一种喹诺酮类化合物的绿色高效合成方法与流程
本发明属于化工技术领域,具体涉及一种高效绿色合成喹诺酮类化合物的方法。
背景技术:
喹诺酮类化合物由其具有抗有丝分裂、抗癌、抗疟、抗菌、抗病毒、抗糖尿病、抗hiv和抗炎性等生物活性可作为多种药物的核心模块。例如,其是已被用作临床治疗药物有沙拉沙星、诺氟沙星、帕珠沙星、安托沙星、普鲁沙星、德拉沙星和埃替格韦等。
喹诺酮类药物结构代表
文献报道,工业生产喹诺酮药物主要基于conradlimpach、gouldjacobs等方法。虽然该方法在过去几十年里为生产喹诺酮家族药物提供了有效的合成途径,但是由于合成路线需要复杂的原料,不够绿色的反应介质,酸或者碱做催化剂,比较长的工艺路径在一定程度上使得喹诺酮类药物成本高居不下并且最重要的是对环境的迫害是不可逆的。因此开应用更为现代的合成模式发新的绿色合成途径具有重要的意义。
conradlimpach反应
上述反应存在第一步席夫碱反应需要使用强酸作为催化剂,第二步脱羧偶联时副产物多、产品分离难度大、原子经济性差等缺点。
gouldjacobs反应
上述工艺存在乙氧基甲叉丙二酸二乙酯使用使得生产成本高,整个过程具有使用溶剂催化剂使用造成三废排量大,操作难度高,对合成环境要求高等缺点。
技术实现要素:
本发明的目的在于提供一种绿色高效合成喹诺酮类化合物的方法。
本发明的目的是这样实现的,是以二羰基化合物、原甲酸三乙酯和胺类化合物为原料,同时经历condensation反应、substitution反应,再通过friedel-craft反应制备得到,其反应原理如下:
本发明所述的高效绿色合成喹诺酮类化合物的方法第一步是以简单易得的二羰基化合物、原甲酸三乙酯和胺类化合物三组分化合物一起投料。第二步以二苯基醚作为环化试剂,加热回流关环。整个过程中除二苯基醚可以回收重复利用反应过程无任何溶剂和催化剂的使用,从根本上解决了原路线污染较大、操作难度高等问题。
本发明的有益效果主要体现为:
1.原料简单市场可以买到价格便宜;
2.三组分原料一起投料一锅操作,中间体不需要分离操作,反应过程高效省力;
3.整个转变过程效率高,最终产物不需要色谱处理简单洗涤就可得到纯净化合物;
4.安全的反应过程,后处理不需要过柱分离只需要乙酸乙酯和甲醇洗涤并且洗脱剂可以回收利用可预防污染;
5.绿色的反应过程、副产物只有乙醇、原子经济性高;
6.喹诺酮化合物实用价值高,重要的抗菌药物的组成结构。
附图说明
图1为本发明实施例1中1号化合物核磁共振氢谱图谱;
图2为本发明实施例1中1号化合物核磁共振碳谱图谱;
图3为本发明实施例2中1号化合物核磁共振氢谱图谱;
图4为本发明实施例1中1号化合物核磁共振碳谱图谱;
图5为本发明实施例3中1号化合物核磁共振氢谱图谱;
图6为本发明实施例1中1号化合物核磁共振碳谱图谱;
图7为本发明实施例4中1号化合物核磁共振氢谱图谱;
图8为本发明实施例1中1号化合物核磁共振碳谱图谱。
具体实施方式
下面结合实施例和附图对本发明作进一步的说明,但不以任何方式对本发明加以限制,基于本发明教导所作的任何变换或替换,均属于本发明的保护范围。
本发明所述的绿色高效合成喹诺酮类化合物的方法,是本发明公开了喹诺酮类化合物及其新颖的三组分一锅投料绿色合成方法。方法如下:步骤一,二羰基化合物、原甲酸三乙酯与苯胺类化合物三组分原料在无溶剂和催化剂条件下反应得到烯胺酯中间体;步骤二,所述的烯胺酯中间体再在环化试剂(二苯基醚、多聚磷酸、伊顿试剂或硫酸)作用下发生分子内环合反应得到喹诺酮主环化合物,产物的纯度高达98.8%以上;
其反应原理如下:
本发明所述的高效绿色合成喹诺酮类化合物的方法制备得到喹诺酮化合物产品的纯度能达到98.9%以上。
本发明所述的目标物喹诺酮类化合物结构通式为:
其中,r1为苯、叔丁基、环丙基,乙氧基;r2为氢、甲基、三氟甲基、氰基、硝基、卤素、甲基、甲氧基、叔丁基、乙基等烷基或芳基。
本发明所述的高效绿色合成喹诺酮化合物的技术路线如下:
下面以具体实施案例对本发明做进一步说明:
本发明实施例中所用的材料或试剂的规格及检测仪器如无特别说明均为市售。
核磁仪:brukeravanceiii400mhz核磁仪
红外波谱仪:thermonicolets10ft-ir红外波谱仪
质谱仪:agilentlc/msdtofinstrument质谱仪器
熔点仪器:北京泰克techx-5熔点仪器
实施例1
实施例反应式如下:
该制备方法包括以下步骤:
第一步,搅拌下,以苯甲酰乙酸乙酯、原甲酸三乙酯、苯胺为原料,三组分一起投入反应器中,升温到130℃保温反应24h,继续加入二苯基醚回流,待有大量固体析出后停止反应得到喹诺酮化合物。减压抽滤,用石油醚、乙酸乙酯洗涤和甲醇洗涤,干燥得到喹诺酮化合物。
实施例中胺类化合物变化制备得到喹诺酮类化合物的得率情况如下:
上述化合物数据如下:
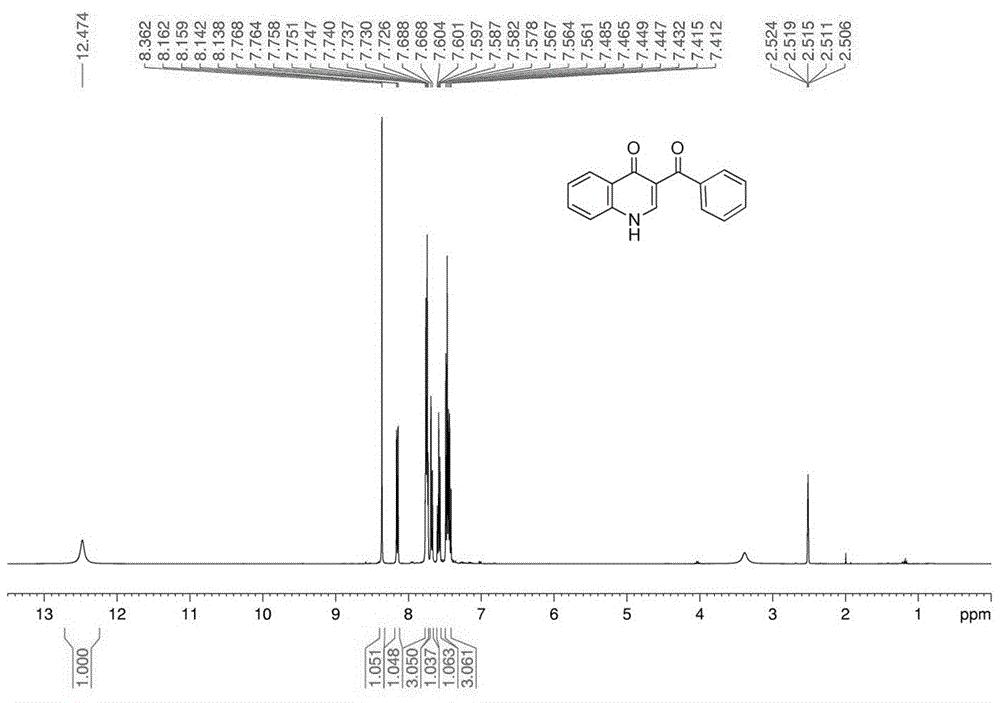
