127.21,126.55,125.95,125.64,114.18,112.21,65.57,47.04,33.02,32.82,23.90.
实施例36
4'-异丙基-4-((2-甲氧基乙基)氨基)-[1,1'-联苯]-3-甲酰胺(36)的制备
本品制备方法同实施例30。由4-氟-4'-异丙基-[1,1'-联苯]-3-苯甲腈(150mg,0.63mmol)和2-甲氧基乙胺(212mg,2.82mmol)反应制备,得淡黄色固体118mg,产率:60.0%。1H NMR(300MHz,Chloroform-d)δ7.62–7.58(m,2H),7.47(d,J=8.9Hz,2H),7.31(s,1H),6.88(d,J=8.6Hz,1H),5.80(s,2H),3.68(t,J=5.6Hz,2H),3.46–3.42(m,5H),3.00–2.91(m,1H),1.30(d,J=6.9Hz,6H).HRMS(ESI):calcd for C19H25N2O2[M+H]+313.1838,found 313.1913.13C NMR(75MHz,DMSO-d6)δ171.98,149.28,146.63,138.03,130.93,127.50,127.06,126.48,126.12,114.94,112.06,70.99,58.54,42.42,33.51,24.40.
实施例37
4-(1-乙酰基哌啶-4-氨基)-4'-异丙基-[1,1'-联苯]-3-甲酰胺(37)的制备
本品制备方法同实施例30。由4-氟-4'-异丙基-[1,1'-联苯]-3-苯甲腈(150mg,0.63mmol)和1-乙酰基-4-氨基哌啶(401mg,2.82mmol)反应制备,得淡黄色固体50mg,产率:20.9%。1H NMR(300MHz,DMSO-d6)δ8.18(d,J=7.9Hz,1H),7.96(s,1H),7.80(d,J=2.3Hz,1H),7.47(d,J=8.2Hz,3H),7.17(d,J=8.0Hz,2H),7.12(s,1H),6.77(d,J=8.9Hz,1H),4.04–3.99(m,1H),3.66–3.56(m,2H),3.16(t,J=11.1Hz,1H),2.89–2.76(m,2H),1.92(s,3H),1.90–1.82(m,2H),1.33–1.20(m,2H),1.13(d,J=6.8Hz,6H).HRMS(ESI):calcd for C23H30N3O2[M+H]+380.226,found 380.2335.13C NMR(75MHz,DMSO-d6)δ171.65,168.01,147.79,146.17,137.51,130.50,127.21,126.56,126.03,125.66,114.22,112.27,47.78,44.21,33.01,32.11,31.45,23.90,21.28.
实施例38
4-(1-叔丁氧羰基-4-氨基)-4'-异丙基-[1,1'-联苯]-3-甲酰胺(38)的制备
本品制备方法同实施例30。由4-氟-4'-异丙基-[1,1'-联苯]-3-苯甲腈(1.67g,7.0mmol)和1-Boc-4-氨基哌啶(6.3g,31.5mmol)反应制备,得白色固体2.2g,产率:71.9%。1H NMR(300MHz,DMSO-d6)δ8.26(d,J=7.8Hz,1H),8.02(s,1H),7.89(d,J=2.2Hz,1H),7.56(d,J=8.1Hz,3H),7.27(d,J=7.9Hz,2H),7.18(s,1H),6.84(d,J=8.7Hz,1H),3.81–3.77(m,2H),3.63–3.58(m,1H),3.08–2.99(m,2H),2.95–2.86(m,1H),1.95–1.91(m,2H),1.41(s,9H),1.30–1.26(m,2H),1.22(d,J=7.0Hz,6H).HRMS(ESI):calcd for C26H36N3O3[M+H]+438.2678,found 438.2751.
