Ovatodiolide衍生物及其盐,制备方法及其在制备抗癌药物中的用途与流程
本本发明提供了一种ovatodiolide衍生物及其盐,并涉及ovatodiolide衍生物及其盐的制备方法,及其在制备治疗癌症药物中的用途,本发明属于药物技术领域。
背景技术:
二萜内酯化合物ovatodiolide是从防风草中提取的化合物。防风草作为传统中药,被用于祛风解表,解毒除湿,强壮筋骨,治感冒身热等。近期研究表明,ovatodiolide可在体外抑制肝癌,肾癌,口腔癌,鼻咽癌,结肠癌细胞,胰腺癌等癌细胞的生长,同时其可选择性抑制包括造血干细胞,乳腺癌干细胞等干细胞的生长。但ovatodiolide水溶性差,不利于药物被机体吸收,进入体内易被代谢分解,生物利用度差。对于此类化合物的修饰及其成盐处理,可能有利于增强其成药性。本发明在已有发现的基础上,合成了ovatodiolide衍生物,其药物组合物,及其在制备药物中的用途,特别是在制备治疗癌症的药物中的用途。
目前未见关于式(ⅰ)化合物,其药物组合物,及其制备方法,及在制备药物中的用途,特别是在制备治疗癌症的药物中的用途的报道。
技术实现要素:
本发明提供了式(i)化合物,其药物组合物及其制备方法和用途,特别是式(i)化合物用于治疗癌症的用途。
为了实现本发明的上述目的,本发明提供如下的技术方案:
一种如式(i)所示的ovatodiolide衍生物及其盐,
式(i)中r1为碳原子,氧原子;r2为碳原子,氧原子;r3为氢原子,羟基;r4和r5可以相同或不同,分别为氢、烷基、环烷基、羟基取代烷基、烯基、炔基、芳基、烷基芳基、芳基烷基、芳基烯基、芳基炔基、杂环基、三氟甲基、多氟取代烷基、腈基、腈基甲基、酰基、氨基甲酰基、磺酰基、磺酰胺基或芳氧烷基;r4、r5和n原子形成环状结构,环优选为3–9元环,环状结构上可以被一个或多个取代基取代,包括氢、烷基、环烷基、烯基、炔基、芳基、烷基芳基、芳基烷基、芳基烯基、芳基炔基或杂环基;
其与无机酸或有机酸形成的在药学上可接受的盐,包括与r6z形成的季铵盐,包括氢氟酸、盐酸、氢溴酸、氢碘酸、硫酸、磷酸、硝酸、亚磷酸、亚硫酸、碳酸、硼酸、磷钼酸、亚硒酸、甲基磺酸、取代甲基磺酸、苯基磺酸、取代苯基磺酸、富马酸、柠檬酸、马来酸、酒石酸、草酸、d-苹果酸、l-苹果酸、dl-苹果酸、l-乳酸、d-乳酸、dl-乳酸、甲酸、取代甲酸、乙酸、丙酸、丁酸、戊酸、油酸、月桂酸、对甲基苯磺酸、1-萘磺酸、2-萘磺酸、酞酸、丙二酸、丁二酸、乙醇酸、硫醇酸、甘氨酸、肌氨酸、磺酸、烟酸、甲基吡啶酸、异烟酸、二氯乙酸、苯甲酸、取代苯甲酸;r6为烃基、环烷基、羟基取代烷基、烯基、炔基、芳基、杂环基、芳基取代烷基、芳基烯基、芳基炔基、氰基取代甲基、烷氧基取代烷基或芳氧取代烷基;z为氟、氯、溴、碘、对甲基磺酸基、甲基磺酸基、苯基磺酸基、取代苯基磺酸基、三氟甲基磺酸基。
一种制备式(i)所示ovatodiolide衍生物及其盐的方法,其特征在于以式(ii)所示的化合物为原料,与(iii)所示的化合物反应得式(i)所示的化合物;
一种如式(i)所示的ovatodiolide衍生物及其盐,在制备治疗癌症或治疗癌症的辅助药物中的用途,其中癌症为白血病、乳腺癌、前列腺癌、鼻咽癌、大肠癌、肺癌、肝癌、食道癌、胃癌、肠道癌、肾癌、口腔癌、何杰金淋巴癌、胰腺癌、直肠结肠癌、子宫颈癌、非何杰金淋巴癌、神经胶质瘤、黑瘤、膀胱癌、卵巢癌、甲状腺癌或卡波西肉瘤。
一种用于治疗癌症的药物组合物,其中含有有效量的式(i)ovatodiolide衍生物及其盐在药学上可接受的载体或与其他抗癌药物的组合物。
本发明化合物用作药物时,可以直接使用,或者以药物组合物的形式使用。该药物组合物含有0.1–99%,优选为0.5–90%的本发明化合物,其余为药物学上可接受的,对人和动物无毒和惰性的可药用载体和/或赋形剂或与其他抗癌药物联合用药。本发明的组合物可以制备成注射液、片剂和胶囊等。
所述的药用载体或赋形剂是一种或多种固体、半固体和液体稀释剂、填料以及药物制品辅剂。将本发明的药物组合物以单位体重服用量的形式使用。本发明的药物可经注射和口服两种形式给药,注射如静脉注射和肌肉注射,口服的剂型可以是片剂和胶囊剂。
附图说明
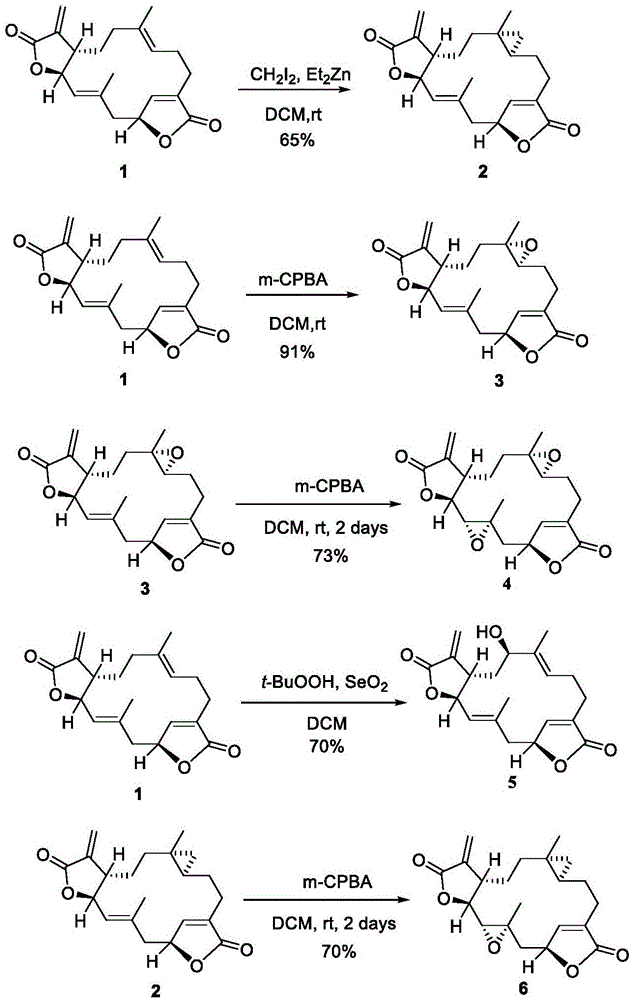
图1.化合物2-6的合成
图2.化合物7-12的合成
图3.化合物13-18的合成
图4.化合物19-23的合成
图5.化合物24-28的合成
图6.化合物29-34的合成
具体实施方式
为了理解本发明,下面以实施例进一步说明本发明,但不意于限制本发明的保护范围。
实施例1:ovatodiolide衍生物及其盐的合成
具体合成路线见图1,图2,图3,图4,图5,图6,具体步骤如下:
