一种多取代γ-丁内酰胺的合成方法
一种多取代
γ
‑
丁内酰胺的合成方法
技术领域
1.本发明属于有机合成技术领域,具体涉及一种合成多取代γ
‑
丁内酰胺的方法。
背景技术:
2.γ
‑
丁内酰胺是一类重要的含氮杂环化合物,广泛存在于生物活性天然产物和药物结构中。例如,吡拉西坦、茴拉西坦、奥拉西坦等治疗脑供血不足药物都含有γ
‑
丁内酰胺结构。此外,γ
‑
丁内酰胺类化合物也是有机合成、药物和生物活性分子的重要中间体。γ
‑
丁内酰胺类化合物的合成在药物研发和有机化学研究等相关产业方面一直是重要的研究课题。迄今为止,关于γ
‑
丁内酰胺的构建方法已有众多文献报道。γ
‑
丁内酰胺的传统合成方法一般是γ
‑
丁内酯和胺在高温高压条件下制备。除此之外,还有一些在使用钯、铜等过渡金属催化酰胺分子内环化合成γ
‑
丁内酰胺化合物的方法,但都存在反应条件苛刻、使用过渡金属催化剂、底物预制备困难、结构多样性受限和产率低等局限性。因此,开发原料简单易得、反应条件温和、后处理简单、简洁高效地实现具有多功能结构的γ
‑
丁内酰胺,如含额外的胺基、磺酰基等生物活性位点的γ
‑
丁内酰胺合成,可望发现新的多用途有机合成子、开发新的有机合成策略均具有重要意义。
技术实现要素:
3.本发明的目的是为了解决现有技术中反应条件苛刻、使用过渡金属催化剂、底物预制备困难、结构多样性受限等问题,提供克服上述局限的多取代γ
‑
丁内酰胺的合成方法,通过温和的反应条件和简单的后处理,基于简单易得起始物,简洁高效地实现具有胺基和磺酰基的多功能结构γ
‑
丁内酰胺。
4.本发明是这样来实现的:
5.在反应器中加入1,3
‑
二羰基化合物1、伯胺2和亚磺酸钠3,以碘化钾和叔丁基过氧化氢(tbhp)为催化体系,在苯甲醚(anisole)和醋酸的混合溶剂中,在空气、室温条件下反应,薄层色谱监测反应进程,直至反应完全;反应物用乙酸乙酯、饱和碳酸氢钠溶液萃取,收集有机相用无水硫酸钠干燥,然后减压蒸出溶剂,残渣经硅胶柱层析分离纯化得到目标化合物多取代γ
‑
丁内酰胺4,反应方程式如下:
[0006][0007]
其中r1选自氢、烷基、卤素、芳基;r2选自氢、烷基、卤素、芳基;r3选自烷基、芳基;r4选自烷基、芳基。
[0008]
所述1,3
‑
二羰基化合物1、伯胺2和亚磺酸钠3的摩尔比为1:(1
‑
5):(1
‑
5);碘化钾与1,3
‑
二羰基化合物1的摩尔比为1:(1
‑
50),叔丁基过氧化氢与1,3
‑
二羰基化合物1的摩尔比为(1
‑
5):1;苯甲醚和醋酸的混合溶剂是苯甲醚和醋酸按体积比1:5
‑
5:1的比例混合制
得;硅胶柱层析中使用石油醚
‑
乙酸乙酯混合物洗脱,石油醚
‑
乙酸乙酯混合物是石油醚和乙酸乙酯按体积比30:1
‑
1:5的比例混合制得。
[0009]
所述产物结构经过核磁共振、高分辨质谱以及代表性产物的单晶衍射测试等确证。
[0010]
所述目标产物即γ
‑
丁内酰胺产物的结构特征是α
‑
位具有胺基取代以及γ
‑
位具有磺酰亚甲基。
[0011]
目标产物γ
‑
丁内酰胺的结构式为下列中任一种:
[0012][0013]
本发明的有益技术效果是:1,3
‑
二羰基化合物、伯胺和亚磺酸钠为起始原料,均为来源广泛、结构多样、价廉易得的商品化产品,提供一种具有多官能结构、结构多样和潜在反应活性位点的γ
‑
丁内酰胺衍生物的高通用性合成方法;反应在室温中进行,条件温和、实验操作过程简便,且具有较高产率,合成方法适合工业放大,有良好的产业应用前景。
附图说明
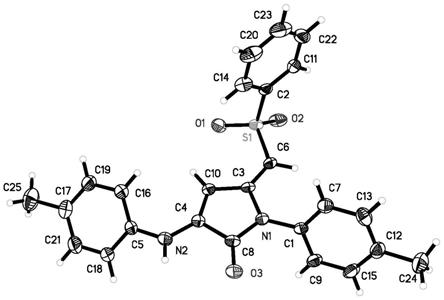
[0014]
图1为实施例2化合物4b的单晶结构图。
具体实施方式
[0015]