实施例39
4'-异丙基-4-(哌啶-4-氨基)-[1,1'-联苯]-3-甲酰胺(39)的制备
本品由化合物38脱去Boc保护基得到,具体操作如下。将38(2.2g,5.26mmol)溶于120ml二氯甲烷中,加入三氟乙酸(3.9ml,52.6mmol),室温下反应48h。将反应液旋干后分散于50ml水中,1M/L NaOH溶液调节PH至9–10,产物析出,抽滤得到淡黄色固体1.3g,产率73.4%:。1H NMR(300MHz,DMSO-d6)δ8.13(d,J=7.9Hz,1H),7.93(s,1H),7.78(d,J=2.2Hz,1H),7.48–7.45(m,3H),7.17(d,J=8.0Hz,2H),7.09(s,1H),6.70(d,J=8.8Hz,1H),3.44–3.37(m,2H),2.88–2.75(m,3H),2.57–2.50(t,J=11.2Hz,2H),1.85–1.81(m,2H),1.24–1.17(m,2H),1.13(d,J=6.9Hz,6H).HRMS(ESI):calcd for C21H28N3O[M+H]+338.2154,found 338.2232.
实施例40
4'-异丙基-4-(哌啶-4-氨基)-[1,1'-联苯]-3-甲酰胺(40)的制备
本品通过以下反应步骤得到。将39(120mg,0.36mmol)溶于5ml DMF中,加入Boc-甘氨酸(95mg,0.54mmol),BOP(239mg,0.54mmol),DIEA(189μL,1.08mmol),室温下反应5h。反应液旋干后分散于20ml二氯甲烷中,用20ml水洗2次,20ml饱和氯化钠溶液洗1次,无水硫酸钠干燥。柱层析(石油醚:乙酸乙酯=5:1~2:1)得白色固体160mg。将以上固体溶于10ml二氯甲烷中,加入三氟乙酸(238μL,3.2mmol),室温下反应36h。反应液旋干后分散于20ml饱和Na2CO3溶液和20ml乙酸乙酯中,乙酸乙酯层用20ml水洗3次,无水硫酸钠干燥。柱层析(二氯甲烷:甲醇=50:1~30:1),得白色固体50mg,产率:35.2%。1H NMR(300MHz,DMSO-d6)δ8.28(d,J=7.2Hz,1H),8.05(s,1H),7.89(s,1H),7.57(d,J=8.0Hz,3H),7.27(d,J=8.2Hz,3H),6.87(d,J=8.8Hz,1H),4.17–4.12(m,1H),3.69–3.64(m,2H),3.50–3.44(m,2H),3.23–3.15(m,1H),3.03–2.96(m,1H),2.94–2.85(m,1H),1.99–1.96(m,2H),1.41–1.31(m,2H),1.22(d,J=6.7Hz,6H).HRMS(ESI):calcd for C23H31N4O2[M+H]+395.2369,found 395.2446.
实施例41
4-(1-(2-氨基丙酰基)哌啶-4-氨基)-4'-异丙基-[1,1'-联苯]-3-甲酰胺(41)的制备
本品由39(120mg,0.36mmol)和Boc-DL-α-丙氨酸(102mg,0.54mmol)按照实施例41的方法制备,得淡黄色固体63mg,产率42.9%:。1H NMR(300MHz,Chloroform-d)δ7.94(s,1H),7.53(s,1H),7.47(d,J=8.8Hz,1H),7.36(d,J=7.9Hz,2H),7.21–7.18(m,2H),6.70(d,J=8.8Hz,1H),5.81(brs,2H),4.17(brs,1H),3.81–3.60(m,3H),3.22–3.07(m,2H),2.90–2.81(m,1H),2.19(brs,2H),2.00–1.97(m,2H),1.50(brs,2H),1.21–1.19(m,9H).HRMS(ESI):calcd for C24H33N4O2[M+H]+409.2525,found 409.2603.