0℃条件下,将et2zn(1.0minhexane,1.52mmol,1.52ml)溶于无水dcm(3.0ml)中,然后将ch2i2(260.9ul,3.04mmol)缓慢滴加到体系中,然后搅拌30min,将化合物1(250mg,0.76mmol,1.0eq)的dcm溶液(3.0ml)缓慢滴加到体系中。室温下搅拌过夜,向体系中加入饱和nh4cl(5.0ml),分离有机相。将水相用ch2cl2(3×5ml)萃取,并将合并的有机相用饱和nacl(5ml)洗涤。有机相无水na2so4干燥,过滤并在减压下浓缩,然后通过柱色谱法(80%etoac/hexane)纯化,得化合物2(162mg,65%,dr=85:15白色固体)。1hnmr(400mhz,cdcl3)δ6.99(s,1h),6.21(s,1h),5.61(s,1h),5.32–5.03(m,2h),4.78(d,j=10.2hz,1h),2.94(dd,j=14.6,3.2hz,1h),2.67(t,j=9.5hz,1h),2.60–2.42(m,1h),2.29(ddd,j=19.0,18.5,7.7hz,2h),2.18–2.07(m,1h),1.98(dt,j=14.4,4.2hz,1h),1.66(s,3h),1.64–1.52(m,2h),1.34–1.25(m,1h),1.04(s,3h),0.68–0.54(m,1h),0.32(dd,j=8.2,4.3hz,1h),0.14–0.03(m,1h),-0.02(t,j=4.6hz,1h);13cnmr(101mhz,cdcl3)δ173.2,170.3,146.6,139.5,135.0,132.1,128.4,122.6,79.2,78.0,43.8,39.8,37.1,32.2,26.1,25.6,21.3,19.9,19.8,17.0,16.9;ms-esi(m/z):calcd.forc21h26o4na[m+na]+:365.1729,found365.1728.
室温条件下,将1(1.2g,3.6mmol,1.0eq)溶于dcm(37ml)中,然后将m-cpba(1.1g,5.4mmol,85%,1.5eq)加入到体系中。室温下搅拌1h,将混合物倒入饱和na2s2o3溶液(10ml)中,分离有机相。将水相用ch2cl2(3×20ml)萃取,并将合并的有机相用饱和nahco3(2×20ml)洗涤,饱和nacl(15ml)洗涤。有机相无水na2so4干燥,过滤并在减压下浓缩,然后通过柱色谱法(80%etoac/hexane)纯化,得化合物3(1.0g,91%,白色固体)。[α]26d=-23.3(c=1.0,chcl3);ir(neat)2926,2855,1754,1661,1435,1389,1329,1266,1237,1206,1116,971,944,797,760cm-1;1hnmr(400mhz,cdcl3)δ7.10(s,1h),6.26(d,j=1.6hz,1h),5.65(d,j=1.3hz,1h),5.21(d,j=10.5hz,1h),5.18(d,j=1.2hz,1h),4.81(dd,j=10.2,1.9hz,1h),2.96(dd,j=14.7,3.3hz,1h),2.59(d,j=4.7hz,1h),2.57(d,j=3.4hz,1h),2.48–2.42(m,1h),2.42–2.34(m,1h),2.31(dd,j=10.6,3.7hz,1h),2.29–2.26(m,1h),2.13(ddd,j=14.3,5.3,2.8hz,1h),1.71(s,3h),1.70–1.63(m,2h),1.58–1.45(m,1h),1.29(s,3h),1.21–1.09(m,1h);13cnmr(101mhz,cdcl3)δ172.9,169.8,147.8,139.1,134.1,132.5,128.5,123.4,79.4,77.8,60.7,58.6,44.0,39.9,35.6,31.6,25.1,23.4,19.9,16.4;ms-esi(m/z):calcd.forc20h24o5na[m+na]+:367.1521,found367.1520.
室温条件下,将化合物3(10.0g,30.5mmol,1.0equiv)溶于二氯甲烷(300ml),然后将m-cpba(24.7g,121.8mmol,85%,4.0eq)加入到体系中,室温条件下继续搅拌2天,体系析出大量白色固体,过滤,滤液用饱和na2s2o3(100ml)淬灭反应,体系用dcm(3x150ml)萃取,合并有机相,饱和nacl(30ml)洗涤,无水na2so4干燥,过滤并在减压下浓缩,然后通过柱色谱法(100%etoac/hexane),得化合物4(8.0g,73%,白色固体)。[α]22d=-22.5(c=0.52,chcl3);ir(neat)2930,1754,1643,1435,1390,1326,1263,1201,1117,1082,1040,986,957,838,639cm-1;1hnmr(400mhz,cdcl3)δ7.34(s,1h),6.29(s,1h),5.79(s,1h),5.17(s,1h),3.87(d,j=9.3hz,1h),2.84(d,j=9.3hz,1h),2.57(dd,j=12.7,7.3hz,3h),2.48(dt,j=8.2,4.3hz,2h),2.17–1.90(m,4h),1.62–1.35(m,3h),1.34(s,3h),1.32(s,3h);13cnmr(101mhz,cdcl3)δ173.02,169.35,148.56,137.60,133.71,124.49,81.58,78.74,77.48,77.16,76.84,61.97,60.17,58.59,55.99,41.65,41.05,33.01,29.83,25.96,21.87,19.77,17.29;hrms-esi(m/z):calcd.forc20h24o6na[m+na]+:383.1471,found383.1470.