为了能够更清楚地理解本发明的上述目的、特征和优点,下面结合附图和具体实施方式对本发明进行进一步的详细描述。在下面的描述中阐述了很多具体细节以便于充分理解本发明,但是,本发明还可以采用其他不同于在此描述的其他方式来实施,因此,本发明并不限于下面公开的具体实施例的限制。
[0016]
下述实施例中制备工艺如下:在空气、室温下,在15ml反应管中加入1,3
‑
二羰基化合物(0.5mmol)、伯胺(1.0mmol)、亚磺酸钠(1.0mmol)、ki(0.1mmol)和70%tbhp水溶液(tbhp 1.0mmol),加入5.0ml苯甲醚和醋酸混合液(体积比为1:1),磁力搅拌下反应,tlc监测反应,待原料点完全消失后,反应物加入饱和碳酸氢钠溶液,之后用有机溶剂乙酸乙酯对反应液进行萃取,取有机层,然后用无水na2so4进行干燥,将经过干燥的液体浓缩蒸干,之后对浓缩蒸干物进行硅胶柱层析分离,所述的柱色谱分离采用的溶剂为石油醚
‑
乙酸乙酯的混合溶剂,得目标化合物γ
‑
丁内酰胺;产物结构经过核磁共振、高分辨质谱以及代表性产物的单晶衍射测试等确证无误。反应通式如下:
[0017][0018]
实施例1:本实施例制备如式4a所示的化合物
[0019][0020]
本实施例的柱色谱洗脱体系为石油醚/乙酸乙酯(v/v=18/1),产率88%;式4a所示的化合物的结构表征如下:黄色固体;熔点:204
‑
205℃;1h nmr(600mhz,cdcl3):δ=7.90(d,j=7.6hz,2h,arh),7.60
–
7.57(m,1h,arh),7.53
–
7.51(m,2h,arh),7.48(d,j=7.5hz,2h,arh),7.46
–
7.42(m,3h,arh),7.25(s,2h,arh),7.22(d,j=7.3hz,2h,arh),7.16(d,j=7.3hz,1h,arh),7.13(s,1h,c=ch),5.68(s,1h,c=ch);
13
c nmr(150mhz,cdcl3):δ=165.7,151.7,142.6,138.9,135.7,133.1,132.4,129.8,129.8,129.8,129.8,129.3,129.3,129.1,128.1,128.1,126.8,126.8,123.8,118.3,118.3,105.8,92.7;hrms(tof es
+
):m/z calcd for c
23
h
19
n2o3s[(m+h)
+
],403.1111,found,403.1115.
[0021]
实施例2:本实施例制备如式4b所示的化合物
[0022][0023]
本实施例的柱色谱洗脱体系为石油醚/乙酸乙酯(v/v=18/1),产率为95%;式4b所示的化合物的结构表征如下:黄色固体;熔点:204
–
205℃;化合物4b的单晶结构入图1所
示;1hnmr(600mhz,cdcl3):δ=7.89(d,j=7.6hz,2h,arh),7.59
–
7.56(t,j=7.3hz,1h,arh),7.52
–
7.49(t,j=7.6hz,2h,arh),7.28(s,1h,c=ch),7.22(d,j=8.0hz,2h,arh),7.16
–
7.13(m,j=7.3hz,3h,arh)7.08(d,j=8.1hz,3h,arh),5.63(s,1h,arh),2.40(s,3h,ch3),2.37(s,3h,ch3);
13
c nmr(150mhz,cdcl3):δ=165.9,152.1,142.8,139.3,136.5,136.1,133.6,133.0,130.4,130.4,130.3,130.3,129.7,129.3,129.3,127.9,127.9,126.9,126.9,118.4,118.4,105.3,92.0,21.3,20.9;hrms(tof es
+
):m/z calcd for c
25
h
23
n2o3s[(m+h)
+
],431.1424,found,431.1430.