3-benzoylquinolin-4(1h)-one(1a):whitesolid;yield86%;m.p.260-262oc;1hnmr(400mhz,dmso-d6,ppm):δ12.47(brs,1h),8.36(s,1h),8.16(dd,1h,j=1.32,1.28hz),7.76-7.72(m,3h),7.68(d,1h,j=7.12hz),7.60-7.56(m,1h)7.48-7.41(m,3h);13cnmr(100mhz,dmso-d6,ppm):δ194.8,174.9,143.7,139.9,139.0,132.9,132.6,129.5,128.5,127.5,126.0,125.0,119.9,119.3;ir(kbr):vmax(cm-1)3445,2330,1644,1554,1473,1440,1371,1295,1068,857,758,624;hrms(esi-tof+):m/zcalcdforc16h12no2+[(m+h)+]250.0863;found,250.0862.
3-benzoyl-6-fluoroquinolin-4(1h)-one(1b):whitesolid;yield74%;m.p.>300oc;1hnmr(400mhz,dmso-d6,ppm):δ12.61(brs,1h),8.39(s,1h),7.80-7.72(m,4h),7.68-7.63(m,1h),7.60-7.56(m,1h),7.48-7.44(m,2h);13cnmr(100mhz,dmso-d6,ppm):δ194.6,174.0,173.9,160.7,158.3,143.7,138.8,136.6,132.7,129.5,128.9,128.8,128.5,122.2,122.1,121.7,121.5,119.1,110.3,110.1.
3-benzoyl-6-chloroquinolin-4(1h)-one(1c):whitesolid;yield71%;m.p.>300oc;1hnmr(400mhz,dmso-d6,ppm):δ12.64(brs,1h),8.40(s,1h),8.05(d,1h,j=2.24hz),7.79(m,1h),7.72(t,3h,j=8.36hz),7.59(t,1h,j=7.28hz),7.46(t,2h,j=7.60hz);13cnmr(100mhz,f3cood,ppm):δ201.9,176.9,150.0,141.2,140.0,139.6,137.0,136.1,131.4,131.1,126.3,123.7,123.7.
3-benzoyl-6-bromoquinolin-4(1h)-one(1d):whitesolid;yield67%;m.p.>300oc;1hnmr(400mhz,dmso-d6,ppm):δ12.60(brs,1h),8.39(d,1h,j=5.32hz),8.19(s,1h),7.91(d,1h,j=8.68hz),7.74-7.44(m,6h);13cnmr(100mhz,dmso-d6,ppm):δ194.5,173.6,143.9,138.9,138.6,135.6,132.8,129.5,128.9,128.5,128.1,121.9,120.2,117.7.
3-benzoyl-6-iodoquinolin-4(1h)-one(1e):paleyellowsolid;m.p.>300oc;yield66%;1hnmr(400mhz,dmso-d6,ppm):δ12.53(brs,1h),8.39(dd,2h,j=2.00,4.88hz),8.03(dd,1h,j=2.04,2.04hz),7.71(t,2h,j=7.04hz),7.59-7.56(m,1h),7.49-7.43(m,3h);13cnmr(100mhz,dmso-d6,ppm):δ194.5,173.4,143.9,141.0,139.2,138.6,134.4,132.8,129.5,129.1,128.5,121.7,120.4,89.8.
3-benzoyl-6-nitroquinolin-4(1h)-one(1f):brownsolid;yield82%;m.p.235-238oc;1hnmr(400mhz,dmso-d6,ppm):δ12.90(brd,1h,j=4.00hz),8.85(d,1h,j=2.64hz),8.52(dd,1h,j=2.68,2.72hz),8.47(s,1h),7.86(d,1h,j=9.12hz),7.78-7.76(m,2h),7.64-7.59(m,1h),7.50-7.46(m,2h);13cnmr(100mhz,dmso-d6,ppm):δ194.0,174.4,144.6,144.0,143.8,138.2,133.1,129.6,128.6,127.0,126.7,122.3,121.3,121.2;ir(kbr):vmax(cm-1)3444,2338,1662,1585,1510,1474.1345,1203,1080,905,843,746,563;hrms(esi-tof+):m/zcalcdforc16h11n2o4+[(m+h)+]295.0713;found,295.0716.