室温条件下,将化合物1(100mg,0.304mmol,1.0eq)溶于无水dcm(2.0ml),然后将seo2(18.9mg,0.17mmol,0.56eq)和t-buooh(0.104ml,1.03mmol,3.4eq)加入到体系中,室温条件下继续搅拌过夜,向体系中加入饱和na2s2o4溶液和饱和nahco3溶液(5ml:5ml),搅拌10min,体系用dcm(3x15ml)萃取,有机相饱和nacl(5ml)洗涤,无水na2so4干燥,减压真空浓缩,柱层析(50%etoac/hexane),得化合物5(70mg,70%,白色固体)。[α]22d=-23.1(c=0.2,chcl3);1hnmr(400mhz,cdcl3)δ7.01(s,1h),6.25(d,j=1.8hz,1h),5.63(d,j=1.6hz,1h),5.09(d,j=9.6hz,3h),4.88(dd,j=10.3,1.2hz,1h),4.04(dd,j=11.2,4.0hz,1h),3.02–2.90(m,1h),2.88(d,j=3.7hz,1h),2.62–2.52(m,1h),2.51–2.40(m,1h),2.27(dd,j=14.4,3.6hz,2h),2.22–2.13(m,1h),1.89(td,j=13.3,4.0hz,1h),1.80(s,1h),1.72(d,j=1.2hz,4h),1.64(s,3h);13cnmr(101mhz,cdcl3)δ172.9,170.1,147.8,138.9,137.2,134.2,131.3,129.2,128.0,123.7,78.9,78.5,75.8,42.1,41.0,40.3,25.0,23.3,19.4,10.3.hrms-esi(m/z):calcd.forc20h24o5na[m+na]+:367.1521,found367.1520.
室温条件下,将化合物2(1.5g,4.38mmol,1.0equiv)溶于二氯甲烷(30ml),然后将m-cpba(1.78g,8.76mmol,85%,2.0eq)加入到体系中,室温条件下继续搅拌2天,体系析出大量白色固体,过滤,滤液用饱和na2s2o3(30ml)淬灭反应,体系用dcm(3x50ml)萃取,合并有机相,饱和nacl(20ml)洗涤,无水na2so4干燥,过滤并在减压下浓缩,然后通过柱色谱法(60%etoac/hexane),得化合物6(1.2g,70%,白色固体)。[α]18d=-15.9(c=1.0,chcl3);1hnmr(400mhz,cdcl3)δ7.22(s,1h),6.26(s,1h),5.77(s,1h),5.14(d,j=1.5hz,1h),3.85(d,j=9.4hz,1h),2.85(d,j=9.5hz,1h),2.79–2.62(m,1h),2.61–2.39(m,3h),2.06(dd,j=15.4,3.4hz,1h),1.99–1.87(m,2h),1.78(dd,j=9.1,5.1hz,1h),1.71–1.41(m,3h),1.32(s,3h),1.08(s,3h),0.66–0.50(m,1h),0.42–0.27(m,1h),0.08–-0.05(m,1h);13cnmr(101mhz,cdcl3)δ173.0,169.7,147.3,138.5,134.6,123.8,81.0,78.6,61.2,56.5,41.6,40.5,36.7,32.2,25.8,25.3,21.6,19.9,18.9,16.9,16.8;hrms-esi(m/z):calcd.forc21h26o5na[m+na]+:381.1678,found381.1677.
室温条件下,将化合物1(1.0g,3.04mmol,1.0eq)溶于无水thf(15.0ml),然后将二甲胺溶液(2.0minthf,7.1ml,15.2mmol,5.0eq)加入到体系中,室温条件下继续搅拌4h,体系直接浓缩,柱层析(50%hexane/etoac),得化合物7(0.5g,53%,白色固体)。1hnmr(400mhz,cdcl3)δ6.98(d,j=1.2hz,1h),5.35(d,j=10.7hz,1h),5.11(s,1h),4.85(dd,j=6.8,5.3hz,1h),4.80(d,j=10.6hz,1h),2.99(dd,j=16.4,8.4hz,1h),2.94–2.77(m,2h),2.53(d,j=1.9hz,2h),2.51(s,1h),2.46–2.32(m,2h),2.23(s,6h),2.19–1.88(m,5h),1.75(d,j=1.4hz,3h),1.58(s,3h),1.21–1.10(m,1h);13cnmr(101mhz,cdcl3)δ178.1,173.1,147.0,136.0,134.9,131.4,127.6,124.4,78.8,78.4,55.0,45.2,40.4,40.3,39.7,36.4,25.2,23.8,23.4,19.5,14.8;hrms-esi(m/z):calcd.forc22h32o4n[m+h]+:374.2331,found374.2330.
室温条件下,将化合物1(1.0g,3.04mmol,1.0eq)溶于无水thf(15.0ml),然后将吗啉(15.2ml,15.2mmol,5.0eq)加入到体系中,室温条件下继续搅拌4h,体系直接浓缩,柱层析(50%hexane/etoac),得化合物8(1.01g,80%,白色固体)。[α]18d=-33.1(c=0.32,chcl3);1hnmr(400mhz,cdcl3)δ6.99(s,1h),5.32(dd,j=27.1,9.2hz,1h),5.12(s,1h),4.92–4.76(m,2h),3.83–3.61(m,4h),3.20–3.02(m,1h),3.00–2.74(m,2h),2.71–2.46(m,5h),2.46–2.19(m,5h),2.19–1.84(m,4h),1.76(s,3h),1.68(s,3h),1.23–1.12(m,1h);13cnmr(101mhz,cdcl3)δ178.0,173.3,146.9,135.8,134.9,131.0,127.5,124.5,78.9,78.5,67.2,54.0,40.2,38.6,36.2,25.0,23.8,23.2,19.5,15.3.hrms-esi(m/z):calcd.forc24h34o5n[m+h]+:416.2437,found416.2435.
室温条件下,将化合物1(1.0g,3.04mmol,1.0eq)溶于无水thf(15.0ml),然后将n-甲基哌嗪(1.69ml,15.2mmol,5.0eq)加入到体系中,室温条件下继续搅拌4h,体系直接浓缩,柱层析(1.2%meoh/dcm-2.5%meoh/dcm),得化合物9(1.1g,89%,白色固体)。[α]23d=-44.3(c=0.56,chcl3);ir(neat)2929,2874,2842,2798,1753,1666,1450,1091,980,960,883,861cm-1;1hnmr(400mhz,cdcl3)δ6.98(s,1h),5.35(d,j=10.7hz,1h),5.11(s,1h),4.84(dd,j=17.8,8.8hz,2h),3.07(s,1h),2.90(dd,j=14.3,3.5hz,1h),2.85–2.75(m,1h),2.63(dd,j=12.6,5.0hz,2h),2.59–2.47(m,4h),2.47–2.36(m,4h),2.29(s,3h),2.27–2.18(m,3h),δ2.17–2.07(m,2h),2.07–1.87(m,3h),1.75(s,3h),1.65(s,3h),1.22–1.10(m,1h);13cnmr(101mhz,cdcl3)δ178.1,173.2,146.9,135.9,134.9,131.0,127.6,124.4,78.9,78.4,55.3,53.6,46.2,40.3,40.2,38.9,36.2,25.1,23.7,23.2,19.5,15.5;hrms-esi(m/z):calcd.forc25h37o4n2[m+h]+:429.2753,found429.2750.