[0024]
实施例3:本实施例制备如式4c所示的化合物
[0025][0026]
本实施例的柱色谱洗脱体系为石油醚/乙酸乙酯(v/v=18/1),产率为96%;式4c所示的化合物的结构表征如下:黄色固体;熔点:217
–
218℃;1h nmr(600mhz,cdcl3):δ=7.89
–
7.88(m,2h,arh),7.59
–
7.56(m,1h,arh),7.52
–
7.49(m,2h,arh),7.21
–
7.18(m,2h,arh),7.13
–
7.10(m,2h,arh),7.07(s,1h,c=ch),7.01(s,1h,nh),6.98
–
6.95(m,4h,arh),5.59(s,1h,c=ch),3.85(s,3h,och3),3.84(s,3h,och3);
13
c nmr(150mhz,cdcl3):δ=166.0,160.0,156.2,152.5,142.8,136.6,132.9,132.2,129.5,129.5,129.3,129.3,126.8,126.8,124.8,120.1,120.1,115.0,115.0,115.0,115.0,104.9,91.0,55.6,55.6.hrms(tof es
+
):m/z calcd for c
25
h
23
n2o5s[(m+h)
+
],463.1322,found,463.1328.
[0027]
实施例4:本实施例制备如式4d所示的化合物
[0028][0029]
本实施例的柱色谱洗脱体系为石油醚/乙酸乙酯(v/v=20/1),产率为73%;式4d所示的化合物的结构表征如下:黄色固体;熔点:245
–
246℃;1h nmr(600mhz,cdcl3):δ=7.90
–
7.89(m,2h,arh),7.62
–
7.59(m,1h,arh),7.54
–
7.52(m,2h,arh),7.47
–
7.45(m,2h,arh),7.39
–
7.38(m,2h,arh),7.18
–
7.15(m,4h,arh),7.12(s,1h,c=ch),5.66(s,1h,c=ch).
13
cnmr(150mhz,cdcl3):δ=165.4,150.8,142.2,137.5,135.5,135.3,133.3,130.7,130.1,130.1,129.9,129.9,129.4,129.4,129.4,129.4,129.0,126.9,126.9,119.5,119.5,106.4,93.3.hrms(tof es
+
):m/z calcd for c
23
h
17
cl2n2o3s[(m+h)
+
],471.0331,found,471.0334.
[0030]
实施例5:本实施例制备如式4e所示的化合物
[0031][0032]
本实施例的柱色谱洗脱体系为石油醚/乙酸乙酯(v/v=1/1),产率为75%;式4e所示的化合物的结构表征如下:黄色固体;熔点:251
–
252℃;1h nmr(600mhz,dmso
‑
d6):δ=9.91(s,1h,oh),9.74(s,1h,oh),9.44(s,1h,nh),7.89(d,j=7.2hz,2h,arh),7.67
–
7.60(m,3h,arh),7.32
–
7.29(t,j=7.7hz,2h,arh),7.23
–
7.21(t,j=7.6hz,2h,arh),6.95(s,1h,c=ch),6.88
–
6.84(m,3h,arh),6.71
–
6.67(m,2h,arh),6.53(d,j=8.2hz,1h,arh),5.53(s,1h,c=ch).
13
c nmr(150mhz,dmso
‑
d6):δ=165.2,158.7,158.6,152.5,143.2,141.6,137.9,133.9,133.7,130.8,130.6,130.1,130.1,126.9,126.9,119.3,116.5,115.8,111.1,110.5,106.6,103.5,91.6.hrms(tof es
+
):m/z calcd for c
23
h
19
n2o5s[(m+h)
+
],435.1009,found,435.1010
[0033]
实施例6:本实施例制备如式4f所示的化合物
[0034][0035]
本实施例的柱色谱洗脱体系为石油醚/乙酸乙酯(v/v=5/1),产率为47%;式4f所示的化合物的结构表征如下:黄色固体;熔点:304
–
306℃;1h nmr(600mhz,dmso
‑
d6):δ=10.04(s,1h,nh),8.09(d,j=8.4hz,2h,arh),8.01(d,j=8.7hz,2h,arh),7.97(d,j=7.5hz,2h,arh),7.71
–
7.68(t,j=7.4hz,1h,arh),7.66
–
7.64(m,3h,arh),7.62(d,j=8.4hz,3h,arh),7.29(s,1h,c=ch),5.94(s,1h,c=ch),3.31(s,3h,ch3),3.24(s,3h,ch3).
13
c nmr(150mhz,dmso
‑
d6):δ=165.0,150.8,145.2,142.8,141.2,137.5,136.9,134.7,133.9,130.1,130.1,129.9,129.9,129.4,129.4,129.0,129.0,127.2,127.2,119.1,119.1,105.9,95.4,44.2,43.8.hrms(tof es
+
):m/z calcd for c
25
h
23
n2o7s3[(m+h)
+
],559.0662,found,559.0662.