3-benzoyl-6-(trifluoromethyl)quinolin-4(1h)-one(1g):whitesolid;yield78%;m.p.>300oc;1hnmr(400mhz,dmso-d6,ppm):δ12.75(brs,1h),8.45(s,1h),8.37(d,1h,j=1.04hz),8.05(dd,1h,j=2.08,2.08hz),7.87(d,1h,j=8.68hz),7.77-7.74(m,2h),7.61-7.57(m,1h),7.48-7.44(m,2h);13cnmr(100mhz,dmso-d6,ppm):δ194.3,174.3,144.5,142.2,138.4,132.9,129.6,128.9,128.6,126.8,123.5,123.4,121.0;ir(kbr):vmax(cm-1)3446,1645,1584,1526,1444,1368,1332,1294,1254,1112,1008,842,783,699;hrms(esi-tof+):m/zcalcdforc17h11f3no2+[(m+h)+]318.0736;found,318.0739.
6-acetyl-3-benzoylquinolin-4(1h)-one(1h):pale-brownsolid;yield80%;m.p.>300oc;1hnmr(400mhz,dmso-d6,ppm):δ12.67(brs,1h),8.68(d,1h,j=1.92hz),8.40(s,1h),8.24(dd,1h,j=2.00,2.00hz),7.76-7.72(m,3h),7.61-7.57(m,1h),7.47(t,2h,j=15.2hz),2.65(s,3h);13cnmr(100mhz,dmso-d6,ppm):δ197.2,194.5,174.9,144.1,142.8,138.5,133.0,132.9,131.5,129.5,128.6,127.4,126.7,121.0,119.8,27.1;ir(kbr):vmax(cm-1)3445,2360,1675,1644,1521,1359,1294,1069,1008,854,780,696;hrms(esi-tof+):m/zcalcdforc18h14no3+[(m+h)+]292.0968;found,292.0969.
3-benzoyl-6-methylquinolin-4(1h)-one(1i):whitesolid;yield64%;m.p.>300oc;1hnmr(400mhz,dmso-d6,ppm):δ12.37(brs,1h),8.29(s,1h),7.91(d,1h,j=0.76hz),7.72-7.70(m,2h),7.58-7.54(m,3h),7.46-7.42(m,2h),2.42(s,3h);13cnmr(100mhz,dmso-d6,ppm):δ194.9,174.7,143.2,138.9,137.8,134.5,134.2,132.6,129.5,128.4,127.4,125.3,119.6,119.2,21.2.
3-benzoyl-6-ethylquinolin-4(1h)-one(1j):whitesolid;yield56%;m.p.282-284oc;1hnmr(400mhz,dmso-d6,ppm):δ12.40(brs,1h),8.30(s,1h),7.93(d,1h,j=0.84hz),7.73-7.70(m,2h),7.62-7.54(m,3h),7.46-7.42(m,2h),2.75(dd,2h,j=7.52,7.56hz),1.22(t,3h,j=15.2hz);13cnmr(100mhz,dmso-d6,ppm):δ194.9,174.7,143.2,140.7,139.0,138.1,133.2,132.5,130.5,129.5,128.4,127.5,123.9,119.6,119.3,119.0,28.3,15.9.
3-benzoyl-6-isopropylquinolin-4(1h)-one(1k):whitesolid;yield69%;m.p.265-267oc;1hnmr(400mhz,dmso-d6,ppm):δ12.41(brs,1h),8.31(s,1h),7.96(d,1h,j=1.96hz),7.73-7.70(m,2h),7.66(dd,1h,j=2.04,2.04hz),7.61-7.54(m,2h),7.46-7.42(m,2h),3.05-2.98(m,1h),1.25(dd,6h,j=6.92hz);13cnmr(100mhz,dmso-d6,ppm):δ194.9,174.8,145.3,143.3,139.0,138.2,132.5,131.9,130.4,129.5,128.4,127.4,122.3,119.6,119.4,119.0,33.6,24.2.
3-benzoyl-6-(tert-butyl)quinolin-4(1h)-one(1l):whitesolid;yield73%;m.p.>300oc;1hnmr(400mhz,dmso-d6,ppm):δ12.43(brs,1h),8.32(s,1h),8.10(d,1h,j=2.24hz),7.86(dd,1h,j=2.32,2.32hz),7.74-7.71(m,2h),7.63(d,1h,j=8.72hz),7.59-7.55(m,1h),7.47-7.43(m,2h),1.34(s,9h);13cnmr(100mhz,dmso-d6,ppm):δ195.0,174.9,147.6,143.2,139.1,137.9,132.5,131.0,129.4,128.4,127.0,121.0,119.7,119.2,34.9,31.4;ir(kbr):vmax(cm-1)3444,3071,2961,1649,1558,1489,1357,1322,1068,838,700,601;hrms(esi-tof+):m/zcalcdforc20h20no2+[(m+h)+]306.1489;found,306.1486.
3-benzoyl-6-methoxyquinolin-4(1h)-one(1m):whitesolid;yield47%;m.p.>300oc;1hnmr(400mhz,dmso-d6,ppm):δ12.44(brs,1h),8.28(s,1h),7.72(d,2h,j=8.04hz),7.63(d,1h,j=8.96hz),7.58-7.52(m,2h),7.44(t,2h,j=7.56hz),7.38(dd,1h,j=2.76,2.84hz),3.84(s,3h);13cnmr(100mhz,dmso-d6,ppm):δ195.0,174.2,157.0,142.5,139.1,134.3,132.5,129.5,128.7,128.4,123.0,121.0,118.8,105.6,55.9.
3-benzoyl-8-bromoquinolin-4(1h)-one(1n):whitesolid;yield76%;m.p.248-250oc;1hnmr(400mhz,dmso-d6,ppm):δ11.76(brs,1h),8.19-8.14(m,2h),8.10(dd,1h,j=1.28,1.32hz),7.75-7.73(m,2h),7.62-7.57(m,1h),7.49-7.45(m,2h),7.37(t,1h,j=7.84hz);13cnmr(100mhz,dmso-d6,ppm):δ194.2,174.4,144.2,138.5,137.5,136.5,132.9,129.5,129.0,128.6,126.0,125.8,120.4,112.3;ir(kbr):vmax(cm-1)3445,2360,1646,1523,1432,1328,1278,1069,1009,162,699,547;hrms(esi-tof+):m/zcalcdforc16h11brno2+[(m+h)+]327.9968;found,327.9968.