室温条件下,将化合物3(300mg,0.87mmol,1.0eq)溶于无水thf(10ml),然后将n-甲基哌嗪(0.48ml,4.36mmol,5.0eq)加入到体系中,室温条件下继续搅拌过夜,体系直接浓缩,然后通过柱色谱法(2%meoh/dcm),得化合物10(309mg,80%,白色固体)。1hnmr(400mhz,cdcl3)δ7.07(s,1h),5.39(d,j=10.7hz,1h),5.18(s,1h),4.78(d,j=10.7hz,1h),3.23–3.08(m,1h),2.94(dd,j=14.6,3.3hz,1h),2.89–2.42(m,8h),2.40–2.30(m,2h),2.29(s,3h),2.25(dd,j=10.2,4.5hz,3h),2.19–2.11(m,1h),2.10–1.70(m,4h),1.68(s,3h),1.33(s,3h),1.30–1.15(m,2h),1.03(td,j=13.9,1.9hz,1h).13cnmr(101mhz,cdcl3)δ177.1,173.0,147.4,134.0,131.3,126.9,79.2,78.4,61.3,58.8,55.2,53.3,45.9,41.4,39.6,38.9,35.6,24.9,23.3,22.7,19.7,16.1;hrms-esi(m/z):calcd.forc25h37o5n2[m+h]+:445.2702,found445.2699.
室温条件下,将化合物3(300mg,0.87mmol,1.0equiv)溶于无水thf(10ml),然后将吗啉(0.38ml,4.36mmol,5.0eq)加入到体系中,室温条件下继续搅拌过夜,体系直接浓缩,然后通过柱色谱法(1%meoh/dcm),得化合物11(311mg,83%,白色固体)。1hnmr(400mhz,cdcl3)δ7.08(d,j=1.2hz,1h),5.41(d,j=10.7hz,1h),5.19(s,1h),4.81(d,j=10.7hz,1h),3.95–3.54(m,4h),3.26–3.08(m,1h),2.96(dd,j=14.6,3.1hz,1h),2.77–2.60(m,3h),2.59–2.46(m,3h),2.37–2.30(m,2h),2.30–2.22(m,2h),2.22–2.14(m,1h),1.99–1.75(m,3h),1.70(d,j=1.3hz,3h),1.36(s,3h),1.33–1.22(m,2h),1.11–1.00(m,1h).13cnmr(101mhz,cdcl3)δ177.1,173.0,147.4,134.1,131.3,127.0,79.24,78.5,67.2,61.4,58.9,53.8,41.5,39.6,38.6,35.6,24.9,23.3,22.8,19.7,16.1;hrms-esi(m/z):calcd.forc24h34o6n[m+h]+:432.2386,found432.2396.
室温条件下,将化合物4(7.47g,20.7mmol,1.0equiv)溶于无水thf(100ml),然后将n-甲基哌嗪(11.5ml,103.6mmol,5.0eq)加入到体系中,室温条件下继续搅拌过夜,体系直接浓缩,然后通过柱色谱法(5%meoh/dcm-6.7%meoh/dcm),得化合物12(5.0g,52%,白色固体)。
[α]22d=-64.8(c=0.53,chcl3);ir(neat)3084,2979,2937,1761,1740,1640,1461,1288,1167,1072,1038,1012,996,903cm-1;1hnmr(400mhz,cdcl3)δ7.20(s,1h),5.14(s,1h),3.80(d,j=10.0hz,1h),3.12(ddd,j=12.4,7.2,5.5hz,1h),3.02(d,j=9.9hz,1h),2.71–2.51(m,6h),2.50–2.31(m,6h),2.28(s,3h),2.12(dd,j=15.4,3.0hz,1h),2.00(t,j=12.7hz,4h),1.79–1.53(m,1h),1.34(s,3h),1.12(d,j=7.9hz,3h),1.12–1.01(m,1h),0.45(t,j=13.8hz,1h),0.28(s,2h);13cnmr(101mhz,cdcl3)δ177.2,172.9,147.3,134.7,81.8,78.4,77.5,77.2,76.8,59.1,57.0,55.5,54.3,46.2,41.8,39.7,37.0,36.6,25.6,25.4,23.3,22.1,20.0,18.8,16.9,16.6;hrms-esi(m/z):calcd.forc25h37o6n2[m+h]+:461.2652,found461.2650.
室温条件下,将化合物4(50mg,0.138mmol,1.0equiv)溶于无水thf(10ml),然后将吗啉(60.4ul,0.70mmol,5.0eq)加入到体系中,室温条件下继续搅拌过夜,体系直接浓缩,然后通过柱色谱法(2%meoh/dcm),得化合物13(40mg,60%,白色固体)。[α]18d=-61.2(c=1.0,chcl3);1hnmr(400mhz,dmso)δ7.95(s,1h),5.75(s,1h),5.31(s,1h),4.00(d,j=9.9hz,1h),3.63(s,3h),3.24–3.11(m,1h),2.99(d,j=9.9hz,1h),2.68(s,2h),2.61–2.51(m,4h),2.46–2.12(m,5h),1.94(dd,j=21.8,10.5hz,2h),1.80(dd,j=36.4,14.5hz,3h),1.34(s,6h),1.13(t,j=12.5hz,1h),0.91(t,j=13.7hz,1h);13cnmr(101mhz,dmso)δ176.9,173.5,150.2,132.0,81.3,79.3,66.8,61.6,58.9,58.7,57.8,54.4,41.2,38.9,36.7,35.4,24.5,22.6,22.2,19.4,15.9.hrms-esi(m/z):calcd.forc24h34o7n[m+h]+:448.2335,found448.2333.