[0036]
实施例7:本实施例制备如式4g所示的化合物
[0037][0038]
本实施例的柱色谱洗脱体系为石油醚/乙酸乙酯(v/v=3/1),产率为41%。式4g所示的化合物的结构表征如下:黄色固体;熔点:150
–
152℃;1h nmr(600mhz,dmso
‑
d6):δ=
7.81(d,j=7.4hz,3h,arh),7.66
–
7.64(t,j=7.3hz,1h,arh),7.63
–
7.57(m,3h,arh),7.52(s,1h,nh),6.46
–
6.45(m,1h,c=ch),6.36
–
6.34(m,2h,c=ch),6.31(d,j=2.9hz,1h,c=ch),6.27(s,1h,c=ch),6.12(s,1h,c=ch),4.75(s,2h,ch2),4.33(m,2h,ch2),
13
c nmr(150mhz,dmso
‑
d6):δ=165.2,151.1,151.0,149.9,143.8,143.2,143.2,143.1,143.1,133.3,129.9,129.9,126.6,126.6,110.9,110.9,108.8,108.7,102.4,88.3,36.0.hrms(tof es
+
):m/z calcd for c
21
h
19
n2o5s[(m+h)
+
],411.1009,found,411.1009.
[0039]
实施例8:本实施例制备如式4h所示的化合物
[0040][0041]
本实施例的柱色谱洗脱体系为石油醚/乙酸乙酯(v/v=3/1),产率为51%;式4h所示的化合物的结构表征如下:黄色固体;熔点:151
–
153℃;1h nmr(600mhz,dmso
‑
d6):δ=7.89(d,j=7.4hz,2h,arh),7.65(d,j=6.9hz,1h,arh),7.62
–
7.60(t,j=7.2hz,2h,arh),7.21
–
7.19(t,j=6.2hz,1h,nh),6.20(s,1h,c=ch),6.09(s,1h,c=ch),4.02
–
3.98(m,1h,ch),3.93
–
3.89(m,1h,ch),3.77
–
3.73(m,1h,ch2),3.65
–
3.61(m,1h,ch2),3.60
–
3.55(m,2h,ch2),3.53
–
3.48(m,2h,ch2),3.20
–
3.11(m,2h,ch2),1.93
–
1.89(m,1h,ch2),1.87
–
1.81(m,3h,ch2),1.77
–
1.74(m,1h,ch2),1.73
–
1.69(m,1h,ch2),1.60
–
1.56(m,1h,ch2),1.47
–
1.43(m,1h,ch2).
13
c nmr(150mhz,dmso
‑
d6):δ=165.8,152.2,144.1,144.0,133.2,129.8,129.8,126.6,126.6,101.7,86.7,77.1,76.4,67.6,67.4,48.3,43.32,29.1,28.7,25.6,25.3.hrms(tof es
+
):m/z calcd for c
21
h
27
n2o5s[(m+h)
+
],419.1635,found,419.1635.
[0042]
实施例9:本实施例制备如式4i所示的化合物
[0043][0044]
本实施例的柱色谱洗脱体系为石油醚/乙酸乙酯(v/v=10/1),产率为46%;式4i所示的化合物的结构表征如下:黄色固体;熔点:152
–
154℃;1h nmr(600mhz,dmso
‑
d6):δ=7.90(d,j=7.3hz,2h,arh),7.67
–
7.65(t,j=7.3hz,1h,arh),7.62
–
7.60(t,j=7.6hz,2h,arh),7.33
–
7.31(t,j=5.8hz,1h,nh),6.15(s,1h,c=ch),5.97(s,1h,c=ch),3.51
–
3.48(t,j=7.1hz,2h,ch2),3.11
–
3.07(q,j=6.8hz,2h,ch2),1.54
–
1.49(m,2h,ch2),1.37
–
1.35(m,2h,ch2),1.33
–
1.29(m,2h,ch2),1.19
–
1.15(m,2h,ch2),0.89(t,j=7.1hz,3h,ch3),0.82(t,j=7.1hz,3h,ch3).
13
c nmr(150mhz,dmso
‑
d6):δ=165.5,152.0,144.1,144.0,133.2,129.8,129.8,126.6,126.6,100.7,85.6,43.7,39.0,30.8,30.3,20.1,19.7,14.2,14.1.hrms(tof es
+
):m/z calcd for c
19
h
27
n2o3s[(m+h)
+
],363.1737,found,363.1738.