3-benzoyl-8-methylquinolin-4(1h)-one(1o):whitesolid;yield70%;m.p.270-272oc;1hnmr(400mhz,dmso-d6,ppm):δ11.77(brs,1h),8.18(s,1h),8.02(d,1h,j=8.00hz),7.73(t,2h,j=15.28hz),7.59-7.56(m,2h),7.46(t,2h,j=7.68hz),7.32(t,1h,j=7.72hz);13cnmr(100mhz,dmso-d6,ppm):δ194.7,175.1,143.5,138.8,138.4,133.7,132.6,129.5,128.5,127.6,127.5,124.6,123.9,119.8,17.5;ir(kbr):vmax(cm-1)3447,2360,1645,1538,1450,1423,1345,1229,1073,1015,793,768,553;hrms(esi-tof+):m/zcalcdforc17h14no2+[(m+h)+]264.1019;found,264.1017.
3-benzoyl-8-isopropylquinolin-4(1h)-one(1p):whitesolid;yield66%;m.p.201-203oc;1hnmr(400mhz,dmso-d6,ppm):δ11.80(brd,1h,j=6.44hz),8.19(d,1h,j=6.76hz),8.05(dd,1h,j=1.04,1.08hz),7.75-7.68(m,3h),7.60-7.56(m,1h),7.48-7.37(m,3h),3.52(m,1h),1.33(d,6h,j=6.72hz);13cnmr(100mhz,dmso-d6,ppm):δ194.6,175.1,143.5,138.8,137.7,137.0,132.6,130.4,129.5,129.1,128.4,127.9,124.9,123.8,119.5,119.0,26.9,23.4;ir(kbr):vmax(cm-1)3448,1646,1540,1431,1388,1323,1233,1196,1072,863,770,712,586;hrms(esi-tof+):m/zcalcdforc19h18no2+[(m+h)+]292.1332;found,292.1329.
3-benzoyl-8-methoxyquinolin-4(1h)-one(1q):brownsolid;yield62%;m.p.238-240oc;1hnmr(400mhz,dmso-d6,ppm):δ12.06(brs,1h),8.11(s,1h),7.74-7.68(m,3h),7.59-7.56(m,1h),7.46(t,2h,j=7.72hz),7.38-7.34(m,2h),4.03(s,3h);13cnmr(100mhz,dmso-d6,ppm):δ194.7,174.6,149.2,142.9,138.9,132.6,130.3,129.5,128.5,128.4,124.9,120.1,117.1,112.7,56.8.
3-benzoyl-7-bromoquinolin-4(1h)-one(1r):whitesolid;yield64%;m.p.>300oc;1hnmr(400mhz,f3ccood,ppm):δ9.15(s,1h),8.50(d,1h,j=8.84hz),8.28(s,1h),8.02(d,1h,j=8.20hz),7.71-7.54(m,5h);13cnmr(100mhz,f3ccood,ppm):δ220.2,178.0,151.0,141.7,137.3,137.1,136.6,136.3,131.6,131.2,128.5,125.2,121.6;ir(kbr):vmax(cm-1)3444,1643,1524,1370,1070,1013,847,695,547;hrms(esi-tof+):m/zcalcdforc16h11brno2+[(m+h)+]327.9968;found,327.9967.
3-benzoyl-7-methylquinolin-4(1h)-one(1s):whitesolid;yield58%;m.p.>300oc;1hnmr(400mhz,dmso-d6,ppm):δ12.34(brt,1h,j=3.36hz),8.29(d,1h,j=8.24hz),8.02(d,1h,j=8.24hz),7.72-7.70(m,2h),7.59-7.55(m,1h),7.47-7.42(m,3h),7.25(dd,1h,j=1.61,1.12hz),2.45(s,3h);13cnmr(100mhz,dmso-d6,ppm):δ194.8,174.7,143.5,143.3,140.0,139.0,132.6,129.5,129.3,128.5,128.4,126.6,125.9,125.4,119.8,118.5,21.7;ir(kbr):vmax(cm-1)3448,3067,1644,1578,1532,1477,1324,1203,1075,1014,848,770,693;hrms(esi-tof+):m/zcalcdforc17h14no2+[(m+h)+]264.1019;found,264.1016.
3-benzoyl-7-methoxyquinolin-4(1h)-one(1t):pale-brownsolid;yield67%;m.p.>300oc;1hnmr(400mhz,dmso-d6,ppm):δ12.23(brs,1h),8.27(s,1h),8.03(d,1h,j=8.92hz),7.72-7.70(m,2h),7.59-7.54(m,1h),7.47-7.43(m,2h),7.05-7.00(m,2h),3.88(s,3h);13cnmr(100mhz,dmso-d6,ppm):δ194.8,174.3,162.8,143.5,141.7,139.0,132.6,129.5,128.4,127.8,121.5,119.9,114.6,100.5,56.0;ir(kbr):vmax(cm-1)3446,1643,1549,1475,1367,1270,1068,1015,950,866,775,725,634;hrms(esi-tof+):m/zcalcdforc17h14no3+[(m+h)+]280.0968;found,280.0962.
3-benzoyl-6,7-dichloroquinolin-4(1h)-one(1u):whitesolid;yield84%;m.p.>300oc;1hnmr(400mhz,dmso-d6,ppm):δ12.53(brs,1h),8.30(s,1h),7.92(d,1h,j=8.96hz),7.76-7.74(m,2h),7.64-7.57(m,2h),7.49-7.45(m,2h);13cnmr(100mhz,dmso-d6,ppm):δ194.6,173.9,142.2,141.1,138.5,133.4,132.8,130.5,129.5,129.3,128.6,124.7,122.2,119.8;ir(kbr):vmax(cm-1)3444,1642,1576,1544,1446,1367,1290,1204,1070,1009,801,777,700,634;hrms(esi-tof+):m/zcalcdforc16h10cl2no2+[(m+h)+]318.0083;found,318.0082.