室温条件下,将化合物5(816mg,2.37mmol,1.0equiv)溶于无水thf(5ml),然后将n-甲基哌嗪(1.3ml,11.85mmol,5.0eq)加入到体系中,室温条件下继续搅拌3h,体系直接浓缩,然后通过柱色谱法(5%meoh/dcm),得化合物14(650mg,75%,白色固体)。[α]18d=-54.5(c=0.48,chcl3);1hnmr(400mhz,cdcl3)δ7.02(s,1h),5.62(s,1h),5.33(d,j=10.4hz,1h),5.12(s,1h),4.65(d,j=10.5hz,1h),4.14(s,1h),3.24–3.07(m,1h),2.86(dd,j=14.1,4.2hz,2h),2.73(t,j=12.5hz,2h),2.68–2.52(m,5h),2.51–2.40(m,2h),2.31(s,3h),2.28(dd,j=14.3,2.8hz,2h),2.24–2.13(m,2h),2.11–1.98(m,2h),1.87–1.62(m,2h),1.60(s,3h),1.55(s,3h);13cnmr(101mhz,cdcl3)δ177.3,173.8,147.7,136.8,135.9,130.9,128.1,125.2,83.5,78.3,73.2,54.4,53.1,46.0,40.9,38.6,37.7,32.4,27.6,24.4,19.2,13.7;hrms-esi(m/z):calcd.forc25h37o5n2[m+h]+:445.2702,found445.2701.
室温条件下,将化合物6(114mg,0.32mmol,1.0eq)溶于无水thf(1.0ml),然后将n-甲基哌嗪(352ul,3.17mmol,10.0eq)加入到体系中,室温条件下继续搅拌5h,体系直接浓缩,然后通过柱色谱法(3%meoh/dcm),得化合物15(71mg,65%,白色固体)。[α]22d=-78.7(c=0.1,chcl3);1hnmr(400mhz,cdcl3)δ7.20(s,1h),5.14(s,1h),3.80(d,j=10.0hz,1h),3.20–3.07(m,1h),3.02(d,j=9.9hz,1h),2.86–2.51(m,6h),2.50–2.32(m,6h),2.28(s,3h),2.12(dd,j=15.4,3.0hz,2h),2.00(t,j=12.7hz,4h),1.66(dd,j=8.4,5.7hz,1h),1.34(s,3h),1.13(s,3h),1.07(td,j=13.2,4.3hz,1h),0.45(t,j=13.8hz,1h),0.28(s,2h),-0.01(s,1h);13cnmr(101mhz,cdcl3)δ177.2,172.9,147.3,134.7,81.8,78.4,59.1,56.9,55.5,54.3,46.2,41.8,39.7,37.0,36.6,25.6,25.4,23.3,22.1,20.0,18.8,16.9,16.6;hrms-esi(m/z):calcd.forc26h39o5n2[m+h]+:459.2859,found459.2858.
室温条件下,将化合物7(100mg,0.26mmol,1.0eq)溶于无水thf(5ml),然后将富马酸(30.6mg,0.26mmol,1.0eq)加入到体系中,室温条件下继续搅拌5h,体系直接浓缩得化合物16(130mg,100%,白色固体)。[α]22d=+25.2°(c=0.14,meoh);1hnmr(400mhz,dmso)δ7.46(s,1h),6.60(s,2h),5.38–5.10(m,2h),4.87(d,j=10.8hz,2h),3.18–2.90(m,1h),2.74–2.53(m,3h),2.45–2.27(m,4h),2.21(s,6h),2.14–2.00(m,3h),1.90–1.74(m,2h),1.72(s,3h),1.50(s,3h),1.06(t,j=13.2hz,1h);13cnmr(101mhz,dmso)δ177.2,173.0,166.2,149.2,134.6,134.1,132.4,132.2,126.3,124.6,78.8,77.8,54.5,44.6,39.6,39.4,38.8,35.5,24.3,23.1,22.8,18.8,14.5.
室温条件下,将化合物8(50mg,0.12mmol,1.0eq)溶于无水thf(0.6ml),然后将富马酸(13.3mg,0.12mmol,1.0eq)加入到体系中,室温条件下继续搅拌5h,体系直接浓缩得化合物17(63mg,100%,白色固体)。1hnmr(400mhz,dmso)δ7.47(s,1h),6.63(s,2h),5.29(d,j=10.7hz,1h),5.22(d,j=1.4hz,1h),4.89(d,j=10.6hz,2h),3.68–3.50(m,4h),3.47–3.23(m,2h),3.18–3.07(m,1h),2.69(dd,j=14.1,3.5hz,1h),2.66–2.56(m,1h),2.47–2.29(m,5h),2.29–2.18(m,2h),2.12(dd,j=16.5,8.6hz,3h),1.88–1.75(m,2h),1.73(s,3h),1.61(s,3h),1.12–1.00(m,1h);13cnmr(101mhz,dmso)δ177.3,173.2,166.0,149.1,134.7,134.0,132.5,131.6,126.4,124.6,79.0,77.9,66.3,53.3,37.7,35.5,24.2,23.1,22.6,18.8,14.9.
室温条件下,将化合物9(1.0g,2.3mmol,1.0eq)溶于无水thf(25ml),然后将富马酸(270.8mg,2.3mmol,1.0eq)加入到体系中,室温条件下继续搅拌5h,体系直接浓缩得化合物18(1.27g,100%,白色固体)。[α]23d=-16.3(c=0.15,meoh);ir(neat)2922,2851,1750cm-1;1hnmr(400mhz,dmso)δ7.47(d,j=0.9hz,1h),6.55(s,2h),5.76(s,1h),5.29(d,j=10.7hz,1h),5.22(d,j=1.4hz,1h),4.88(d,j=10.6hz,2h),3.16–3.03(m,1h),2.69(dd,j=14.2,3.4hz,2h),2.66–2.52(m,5h),2.47(s,1h),2.44–2.34(m,4h),2.33(s,3h),2.32–2.24(m,1h),2.19–1.97(m,4h),1.88–1.74(m,2h),1.73(d,j=0.7hz,3h),1.59(s,3h),1.14–0.96(m,1h);13cnmr(101mhz,dmso)δ177.2,173.2,167.1,149.1,134.7,134.6,132.5,131.7,126.4,124.6,79.0,77.9,54.9,54.1,52.8,44.7,38.1,35.5,24.2,23.1,22.6,18.8,15.0.