[0045]
实施例10:本实施例制备如式4j所示的化合物
[0046][0047]
制备步骤如与实施例1相同,本实施例的柱色谱洗脱体系为石油醚/乙酸乙酯(v/v=4/1),产率为46%;式4j所示的化合物的结构表征如下:黄色固体;熔点:148
–
150℃;1h nmr(600mhz,dmso
‑
d6):δ=7.87(d,j=7.8hz,2h,arh),7.68
–
7.66(m,1h,arh),7.64
–
7.61(t,j=7.4hz,2h,arh),7.32
–
7.30(t,j=7.5hz,2h,arh),7.27
–
7.25(t,j=5.7hz,1h,nh),7.23(d,j=7.3hz,3h,arh),7.18
–
7.15(t,j=7.3hz,2h,arh),7.12(d,j=7.1hz,1h,arh),7.07(d,j=7.3hz,2h,arh),6.15(s,1h,c=ch),6.01(s,1h,c=ch),3.76
–
3.74(t,j=7.0hz,2h,ch2),3.35
–
3.31(m,2h,ch2),2.84
–
2.82(t,j=7.2hz,2h,ch2),2.71
–
2.69(t,j=7.0hz,2h,ch2).
13
cnmr(150mhz,dmso
‑
d6):δ=165.3,151.7,144.0,143.6,139.5,138.5,133.2,129.8,129.8,129.2,129.2,129.1,129.1,128.8,128.8,128.6,128.6,126.8,126.7,126.7,126.7,101.2,86.3,45.5,40.6,34.4,34.0.hrms(tof es
+
):m/z calcd for c
27
h
27
n2o3s[(m+h)
+
],459.1737,found,459.1738.
[0048]
实施例11:本实施例制备如式4k所示的化合物
[0049][0050]
本实施例的柱色谱洗脱体系为石油醚/乙酸乙酯(v/v=18/1),产率为90%;式4k所示的化合物的结构表征如下:黄色固体;熔点:239
–
241℃;h nmr(600mhz,cdcl3):δ=7.77(d,j=8.3hz,2h,arh),7.30(d,j=8.0hz,2h,arh),7.27(s,1h,nh),7.26(s,1h,arh),7.23(d,j=8.1hz,2h,arh),7.16
–
7.11(m,3h,arh),7.08(d,j=8.2hz,2h,arh),7.03(s,1h,c=ch),5.63(s,1h,c=ch),2.41(s,3h,ch3),2.40(s,3h,ch3),2.37(s,3h,ch3).
13
c nmr(150mhz,cdcl3):δ=δ165.8,151.6,143.9,139.8,139.2,136.5,135.9,133.5,130.3,130.3,130.3,130.3,129.9,129.9,129.7,127.9,127.9,126.9,126.9,118.3,118.3,105.8,92.0,21.6,21.2,20.9.hrms(tof es
+
):m/z calcd for c
26
h
25
n2o3s[(m+h)
+
],445.1580,found,445.1580.
[0051]
实施例12:本实施例制备如式4l所示的化合物
[0052][0053]
本实施例的柱色谱洗脱体系为石油醚/乙酸乙酯(v/v=18/1),产率为94%;式4l所示的化合物的结构表征如下:黄色固体;熔点:264
–
266℃;1h nmr(600mhz,cdcl3):δ=7.82(d,j=8.6hz,2h,arh),7.47(d,j=8.6hz,2h,arh),7.28(s,1h,nh),7.27(s,1h,arh),7.23(d,j=8.1hz,2h,arh),7.14(d,j=8.3hz,2h,arh),7.11(s,1h,c=ch),7.08(d,j=8.0hz,3h,arh),5.58(s,1h,c=ch),2.40(s,3h,ch3),2.37(s,3h,ch3).
13
c nmr(150mhz,cdcl3):δ=163.3,150.1,138.9,137.1,137.0,133.9,133.9,131.3,128.0,128.0,127.9,127.9,127.9,127.1,127.1,125.9,125.9,125.5,125.5,116.0,116.0,102.2,89.2,18.8,18.5.hrms(tof es
+
):m/zcalcd for c
25
h
22
cln2o3s[(m+h)
+
],465.1034,found,465.1033.