3-benzoyl-6,7-dimethylquinolin-4(1h)-one(1v):pale-graysolid;yield77%;m.p.230-232oc;1hnmr(400mhz,dmso-d6,ppm):δ11.36(brd,1h,j=3.76hz),8.06(d,1h,j=8.96hz),7.72(t,2h,j=7.00hz),7.58-7.55(m,1h),7.45(t,2h,j=7.72hz),7.40(d,1h,j=7.48hz),7.01(d,1h,j=7.48hz),2.68(s,3h),2.46(s,3h);13cnmr(100mhz,dmso-d6,ppm):δ195.0,178.1,141.9,139.8,138.9,137.9,132.8,132.5,129.3,128.5,127.0,125.8,124.8,121.5,23.6,17.6;ir(kbr):vmax(cm-1)3446;1653,1625,1544,1450,1406,1325,1206,1069,999,854,789,689,557;hrms(esi-tof+):m/zcalcdforc18h16no2+[(m+h)+]278.1176;found,278.1172.
3-benzoyl-6-benzylquinolin-4(1h)-one(1w):whitesolid;yield83%;m.p.284-286oc;1hnmr(400mhz,dmso-d6,ppm):δ12.43(brs,1h),8.31(s,1h),7.95(d,1h,j=0.92hz),7.71(t,2h,j=7.08hz),7.64-7.54(m,3h),7.44(t,2h,j=7.72hz),7.32-7.25(m,4h),7.22-7.18(m,1h),4.08(s,2h);13cnmr(100mhz,dmso-d6,ppm):δ194.8,174.7,143.3,141.3,138.9,138.4,138.3,133.8,132.6,129.5,129.2,129.0,128.4,127.5,126.5,125.2,119.8,119.5;ir(kbr):vmax(cm-1)3445,1639,1578,1522,1487,1358,1328,1291,1199,1069,862,749,696,574;hrms(esi-tof+):m/zcalcdforc23h17no2+[(m+h)+]340.1332;found,340.1328.
3-benzoyl-6-(4-chlorophenoxy)quinolin-4(1h)-one(1x):whitesolid;yield80%;m.p.291-293oc;1hnmr(400mhz,dmso-d6,ppm):δ12.57(brs,1h),8.36(s,1h),7.76-7.70(m,3h),7.58-7.51(m,3h),7.49-7.42(m,4h),7.14-7.10(m,2h);13cnmr(100mhz,dmso-d6,ppm):δ194.7,174.0,155.6,154.2,143.4,138.9,136.1,132.6,130.5,129.4,128.7,128.4,128.4,125.0,121.8,121.4,119.1,112.5;ir(kbr):vmax(cm-1)3448,2326,1641,1525,1477,1368,1330,1294,1236,1088,1009,833,698;hrms(esi-tof+):m/zcalcdforc22h15clno3+[(m+h)+]376.0735;found,376.0733.
3-benzoylbenzo[h]quinolin-4(1h)-one(1y):whitesolid;yield73%;m.p.276-278oc;1hnmr(400mhz,dmso-d6,ppm):δ12.75(brs,1h),8.74(t,1h,j=3.12hz),8.29(s,1h),8.16-8.10(m,2h),7.88(d,1h,j=8.84hz),7.84-7.79(m,4h),7.61(t,1h,j=7.36hz),7.49(t,2h,j=7.76hz);13cnmr(100mhz,dmso-d6,ppm):δ194.8,174.5,141.9,138.5,135.0,133.0,129.6,129.3,128.6,127.7,125.2,122.5,122.3,122.1;ir(kbr):vmax(cm-1)3442,1644,1599,1550,1420,1328,1270,1183,1067,1009,863,801,763,695,534;hrms(esi-tof+):m/zcalcdforc20h15no2+[(m+h)+]300.1019;found,300.1016.
3-benzoylpyrido[2,3-g]quinolin-4(1h)-one(1za):whitesolid;yield81%;m.p.>300oc;1hnmr(400mhz,dmso-d6,ppm):δ12.85(brs,1h),10.40(d,1h,j=8.28hz),8.93(dd,1h,j=1.40,1.40hz),8.39(s,1h),8.28(d,1h,j=9.16hz),7.99(d,1h,j=9.20hz),7.82(d,2h,j=7.32hz),7.66-7.58(m,2h),7.47(t,2h,j=7.64hz);13cnmr(100mhz,dmso-d6,ppm):δ195.4,177.2,149.8,145.6,140.6,140.0,138.5,135.2,134.5,133.0,129.5,128.7,126.9,124.7,123.4,122.5,119.3;ir(kbr):vmax(cm-1)3449,1654,1597,1559,1528,1368,1310,1284,1206,1075,1014,848,553;hrms(esi-tof+):m/zcalcdforc19h13n2o2+[(m+h)+]301.0972;found,301.0971.
3-benzoylbenzo[b][1,5]naphthyridin-4(1h)-one(1zb):yellowsolid;yield75%;m.p.>300oc;1hnmr(400mhz,dmso-d6,ppm):δ13.13(brs,1h),9.92(dd,1h,j=1.04,1.16hz),9.31(s,1h),8.49(s,1h),8.46(dd,1h,j=0.96,0.84hz),7.84-7.79(m,2h),7.76-7.72(m,2h),7.50(t,2h,j=7.76hz);13cnmr(100mhz,dmso-d6,ppm):δ195.1,176.6,145.6,144.1,140.7,138.2,134.7,133.1,129.7,129.5,129.1,128.8,126.4,126.0,124.4,123.0;ir(kbr):vmax(cm-1)3446,2329,1643,1495,1417,1272.1068,998,816,543;hrms(esi-tof+):m/zcalcdforc19h13n2o2+[(m+h)+]301.0972;found,301.0970.