室温条件下,将化合物10(30mg,0.067mmol,1.0eq)溶于无水thf(2ml),然后将富马酸(7.8mg,0.067mmol,1.0eq)加入到体系中,室温条件下继续搅拌5h,体系直接浓缩得化合物19(38mg,100%,白色固体)。1hnmr(400mhz,dmso)δ7.65(s,1h),6.58(s,2h),5.31(d,j=11.4hz,2h),4.92(d,j=10.6hz,1h),3.75–3.37(m,2h),3.11(d,j=4.5hz,2h),2.86–2.60(m,7h),2.42(s,3h),2.38–2.20(m,4h),2.12–1.85(m,4h),1.84–1.71(m,4h),1.68(s,3h),1.27(s,3h),1.10(dt,j=26.7,13.4hz,2h);13cnmr(101mhz,dmso)δ176.6,173.3,166.7,149.2,134.4,132.2,132.1,125.7,79.5,77.9,60.6,58.4,53.9,52.6,44.1,41.1,38.6,38.3,35.1,24.6,22.2,22.1,19.0,15.7.
室温条件下,将化合物11(30mg,0.069mmol,1.0eq)溶于无水thf(2ml),然后将富马酸(7.8mg,0.069mmol,1.0eq)加入到体系中,室温条件下继续搅拌5h,体系直接浓缩得化合物20(38mg,100%,白色固体)。1hnmr(400mhz,dmso)δ7.65(s,1h),6.63(s,2h),5.31(d,j=10.4hz,2h),4.93(d,j=10.6hz,1h),3.60(s,4h),3.12(d,j=5.2hz,1h),2.75(dd,j=14.4,2.7hz,1h),2.69–2.54(m,2h),2.49–2.38(m,3h),2.37–2.17(m,5h),2.09–1.85(m,3h),1.86–1.70(m,3h),1.68(s,3h),1.30(s,3h),1.28–0.99(m,3h).13cnmr(101mhz,dmso)δ176.7,173.2,166.0,149.2,134.0,132.1,132.1,125.7,79.5,78.1,66.3,60.6,58.4,53.3,41.2,38.6,37.9,35.2,24.5,22.3,22.1,19.0,15.5.
室温条件下,将化合物12(3.0g,6.5mmol,1.0eq)溶于无水thf(30ml),然后将富马酸(1.4g,12.3mmol,1.9eq)加入到体系中,室温条件下继续搅拌3h,体系析出白色固体,过滤,滤饼用thf(3x15ml)洗涤,无水et2o(3x10ml)洗涤,收集滤饼得化合物21(3.3g,89%,白色固体)。[α]22d=-39.5(c=0.5,meoh);ir(neat)2956,2929,2835,1751,1703,1458,1390,1145,1103,981,645cm-1;1hnmr(400mhz,dmso)δ7.95(s,1h),6.57(s,2h),5.32(s,1h),3.99(d,j=9.7hz,1h),3.18(s,1h),2.99(d,j=9.9hz,1h),2.80–2.45(m,9h),2.46–2.18(m,8h),2.04–1.60(m,6h),1.33(s,3h),1.31(s,3h),1.13(t,j=11.2hz,1h),0.90(t,j=13.4hz,1h);13cnmr(101mhz,dmso)δ176.2,173.1,167.2,149.8,134.6,131.5,80.7,78.9,61.1,58.4,58.2,57.3,53.7,53.2,43.8,40.7,38.7,36.2,34.9,24.2,22.1,21.8,18.9,15.5.
室温条件下,将化合物12(20.0mg,0.043mmol,1.0eq)溶于无水thf(1.0ml),然后将二氯乙酸(5.3mg,0.043mmol,1.0eq)加入到体系中,室温条件下继续搅拌3h,体系直接浓缩得化合物22(25.3mg,100%,白色固体)。[α]18d=-29.9(c=0.35,meoh);1hnmr(400mhz,dmso)δ7.97(s,1h),6.14(s,1h),5.75(s,1h),5.47–5.03(m,1h),4.01(d,j=10.0hz,2h),3.84–3.74(m,2h),3.31–3.17(m,2h),3.02(d,j=10.0hz,2h),2.79(s,3h),2.66–2.52(m,3h),2.48–2.39(m,1h),2.36–2.17(m,3h),2.03–1.76(m,5h),1.74–1.62(m,2h),1.33(s,3h),1.29(s,3h),1.18–1.00(m,1h),1.00–0.84(m,1h);13cnmr(101mhz,dmso)δ176.10,173.31,165.53,150.03,131.48,106.98,80.64,79.03,70.12,66.61,61.14,58.46,58.19,57.30,53.09,52.72,40.64,38.75,36.21,34.79,28.89,24.23,23.57,22.09,21.85,18.87,15.47.
室温条件下,将化合物12(20.0mg,0.043mmol,1.0eq)溶于无水thf(1.0ml),然后将l-苹果酸(5.5mg,0.043mmol,1.0eq)加入到体系中,室温条件下继续搅拌3h,体系直接浓缩得化合物23(25.3mg,100%,白色固体)。[α]18d=-40.0(c=0.27,meoh);1hnmr(400mhz,dmso)δ7.96(s,1h),5.75(s,1h),5.46–4.90(m,1h),4.07(t,j=6.5hz,1h),4.00(d,j=10.0hz,1h),3.80(ddd,j=14.8,9.8,6.3hz,1h),3.26–3.11(m,1h),3.00(d,j=10.0hz,1h),2.73(s,3h),2.63–2.51(m,5h),2.45(s,3h),2.43–2.21(m,6h),2.01–1.67(m,7h),1.33(s,3h),1.31(s,3h),1.20–1.03(m,1h),0.99–0.81(m,1h);13cnmr(101mhz,dmso)δ176.34,175.63,173.17,172.01,149.89,131.53,107.01,80.75,78.94,66.63,66.55,61.13,58.49,58.20,57.38,54.20,53.28,44.35,40.68,40.46,38.68,36.20,34.89,28.90,24.17,23.58,22.11,21.78,18.90,15.54.
室温条件下,将化合物13(15.0mg,0.033mmol,1.0eq)溶于无水thf(2.0ml),然后将富马酸(3.9mg,0.033mmol,1.0eq)加入到体系中,室温条件下继续搅拌3h,体系直接浓缩得化合物24(19mg,100%,白色固体)。[α]18d=-62.1(c=0.165,meoh:chcl3=1:1);1hnmr(400mhz,dmso)δ7.95(s,1h),6.63(s,2h),5.38(d,j=5.9hz,1h),5.32(s,1h),4.01(d,j=9.9hz,1h),3.64(s,3h),3.27–3.14(m,1h),3.00(d,j=9.9hz,1h),2.69(s,2h),2.63–2.52(m,4h),2.47–2.23(m,5h),2.04–1.90(m,2h),1.88–1.77(m,3h),1.35(s,6h),1.20–1.07(m,1h),0.91(t,j=13.8hz,1h);13cnmr(101mhz,dmso)δ176.4,173.1,166.0,149.8,134.0,131.6,80.9,78.9,66.4,61.1,58.5,58.2,57.4,54.0,40.7,38.4,36.2,34.9,24.1,22.2,21.8,18.9,15.4.