[0054]
实施例13:本实施例制备如式4m所示的化合物
[0055][0056]
制备步骤如与实施例1相同,本实施例的柱色谱洗脱体系为石油醚/乙酸乙酯(v/v=18/1),产率为93%;式4m所示的化合物的结构表征如下:黄色固体;熔点:242
–
244℃;1h nmr(600mhz,cdcl3):δ=7.32(d,j=7.9hz,2h,arh),7.20(s,1h,nh),7.19(s,1h,c=ch),7.15(d,j=8.1hz,2h,arh),7.11(d,j=8.5hz,3h,arh),6.96(s,1h,c=ch),5.62(s,1h,c=ch),3.04(s,3h,ch3),2.43(s,3h,ch3),2.35(s,3h,ch3).
13
c nmr(150mhz,cdcl3):δ=δ165.9,153.1,139.5,136.3,136.2,133.6,130.5,130.5,130.3,130.3,129.7,128.0,128.0,118.4,118.4,104.1,91.4,45.4,21.3,20.9.hrms(tof es
+
):m/z calcd for c
20
h
21
n2o3s[(m+h)
+
],369.1267,found,369.1268.
[0057]
实施例14:本实施例制备如式4n所示的化合物
[0058][0059]
本实施例的柱色谱洗脱体系为石油醚/乙酸乙酯(v/v=13/1),产率为50%;式4n所示的化合物的结构表征如下:黄色固体;熔点:205
–
207℃;1h nmr(600mhz,dmso
‑
d6)δ=1hnmr(600mhz,dmso
‑
d6)δ=9.34(s,1h,nh),7.75(d,j=7.7hz,2h,arh),7.66
–
7.63(t,j=7.1hz,1h,arh),7.59
–
7.56(t,j=7.6hz,2h,arh),7.46
–
7.44(t,j=7.8hz,2h,arh),7.36
–
7.32(m,2h,arh),7.31(s,1h,c=ch),7.30
–
7.24(m,3h,arh),7.11(d,j=7.6hz,6h,arh),
6.78(d,j=6.5hz,2h,arh),3.45(s,2h,ch2).
13
c nmr(150mhz,dmso
‑
d6):δ=167.4,149.2,142.9,140.8,138.7,135.9,135.7,133.7,129.9,129.9,129.9,129.5,129.5,129.3,129.3,129.2,129.2,128.4,128.4,128.1,128.1,127.1,127.1,126.4,123.3,119.2,119.2,117.4,97.3,33.2.hrms(tof es
+
):m/z calcd for c
30
h
25
n2o3s[(m+h)
+
],493.1580,found,493.1580.
[0060]
实施例15:本实施例制备如式4o所示的化合物
[0061][0062]
本实施例的柱色谱洗脱体系为石油醚/乙酸乙酯(v/v=13/1),产率为62%;式4o所示的化合物的结构表征如下:黄色固体;熔点:248
–
250℃;1h nmr(600mhz,dmso
‑
d6)δ=9.45(s,1h,nh),7.66
–
7.63(t,j=7.3hz,1h,arh),7.61(d,j=7.6hz,2h,arh),7.57
–
7.54(m j=7.7hz,3h,arh),7.49
–
7.46(t,j=7.8hz,2h),7.42(s,1h,c=ch),7.40(s,1h,arh),7.13
–
7.11(t,j=7.3hz,1h,arh),7.13
–
7.11(t,j=7.3hz,1h,arh),7.03
–
7.01(t,j=7.4hz,2h,arh),6.87(dd,j=7.8,3.4hz,4h,arh),6.72(d,j=8.4hz,2h,arh).
13
c nmr(150mhz,dmso
‑
d6):δ=167.5,149.2,141.5,140.8,136.5,135.5,135.2,135.2,133.8,133.5,130.1,129.9,129.9,129.9,129.9,129.9,129.8,129.7,128.7,128.7,127.8,127.4,127.4,127.4,123.5,119.4,119.4,117.8,96.5.hrms(tof es
+
):m/z calcd for c
29
h
22
cln2o3s[(m+h)
+
],513.1034,found,513.1039.
[0063]
实施例16:本实施例制备如式4p所示的化合物
[0064][0065]
本实施例的柱色谱洗脱体系为石油醚/乙酸乙酯(v/v=15/1),产率为80%;式4p所示的化合物的结构表征如下:黄色固体;熔点:154
–
156℃;1h nmr(600mhz,dmso
‑
d6)δ=9.21(s,1h,nh),7.89(d,j=7.4hz,2h,arh),7.74
–
7.68(m,1h,arh),7.68
–
7.66(t,j=7.3hz,2h),7.50(d,j=7.3hz,1h,arh),7.48(s,1h,c=ch),7.48(s,1h,arh),7.47
–
7.46(m,4h,arh),7.25(d,j=8.7hz,3h,arh),7.07
–
7.05(t,j=7.3hz,1h,arh),1.88
–
1.85(m,2h,ch2),1.27
–
1.18(m,6h,ch2),1.14
–
1.10(m,2h,ch2),1.07
–
1.01(m,4h,ch2),0.92
–
0.89(m,2h,ch2),0.72
–
0.84(t,j=7.1hz,3h,ch3),0.55
–
0.51(m,2h,ch2),.