实施例2
实施例反应式如下:
该制备方法包括以下步骤:
第一步,搅拌下,以4,4-二甲基-3-氧代戊酸乙酯、原甲酸三乙酯、苯胺为原料,三组分一起投入反应器中,升温到130℃保温反应24h,继续加入二苯基醚回流,待有大量固体析出后停止反应得到喹诺酮化合物。减压抽滤,用石油醚、乙酸乙酯洗涤和甲醇洗涤,干燥得到喹诺酮化合物。
实施例中胺类化合物变化制备得到喹诺酮类化合物的得率情况如下:
上述化合物数据如下:
3-pivaloylquinolin-4(1h)-one(2a):whitesolid;yield70%;m.p.198-201oc;1hnmr(400mhz,dmso-d6,ppm):δ12.21(brd,1h,j=4.76hz),8.17(dd,1h,j=1.24,1.24hz),7.04(d,1h,j=6.32hz),7.70-7.66(m,1h),7.60(d,1h,j=7.88hz),7.40-7.36(m,1h),1.23(s,9h);13cnmr(100mhz,dmso-d6,ppm):δ210.3,174.5,140.7,139.7,132.6,126.9,125.8,124.5,123.1,119.0,118.9,44.3,26.7;ir(kbr):vmax(cm-1)3444,2327,1655,1622,1559,1468,1437,1351,1300,1068,845,791,763,545;hrms(esi-tof+):m/zcalcdforc14h16no2+[(m+h)+]230.1176;found,230.1172.
6-fluoro-3-pivaloylquinolin-4(1h)-one(2b):whitesolid;yield81%;m.p.209-211oc;1hnmr(400mhz,dmso-d6,ppm):δ12.38(brs,1h),8.09(s,1h),7.83(dd,1h,j=2.92,2.92hz),7.71-7.67(m,1h),7.63-7.58(m,1h),1.23(s,9h);13cnmr(100mhz,dmso-d6,ppm):δ210.1,173.6,173.5,160.5,158.1,140.9,136.4,128.2,128.1,122.2,121.8,121.7,121.5,121.3,110.1,109.9,44.3,26.6;ir(kbr):vmax(cm-1)3127,2970,1658,1588,1563,1531,1482,1401,1361,1300,1188,1103,1010,920,801,565;hrms(esi-tof+):m/zcalcdforc14h15fno2+[(m+h)+]248.1081;found,248.1079.
6-chloro-3-pivaloylquinolin-4(1h)-one(2c):whitesolid;yield75%;m.p.260-262oc;1hnmr(400mhz,dmso-d6,ppm):δ12.38(brs,1h),8.03(s,1h),8.02(s,1h),7.68(dd,1h,j=2.44,2.40hz),7.59(d,1h,j=8.84hz),1.17(s,9h);13cnmr(100mhz,dmso-d6,ppm):210.0,173.2,141.0,138.4,132.7,130.4,129.2,127.8,124.8,123.2,121.4,119.0,44.3,26.7;hrms(esi-tof+):m/zcalcdforc14h15clno2+[(m+h)+]264.0876;found,264.0873.
6-bromo-3-pivaloylquinolin-4(1h)-one(2d):whitesolid;yield78%;m.p.279-281oc;1hnmr(400mhz,dmso-d6,ppm):δ12.37(brd,1h,j=5.4hz),8.25(d,1h,j=2.32hz),8.10(d,1h,j=6.28hz),7.86(dd,1h,j=2.36,2.36hz),7.60(d,1h,j=8.80hz),1.24(s,9h);13cnmr(100mhz,dmso-d6,ppm):δ210.0,173.2,140.9,138.7,135.3,128.2,128.0,123.3,121.6,117.2,44.3,26.7;ir(kbr):vmax(cm-1)3445,1665,1578,1518,1466,1393,1299,1068,1006,894,823,648,559;hrms(esi-tof+):m/zcalcdforc14h15brno2+[(m+h)+]308.0281;found,308.0280.
6-nitro-3-pivaloylquinolin-4(1h)-one(2e):whitesolid;yield80%;m.p.260-262oc;1hnmr(400mhz,dmso-d6,ppm):δ12.61(brs,1h),8.82(d,1h,j=2.60hz),8.14-8.10(m,2h),7.73(d,1h,j=9.12hz),1.17(s,9h);13cnmr(100mhz,dmso-d6,ppm):δ209.7,174.1,143.7,143.6,141.3,126.7,125.8,125.5,124.3,122.3,120.9,112.8,44.5,26.6;ir(kbr):vmax(cm-1)3447,1664,1614,1518,1469,1360,1300,1076,1007,825,654,561;hrms(esi-tof+):m/zcalcdforc14h15n2o4+[(m+h)+]264.0786;found,264.0784.
3-pivaloyl-6-(trifluoromethyl)quinolin-4(1h)-one(2f):whitesolid;yield83%;m.p.274-276oc;1hnmr(400mhz,dmso-d6,ppm):δ12.53(brs,1h),8.41(d,1h,j=0.92hz),8.15(s,1h),8.01(dd,1h,j=2.61,2.12hz),7.81(d,1h,j=8.68hz),1.23(s,9h);13cnmr(100mhz,dmso-d6,ppm):δ209.9,173.9,142.0,141.3,128.6,128.5,126.1,125.9,124.9,124.5,124.1,123.4,123.4,123.3,123.2,120.7,44.4,26.6;ir(kbr):vmax(cm-1)3168,1669,1616,1566,1472,1408,1327,1203,1120,844,605;hrms(esi-tof+):m/zcalcdforc15h15f3no2+[(m+h)+]298.1049;found,298.1043.
6-acetyl-3-pivaloylquinolin-4(1h)-one(2g):yellowsolid;yield66%;m.p.244-246oc;1hnmr(400mhz,dmso-d6,ppm):δ12.47(brd,1h,j=8.68hz),8.73(d,1h,j=1.88hz),8.20(dd,1h,j=2.04,2.04hz),8.10(d,1h,j=6.16hz),7.68(d,1h,j=8.72hz),2.66(s,3h),1.24(s,9h);13cnmr(100mhz,dmso-d6,ppm):δ210.2,197.2,174.6,142.6,140.9,132.6,131.1,127.5,125.9,124.1,119.5,44.4,27.0,26.7;ir(kbr):vmax(cm-1)3446,1660,1584,1516,1473,1363,1070,831,554;hrms(esi-tof+):m/zcalcdforc16h18no3+[(m+h)+]272.1281;found,272.1281.