室温条件下,将化合物13(15.0mg,0.033mmol,1.0eq)溶于无水thf(2.0ml),然后将二氯乙酸(4.2mg,0.033mmol,1.0eq)加入到体系中,室温条件下继续搅拌3h,体系直接浓缩得化合物25(19mg,100%,白色固体)。[α]19d=-25.4(c=0.24,meoh);1hnmr(400mhz,dmso)δ7.94(s,1h),6.61(s,1h),5.37(d,j=5.9hz,1h),5.31(s,1h),4.01(d,j=9.9hz,1h),3.65(s,3h),3.27–3.16(m,1h),3.01(d,j=9.9hz,1h),2.74(s,2h),2.65–2.51(m,4h),2.37(m,5h),2.03–1.89(m,2h),1.90–1.77(m,3h),1.33(s,6h),1.19–1.02(m,1h),0.91(t,j=13.9hz,1h);13cnmr(101mhz,dmso)δ176.7,173.6,166.2,150.3,132.0,81.3,79.4,66.6,66.6,61.5,58.9,58.7,57.8,54.2,41.1,38.8,36.8,35.4,24.5,22.6,22.2,19.3,15.9.
室温条件下,将化合物13(15.0mg,0.033mmol,1.0eq)溶于无水thf(2.0ml),然后将l-苹果酸(4.5mg,0.033mmol,1.0eq)加入到体系中,室温条件下继续搅拌3h,体系直接浓缩得化合物26(19mg,100%,白色固体)。1hnmr(400mhz,dmso)δ7.96(s,1h),5.76(s,1h),5.32(s,1h),4.25(dd,j=7.7,4.9hz,1h),4.01(d,j=10.0hz,1h),3.73–3.49(m,5h),3.38(dd,j=9.6,4.7hz,3h),3.26–3.14(m,1h),3.00(d,j=10.0hz,1h),2.66–2.53(m,3h),2.48–2.17(m,7h),2.05–1.68(m,5h),1.34(s,6h),1.21–1.05(m,1h),0.93(d,j=13.5hz,1h);13cnmr(101mhz,dmso)δ176.5,174.7,173.1,171.9,149.8,131.6,80.9,78.9,67.0,66.4,61.2,58.5,58.2,57.4,55.0,53.9,40.7,38.4,36.2,34.9,24.1,22.2,21.8,18.9,15.4.
室温条件下,将化合物14(500mg,1.12mmol,1.0eq)溶于无水thf(5.0ml),然后将富马酸(130.5mg,1.12mmol,1.0eq)加入到体系中,室温条件下继续搅拌3h,体系直接浓缩得化合物27(630mg,100%,白色固体)。[α]19d=-40.7(c=0.33,meoh);1hnmr(400mhz,dmso)δ7.50(s,1h),6.57(s,2h),5.76(s,1h),5.44–5.14(m,2h),5.13–4.78(m,2h),3.95(d,j=10.5hz,1h),3.15(d,j=13.5hz,1h),3.05–2.50(m,9h),2.47–2.25(m,8h),2.24–2.07(m,1h),1.91(s,1h),1.70(d,j=13.3hz,4h),1.54(d,j=20.8hz,4h),1.19(t,j=12.7hz,1h);13cnmr(101mhz,dmso)δ177.0,173.2,166.9,149.3,138.6,134.5,132.4,131.7,126.4,124.7,79.0,78.6,74.6,55.0,53.8,52.7,44.2,40.0,37.9,31.3,24.1,22.7,21.2,18.9,10.3.
室温条件下,将化合物15(67mg,0.145mmol,1.0eq)溶于无水thf(1.5ml),然后将富马酸(16.8mg,0.145mmol,1.0eq)加入到体系中,室温条件下继续搅拌3h,体系直接浓缩得化合物28(83mg,100%,白色固体)。[α]19d=-50.3(c=0.4,meoh);13cnmr(101mhz,dmso)δ176.6,173.2,166.3,149.7,134.2,132.1,81.0,78.7,67.0,58.3,57.3,54.2,53.3,44.4,40.9,36.2,35.8,25.1,25.1,24.6,22.7,21.5,19.0,18.3,16.4,16.1.
室温条件下,将化合物9(50mg,0.117mmol,1.0eq)溶于无水thf(2.0ml),然后将柠檬酸(22mg,0.11mmol,1.0eq)加入到体系中,室温条件下继续搅拌3h,体系直接浓缩得化合物29(72mg,100%,白色固体)。[α]18d=-15.8(c=0.35,meoh);1hnmr(400mhz,dmso)δ7.50(s,1h),5.76(s,1h),5.30(d,j=10.7hz,1h),5.24(s,1h),4.90(d,j=10.6hz,2h),3.17(s,2h),3.08–2.61(m,11h),2.59–2.48(m,6h),2.49–2.23(m,5h),2.21–1.99(m,3h),1.83–1.74(m,2h),1.73(s,3h),1.58(s,3h),1.06(t,j=13.1hz,1h);13cnmr(101mhz,dmso)δ177.49,176.79,173.87,171.85,149.74,135.20,132.96,132.13,126.83,125.07,79.50,78.28,72.11,54.01,52.72,44.32,43.76,40.27,38.58,35.93,24.59,23.68,23.02,19.26,15.45.
室温条件下,将化合物10(30mg,0.067mmol,1.0eq)溶于无水thf(2.0ml),然后将柠檬酸(12.2mg,0.067mmol,1.0eq)加入到体系中,室温条件下继续搅拌3h,体系直接浓缩得化合物30(42mg,100%,白色固体)。1hnmr(400mhz,dmso)δ7.66(s,1h),5.76(s,2h),5.31(d,j=11.6hz,2h),4.93(d,j=10.7hz,1h),3.29–3.08(m,2h),3.05–2.70(m,6h),2.61(dd,j=21.9,12.0hz,8h),2.54(s,1h),2.45(dd,j=12.6,4.9hz,2h),2.38–2.20(m,3h),2.09–1.89(m,3h),1.74(dd,j=12.8,7.8hz,1h),1.68(s,3h),1.26(s,3h),1.19–1.01(m,2h);13cnmr(101mhz,dmso)δ176.5,176.1,173.4,171.4,149.3,132.2,132.0,125.61,79.6,77.9,71.7,60.6,58.4,55.0,53.7,52.4,43.7,43.5,41.1,38.6,38.3,35.1,24.6,22.2,22.1,19.0,15.6.