实施例42
5-(1-己炔-1-基)-2-(((1r,4r)-4-羟基环己基)氨基)苯甲酰胺(42)的制备
本品采用方法3制备,具体操作如下。
(1)2-氟-5-(1-己炔-1-基)苯甲腈的制备
将2-氟-5-碘苯腈(370mg,1.5mmol)和己炔(246mg,3.0mmol)溶于5ml三乙胺中,加入(PPh3)2PdCl2(53mg,0.075mmol)和CuI(28mg,0.15mmol),氮气保护下室温反应过夜。反应液硅藻土抽滤,滤液旋干后分散于30ml乙酸乙酯中,用30ml水和饱和氯化钠溶液分别洗两次,无水硫酸钠干燥。石油醚柱层析得淡黄色油状液体270mg,产率:89.5%。1H NMR(300MHz,Chloroform-d)δ7.66–7.58(m,2H),7.15(t,J=8.7Hz,1H),2.41(t,J=6.9Hz,2H),1.63–1.46(m,4H),0.96(t,J=7.2Hz,3H).
(2)标题化合物的制备
将上述所得淡黄色油状液体(270mg,1.34mmol)溶于8ml DMSO中,加入反式-4-氨基环己醇(463mg,4.02mmol)和DIEA(1.40ml,8.04mmol),120℃下(冷凝管)加热反应24h。反应液倒入30ml水中,用30ml乙酸乙酯萃取两次,合并乙酸乙酯层,无水硫酸钠干燥。旋干后溶于20ml乙醇中,加入2ml 2M/L NaOH溶液和2ml 30%H2O2,30℃下反应48h。将反应液中的乙醇旋干后分散于60ml乙酸乙酯中,60ml水洗两次,60ml饱和氯化钠溶液洗1次,无水硫酸钠干燥。旋干后用乙酸乙酯打浆得到白色固体166mg,总产率35.2%。1H NMR(300MHz,DMSO-d6)δ8.30(d,J=7.4Hz,1H),7.88(s,1H),7.65(s,1H),7.23(d,J=8.5Hz,1H),7.12(s,1H),6.66(d,J=8.7Hz,1H),4.54(d,J=3.7Hz,1H),3.52–3.44(m,1H),3.29–3.22(m,1H),2.38(t,J=6.4Hz,2H),1.97–1.94(m,2H),1.84–1.80(m,2H),1.56–1.38(m,4H),1.34–1.13(m,4H),0.92(t,J=6.8Hz,3H).HRMS(ESI):calcd for C19H27N2O2[M+H]+315.2067,found 315.2068.
实施例43
2-(((1r,4r)-4-羟基环己基)氨基)-5-(5-甲基-1-己炔-1-基)-苯甲酰胺(43)的制备
本品制备方法同实施例42,将己炔更换为5-甲基-1-己炔(289mg,3.0mmol)。得白色固体240mg,总产率48.7%。1H NMR(300MHz,DMSO-d6)δ8.29(d,J=7.5Hz,1H),7.88(s,1H),7.64(s,1H),7.23(d,J=8.6Hz,1H),7.12(s,1H),6.66(d,J=8.8Hz,1H),4.54(s,1H),3.53–3.42(m,1H),3.31–3.21(m,1H),2.38(t,J=6.9Hz,2H),1.97–1.93(m,2H),1.84–1.80(m,2H),1.76–1.65(m,1H),1.43(q,J=7.2Hz,2H),1.34–1.13(m,4H),0.91(d,J=6.6Hz,6H).HRMS(ESI):calcd for C20H28N2O2[M+H]+329.2224,found 329.2223.
实施例44
5-(3,3-二甲基-1-丁炔-1-基)-2-(((1r,4r)-4-羟基环己基)氨基)苯甲酰胺(44)的制备
本品制备方法同实施例42,将己炔更换为3,3-二甲基-1-丁炔(246mg,3.0mmol)。得白色固体272mg,总产率57.7%。1H NMR(300MHz,DMSO-d6)δ8.27(d,J=7.5Hz,1H),7.90(s,1H),7.60(s,1H),7.20(d,J=8.6Hz,1H),7.11(s,1H),6.65(d,J=8.8Hz,1H),4.55(d,J=4.0Hz,1H),3.51–3.44(m,1H),3.29–3.21(m,1H),1.97–1.93(m,2H),1.83–1.80(m,2H),1.38–1.13(m,13H).HRMS(ESI):calcd for C19H27N2O2[M+H]+315.2067,found 315.2073.
- 还没有人留言评论。精彩留言会获得点赞!