6-methyl-3-pivaloylquinolin-4(1h)-one(2h):whitesolid;yield52%;m.p.243-246oc;1hnmr(400mhz,dmso-d6,ppm):δ12.14(brd,1h,j=5.52hz),7.99(d,1h,j=2.36hz),7.95(s,1h),7.51-7.50(m,2h),2.41(s,3h),1.23(s,9h);13cnmr(100mhz,dmso-d6,ppm):δ210.4,174.3,140.3,137.7,133.9,126.8,125.1,122.8,118.8,44.3,26.7,21.2;ir(kbr):vmax(cm-1)3445,1644,1551,1485,1388,1296,1069,804,573;hrms(esi-tof+):m/zcalcdforc15h18no2+[(m+h)+]224.1332;found,224.1327.
6-ethyl-3-pivaloylquinolin-4(1h)-one(2i):whitesolid;yield43%;m.p.170-173oc;1hnmr(400mhz,dmso-d6,ppm):δ12.19(brd,1h,j=6.04hz),8.02(d,1h,j=6.36hz),7.98(s,1h),7.57-7.51(m,2h),2.74(dd,2h,j=7.56,7.56hz),1.24(s,9h),1.21(m,3h);13cnmr(100mhz,dmso-d6,ppm):δ210.4,174.3,140.4,140.2,137.9,132.9,126.9,123.8,122.8,118.9,44.2,28.3,26.7,15.9;ir(kbr):vmax(cm-1)3442,2961,1659,1567,1486,1385,1296,1066,831,568;hrms(esi-tof+):m/zcalcdforc16h20no2+[(m+h)+]258.1489;found,258.1487.
6-(tert-butyl)-3-pivaloylquinolin-4(1h)-one(2j):whitesolid;yield49%;m.p.255-257oc;1hnmr(400mhz,dmso-d6,ppm):δ12.19(brd,1h,j=6.16hz),8.13(d,1h,j=2.24hz),8.02(d,1h,j=6.4hz),7.80(dd,1h,j=2.20,2.24hz),7.57(d,1h,j=7.56hz),1.32(s,9h),1.24(s,9h);13cnmr(100mhz,dmso-d6,ppm):δ210.4,174.5,147.1,140.5,137.7,130.7,126.4,122.9,120.9,118.8,44.2,34.9,31.4,26.7;ir(kbr):vmax(cm-1)3446,3085,2969,1689,1551,1488,1358,1202,1071,1009,839,697;hrms(esi-tof+):m/zcalcdforc18h24no2+[(m+h)+]286.1802;found,286.1797.
6-methoxy-3-pivaloylquinolin-4(1h)-one(2k):whitesolid;yield56%;m.p.198-200oc;1hnmr(400mhz,dmso-d6,ppm):δ12.21(brd,1h,j=5.60hz),8.00(d,1h,j=6.40hz),7.58-7.55(m,2h),7.34(dd,1h,j=2.92,2.96hz),3.84(s,3h),1.24(s,9h);13cnmr(100mhz,dmso-d6,ppm):δ210.4,173.7,156.6,139.9,134.2,128.1,122.9,121.9,120.7,105.4,55.8,44.2,26.7;ir(kbr):vmax(cm-1)3445,1664,1548,1514,1364,1307,1071,1006,861,570;hrms(esi-tof+):m/zcalcdforc15h18no3+[(m+h)+]260.1281;found,260.1283.
8-bromo-3-pivaloylquinolin-4(1h)-one(2l):whitesolid;yield72%;m.p.211-213oc;1hnmr(400mhz,dmso-d6,ppm):δ11.56(brs,1h),8.20(dd,1h,j=1.24,1.28hz),8.05-8.02(m,1h),7.91(s,1h),7.36-7.31(m,1h),1.23(s,9h);13cnmr(100mhz,dmso-d6,ppm):δ209.7,174.1,141.3,137.3,136.2,128.3,125.9,125.4,123.4,111.9,44.4,26.6;ir(kbr):vmax(cm-1)3446,1611,1544,1438,1333,1300,1071,1001,868,748,543;hrms(esi-tof+):m/zcalcdforc14h15brno2+[(m+h)+]308.0281;found,308.0281.
8-methyl-3-pivaloylquinolin-4(1h)-one(2m):whitesolid;yield68%;m.p.231-233oc;1hnmr(400mhz,dmso-d6,ppm):δ11.57(brs,1h),8.05(d,1h,j=8.08hz),7.09(d,1h,j=4.32hz),7.55(d,1h,j=7.04hz),7.29(t,1h,j=7.72hz),2.50(s,1h),1.24(s,9h);13cnmr(100mhz,dmso-d6,ppm):δ210.2,174.7,140.6,138.3,133.4,127.1,127.1,124.2,123.8,123.0,44.3,26.7,17.5;ir(kbr):vmax(cm-1)3448,1621,1552,1445,1363,1280,1070,1003,886,545;hrms(esi-tof+):m/zcalcdforc15h18no2+[(m+h)+]224.1332;found,224.1329.
8-methoxy-3-pivaloylquinolin-4(1h)-one(2n):whitesolid;yield64%;m.p.227-230oc;1hnmr(400mhz,dmso-d6,ppm):δ11.84(brd,1h,j=5.64hz),7.83(d,1h,j=6.44hz),7.73(dd,1h,j=1.80,1.80hz),7.34-7.27(m,2h),4.00(s,3h),1.23(s,9h);13cnmr(100mhz,dmso-d6,ppm):δ210.2,174.2,149.0,140.1,130.3,127.8,124.4,123.3,117.0,112.1,56.7,44.3,26.6;ir(kbr):vmax(cm-1)3445,2960,2338,1668,1549,1454,1304,1270,1080,862,773,673;hrms(esi-tof+):m/zcalcdforc15h18no3+[(m+h)+]260.1281;found,260.1278.