室温条件下,将化合物11(30mg,0.069mmol,1.0eq)溶于无水thf(2.0ml),然后将柠檬酸(12.7mg,0.069mmol,1.0eq)加入到体系中,室温条件下继续搅拌3h,体系直接浓缩得化合物31(43mg,100%,白色固体)。1hnmr(400mhz,dmso)δ7.54(d,j=86.5hz,1h),5.93–5.27(m,2h),5.39–5.10(m,2h),4.95(t,j=18.7hz,1h),3.60(s,4h),3.48–2.95(m,2h),2.75(d,j=15.3hz,3h),2.60(dd,j=42.6,11.4hz,4h),2.46–2.14(m,7h),2.09–1.85(m,3h),1.84–1.72(m,3h),1.68(s,3h),1.30(s,3h),1.28–0.89(m,3h);13cnmr(101mhz,dmso)δ176.7,174.7,173.3,171.3,149.2,132.2,132.1,125.7,79.5,78.1,72.4,66.3,60.6,58.39,54.9,53.3,42.8,41.2,38.6,37.9,35.2,24.5,22.3,22.1,19.0,15.6.
室温条件下,将化合物12(50mg,0.107mmol,1.0eq)溶于无水thf(2.0ml),然后将柠檬酸(20mg,0.107mmol,1.0eq)加入到体系中,室温条件下继续搅拌3h,体系直接浓缩得化合物32(70mg,100%,白色固体)。[α]18d=-55.9(c=0.185,meoh:chcl3=1:1);1hnmr(400mhz,dmso)δ7.98(s,1h),5.34(s,1h),4.02(d,j=10.0hz,1h),3.28–3.18(m,1h),3.17(s,3h),3.01(d,j=10.0hz,2h),2.82–2.59(m,10h),2.55–2.39(m,6h),2.39–2.16(m,3h),2.08–1.62(m,5h),1.34(s,3h),1.30(s,3h),1.22–1.05(m,1h),0.92(t,j=13.6hz,1h);13cnmr(101mhz,dmso)δ176.30,176.26,173.31,171.41,150.01,131.53,80.72,79.04,71.69,61.17,58.51,58.21,57.39,53.76,52.98,48.64,43.86,43.43,40.67,38.75,36.22,34.86,24.23,22.12,21.84,18.90,15.53.
室温条件下,将化合物13(50mg,0.112mmol,1.0eq)溶于无水thf(2.0ml),然后将柠檬酸(21mg,0.112mmol,1.0eq)加入到体系中,室温条件下继续搅拌3h,体系直接浓缩得化合物33(71mg,100%,白色固体)。
[α]18d=-27.3(c=0.4,meoh);1hnmr(400mhz,dmso)δ7.96(s,1h),5.76(s,1h),5.32(s,1h),4.01(d,j=10.0hz,1h),3.75–3.48(m,5h),3.38(dd,j=9.5,4.6hz,2h),3.27–3.12(m,2h),3.00(d,j=10.0hz,1h),2.70(dd,j=41.7,15.4hz,6h),2.61–2.52(m,3h),2.35(ddd,j=36.5,16.4,7.9hz,5h),2.06–1.68(m,5h),1.34(s,6h),1.21–1.03(m,1h),0.91(t,j=13.5hz,1h);13cnmr(101mhz,dmso)δ176.47,174.66,173.11,171.34,149.83,131.56,80.88,78.93,72.44,66.38,61.17,58.53,58.23,57.41,54.96,53.95,42.77,40.70,38.41,36.23,34.95,24.10,22.19,21.80,18.93,15.44.
室温条件下,将化合物14(50mg,0.117mmol,1.0eq)溶于无水thf(2.0ml),然后将柠檬酸(22mg,0.11mmol,1.0eq)加入到体系中,室温条件下继续搅拌3h,体系直接浓缩得化合物34(72mg,100%,白色固体)。
[α]18d=-34.6(c=0.32,meoh);1hnmr(400mhz,dmso)δ7.51(s,1h),5.76(s,2h),5.40–5.19(m,2h),5.08–4.94(m,1h),4.92(d,j=10.8hz,1h),3.96(dd,j=11.1,3.7hz,1h),3.28–3.07(m,2h),3.08–2.62(m,11h),2.60–2.27(m,10h),2.26–2.09(m,1h),1.91(s,1h),1.71(s,3h),1.69–1.58(m,1h),1.56(s,3h),1.18(td,j=12.8,3.6hz,1h);13cnmr(101mhz,dmso)δ176.87,176.21,173.38,171.39,149.49,138.56,132.35,131.69,126.36,124.71,79.09,78.58,74.52,71.73,54.96,53.51,52.34,43.80,43.29,40.08,37.91,31.26,24.01,22.75,18.84,10.20.
实施例2:ovatodiolide衍生物及其盐对人恶性脑胶质母细胞瘤细胞系u87,人胰腺癌细胞系panc-1,人肝癌细胞系hepg2和人肝癌细胞系smmc7721的抑制作用
将待测试细胞配成2×105/ml细胞悬液,加入96孔板圆底细胞培养板内,分别加入待测化合物,每一测试浓度3孔,置37℃、5%co2饱和湿度条件下培养72小时,用mtt法在酶联检测仪570nm波长测得吸光度(a)值,计算出本发明化合物对测试癌细胞的抑制作用。
如表1所示,所测试化合物对测试的癌细胞系显示出较强的抗癌活性。表1.ovatodiolide衍生物及其盐对各种癌细胞的抑制活性(ic50,μm)
实施例3:ovatodiolide衍生物在肝微粒体中的代谢实验
向冰冷的50mmtris-柠檬酸缓冲液(ph=7.4)加入适量人肝微粒体蛋白、化合物1、4、18、21、mgcl2,整个孵育体积为50μl,包括0.2mg/ml肝微粒体、5mmmgcl2、1μm化合物1、4、18、21,孵育体系甲醇比例小于1%。37℃水浴预孵育3min后,加入1μl50mmnadph启动反应,继续孵育0、0.25、0.5、1h后,另设未加nadph的对照组,相同条件下孵育1h,向孵育体系加入1ml含1ng/ml盐酸丁螺环酮的甲醇溶液终止反应,涡旋混匀2min,12000rpm离心5min,进样2μl检测化合物1、4、18、21剩余量,各样本平行3份。
如表2所示,ovatodiolide修饰的化合物的肝微粒体代谢实验显示,稳定性显著提高。
表2.化合物1、4、18、21的肝微粒体稳定性实验
肝微粒体代谢数据(n=3)
肝微粒体代谢数据(n=3)
肝微粒体代谢数据(n=3)
注:“+”表示加入,“-”表示未加入。
肝微粒体代谢数据(n=3)
注“+”表示加入,“-”表示未加入。
- 还没有人留言评论。精彩留言会获得点赞!