13
c nmr(150mhz,dmso
‑
d6):δ=167.5,147.5,142.9,140.9,136.6,135.2,133.7,130.1,130.1,129.8,129.8,129.8,129.5,129.5,129.4,127.0,123.1,119.8,118.9,118.9,97.6,31.7,31.7,
30.4,29.3,29.2,29.1,28.7,28.2,22.6,22.6,14.5,14.5.hrms(tof es
+
):m/z calcd for c
33
h
39
n2o3s[(m+h)
+
],543.2676,found,543.2677.
[0066]
实施例17:本实施例制备如式4q所示的化合物
[0067][0068]
本实施例的柱色谱洗脱体系为石油醚/乙酸乙酯(v/v=15/1),产率为63%;式4q所示的化合物的结构表征如下:黄色固体;熔点:158
–
160℃;1h nmr(600mhz,dmso
‑
d6)δ=9.21(s,1h,nh),7.88(d,j=7.4hz,2h,arh),7.73
–
7.69(m,1h,arh),7.68
–
7.66(t,j=7.3hz,2h,arh),7.49(d,j=7.4hz,3h,arh),7.42
–
7.37(m,4h,arh),7.26(s,2h,arh),7.24(s,1h,c=ch),7.07
–
7.04(t,j=7.3hz,1h),1.87
–
1.85(m,2h,ch2),1.27
–
1.09(m,18h,ch2),1.06
–
1.03(m,4h,ch2),0.56
–
0.51(m,2h,ch2),0.92
–
0.88(t,j=6.8hz,3h,ch3),0.56
–
0.51(m,2h,ch2).
13
c nmr(150mhz,dmso
‑
d6):δ=167.5,147.58,142.9,140.9,136.6,135.2,133.7,130.0,130.0,129.8,129.8,129.8,129.8,129.5,129.5,129.3,126.9,126.9,123.1,119.8,118.9,118.9,97.6,31.8,30.4,29.5,29.5,29.5,29.5,29.4,29.3,29.2,29.2,28.7,28.2,22.6,14.5.hrms(tof es
+
):m/z calcd for c
37
h
47
cl2n2o3s[(m+h)
+
],599.3302,found,599.3301.
[0069]
实施例18:本实施例制备如式4r所示的化合物
[0070][0071]
本实施例的柱色谱洗脱体系为石油醚/乙酸乙酯(v/v=13/1),产率为68%;式4r所示的化合物的结构表征如下:黄色固体;熔点:234
–
235℃;1h nmr(600mhz,dmso
‑
d6)δ=9.12(s,1h,nh),δ7.60(q,j=7.8hz,1h,arh),7.44
–
7.41(t,j=7.8hz,2h,arh),7.36
–
7.32(m,1h,arh),7.29(d,j=7.4hz,2h,arh),7.24
–
7.19(m,5h,arh),7.10
–
7.08(m,4h,arh),7.87
–
7.84(t,j=7.8hz,2h,arh),6.77(d,j=7.3hz,1h,arh),6.70(d,j=7.5hz,2h,arh),5.68(s,1h,c=ch).
13
c nmr(150mhz,dmso
‑
d6):δ=168.2,150.2,142.8,138.6,136.4,133.2,132.6,131.2,130.2,130.2,129.5,129.5,129.2,129.2,128.8,128.8,128.5,128.5,128.5,128.4,128.0,128.0,126.1,126.1,123.2,121.7,121.7,113.0,104.7.hrms(tof es
+
):m/z calcd for c
29
h
23
n2o3s[(m+h)
+
],479.1424,found,479.1425.
[0072]
实施例19:本实施例制备如式4s所示的化合物
[0073][0074]
本实施例的柱色谱洗脱体系为石油醚/乙酸乙酯(v/v=13/1),产率为70%;式4s所示的化合物的结构表征如下:黄色固体;熔点:210
–
212℃;h nmr(600mhz,dmso
‑
d6)δ=9.21(s,1h,nh),7.89(d,j=7.4hz,2h,arh),7.74
–
7.68(m,1h,arh),7.68
–
7.66(t,j=7.3hz,2h),7.50(d,j=7.3hz,1h,arh),7.48(s,1h,c=ch),7.48(s,1h,arh),7.47
–
7.46(m,4h,arh),7.25(d,j=8.7hz,3h,arh),7.07
–
7.05(t,j=7.3hz,1h,arh),1.88
–
1.85(m,2h,ch2),1.27
–
1.18(m,6h,ch2),1.14
–
1.10(m,2h,ch2),1.07
–
1.01(m,4h,ch2),0.92
–
0.89(m,2h,ch2),0.72
–
0.84(t,j=7.1hz,3h,ch3),0.55
–
0.51(m,2h,ch2),.
13
c nmr(150mhz,dmso
‑
d6):δ=167.5,147.5,142.9,140.9,136.6,135.2,133.7,130.1,130.1,129.8,129.8,129.8,129.5,129.5,129.4,127.0,123.1,119.8,118.9,118.9,97.6,31.7,31.7,30.4,29.3,29.2,29.1,28.7,28.2,22.6,22.6,14.5,14.5.hrms(tof es
+
):m/z calcd for c
29
h
22
cln2o3s[(m+h)
+
],513.1034,found,513.1043.
[0075]
实施例20:本实施例制备如式4t所示的化合物
[0076][0077]
制备步骤如与实施例1相同,本实施例的柱色谱洗脱体系为石油醚/乙酸乙酯(v/v=13/1),产率为73%;式4t所示的化合物的结构表征如下:黄色固体;熔点:215
–
217℃;1h nmr(600mhz,dmso
‑
d6)δ=8.96(s,1h,nh),7.90(d,j=7.4hz,2h,arh),7.72
–
7.69(t,j=7.4hz,1h,arh),7.63
–
7.61(t,j=7.7hz,2h,arh),7.57
–
7.54(t,j=7.6hz,2h,arh),7.51
–
7.48(t,j=7.4hz,1h,arh),7.39(d,j=7.4hz,2h,arh),7.30
–
7.27(t,j=7.8hz,2h,arh),7.00
–
6.98(m,3h,arh),5.61(s,1h,c=ch),1.82(s,3h,ch3).
13
c nmr(150mhz,dmso
‑
d6):δ=165.2,152.6,143.6,140.7,137.3,133.6,133.6,130.2,130.2,130.0,130.0,129.6,129.6,129.5,129.0,129.0,126.7,126.7,122.8,120.8,120.8,108.7,107.5,14.6.hrms(tof es
+
):m/z calcd for c
24
h
21
n2o3s[(m+h)
+
],417.1267,found,417.1275.
[0078]
实施例21:本实施例制备如式4u所示的化合物
[0079]
[0080]
本实施例的柱色谱洗脱体系为石油醚/乙酸乙酯(v/v=13/1),产率为54%;式4u所示的化合物的结构表征如下:黄色固体;熔点:229
–
231℃;1h nmr(600mhz,dmso
‑
d6)δ=9.21(s,1h,nh),7.89(d,j=7.4hz,2h,arh),7.74
–
7.68(m,1h,arh),7.68
–
7.66(t,j=7.3hz,2h),7.50(d,j=7.3hz,1h,arh),7.48(s,1h,c=ch),7.48(s,1h,arh),7.47
–
7.46(m,4h,arh),7.25(d,j=8.7hz,3h,arh),7.07
–
7.05(t,j=7.3hz,1h,arh),1.88
–
1.85(m,2h,ch2),1.27
–
1.18(m,6h,ch2),1.14
–
1.10(m,2h,ch2),1.07
–
1.01(m,4h,ch2),0.92
–
0.89(m,2h,ch2),0.72
–
0.84(t,j=7.1hz,3h,ch3),0.55
–
0.51(m,2h,ch2),.
13
c nmr(150mhz,dmso
‑
d6):δ=167.5,147.5,142.9,140.9,136.6,135.2,133.7,130.1,130.1,129.8,129.8,129.8,129.5,129.5,129.4,127.0,123.1,119.8,118.9,118.9,97.6,31.7,31.7,30.4,29.3,29.2,29.1,28.7,28.2,22.6,22.6,14.5,14.5.hrms(tof es+):m/z calcd for c
30
h
22
n3o3s[(m+h)
+
],504.1376,found,504.1377.
相关技术
网友询问留言
已有0条留言
- 还没有人留言评论。精彩留言会获得点赞!
1