7-methyl-3-pivaloylquinolin-4(1h)-one(2o):whitesolid;yield50%;m.p.246-248oc;1hnmr(400mhz,dmso-d6,ppm):δ12.08(brs,1h),8.07(dd,2h,j=8.24,5.64hz),7.36(s,1h),7.22(dd,1h,j=1.16,1.12hz),2.43(s,3h),1.25(s,9h);13cnmr(100mhz,dmso-d6,ppm):δ210.3,174.3,142.9,140.5,139.9,126.1,125.8,124.9,123.0,118.7,44.2,27.0,26.7;ir(kbr):vmax(cm-1)3445,3228,3095,2874,1692,1630,1558,1524,1475,1363,1307,1070,999,889,775,698,560;hrms(esi-tof+):m/zcalcdforc15h18no2+[(m+h)+]224.1332;found,224.1330.
7-methoxy-3-pivaloylquinolin-4(1h)-one(2p):whitesolid;yield40%;m.p.245-246oc;1hnmr(400mhz,dmso-d6,ppm):δ12.00(brd,1h,j=5.64hz),8.07(d,1h,j=9.60hz),7.97(d,1h,j=6.24hz),7.00-6.97(m,2h),3.86(s,3h),1.23(s,9h);13cnmr(100mhz,dmso-d6,ppm):δ210.4,174.0,162.6,141.5,140.4,127.7,123.0,121.0,114.4,99.9,55.9,44.3,26.7;ir(kbr):vmax(cm-1)3445,2979,1633,1528,1478,1355,1268,1096,1021,568;hrms(esi-tof+):m/zcalcdforc15h18no3+[(m+h)+]260.1281;found,260.1279.
6-benzyl-3-pivaloylquinolin-4(1h)-one(2q):whitesolid;yield57%;m.p.239-240oc;1hnmr(400mhz,dmso-d6,ppm):δ12.17(brs,1h),8.00(d,2h,j=2.16hz),7.59-7.52(m,2h),7.32-7.17(m,5h),4.07(s,2h),1.23(s,9h);13cnmr(100mhz,dmso-d6,ppm):δ210.3,174.3,141.4,140.4,138.1,137.8,133.5,129.2,128.9,126.9,126.5,125.0,122.9,119.1,41.1,26.7;ir(kbr):vmax(cm-1)3488,2970,1689,1620,1551,1488,1413,1358,1202,1073,1010,839,791,616;hrms(esi-tof+):m/zcalcdforc21h22no2+[(m+h)+]320.1645;found,320.1640.
3-pivaloylbenzo[g]quinolin-4(1h)-one(2r):whitesolid;yield51%;m.p.222-224oc;1hnmr(400mhz,dmso-d6,ppm):δ12.40(brs,1h),10.24(d,1h,j=8.44hz),8.17(d,1h,j=8.88hz),7.99(t,2h,j=7.96hz),7.72-7.59(m,3h),1.27(s,9h);13cnmr(100mhz,dmso-d6,ppm):δ211.9,177.0,140.8,135.3,134.1,131.3,130.2,128.7,128.6,128.1,126.7,126.3,118.8,118.6,44.5,27.0;ir(kbr):vmax(cm-1)3445,1688,1634,1600,1492,1357,1260,1204,1069,865,825,574;hrms(esi-tof+):m/zcalcdforc18h18no2+[(m+h)+]280.1332;found,280.1331.
3-pivaloylbenzo[h]quinolin-4(1h)-one(2s):whitesolid;yield74%;m.p.247-249oc;1hnmr(400mhz,dmso-d6,ppm):δ12.55(brd,1h,j=4.80hz),8.70(t,1h,j=3.24hz),8.19-8.00(m,3h),7.84-7.77(m,3h),1.27(s,9h);13cnmr(100mhz,dmso-d6,ppm):δ210.4,174.2,138.8,136.9,134.8,129.2,129.2,127.5,125.4,124.8,123.8,122.5,122.1,44.4,26.7;ir(kbr):vmax(cm-1)3444,1664,1634,1548,1425,1425,1386,1272,1076,1006,861,829,829,570;hrms(esi-tof+):m/zcalcdforc18h18no2+[(m+h)+]280.1332;found,280.1331.
实施例3
实施例反应式如下:
该制备方法包括以下步骤:
第一步,搅拌下,以3-环丙基-3-羰基-丙酸乙酯、原甲酸三乙酯、苯胺为原料,三组分一起投入反应器中,升温到130℃保温反应24h,继续加入二苯基醚回流,待有大量固体析出后停止反应得到喹诺酮化合物。减压抽滤,用石油醚、乙酸乙酯洗涤和甲醇洗涤,干燥得到喹诺酮化合物。
实施例中胺类化合物变化制备得到喹诺酮类化合物的得率情况如下:
上述化合物数据如下:
3-(cyclopropanecarbonyl)quinolin-4(1h)-one(3a):brownsolid;yield75%;m.p.276-278oc;1hnmr(400mhz,dmso-d6,ppm):δ12.49(brs,1h),8.46(s,1h),8.26(dd,1h,j=1.28,1.28hz),7.74-7.70(m,1h),7.63(d,1h,j=8.00hz),7.46-7.42(m,1h),3.69-3.63(m,1h),1.00-0.91(m,4h);13cnmr(100mhz,dmso-d6,ppm):δ199.6,175.9,144.4,139.4,132.9,128.4,126.2,125.3,119.3,118.1,19.4,11.7;ir(kbr):vmax(cm-1)3441,1646,1559,1523,1474,1441,1354,1274,1067,1009,910,850,772,586;hrms(esi-tof+):m/zcalcdforc13h12no2+[(m+h)+]214.0863;found,214.0865.
3-(cyclopropanecarbonyl)quinolin-4(1h)-one(3b):gray-whitesolid;yield76%;m.p.275-277oc;1hnmr(400mhz,dmso-d6,ppm):δ12.61(brs,1h),8.47(s,1h),7.88(dd,1h,j=2.92,2.92hz),7.72-7.59(m,2h),3.65-3.59(m,1h),1.00-0.97(m,4h);13cnmr(100mhz,dmso-d6,ppm):δ199.4,175.0,174.9,160.9,158.5,144.4,136.1,130.0,129.9,122.2,121.7,121.4,117.3,110.7,110.5,19.4,11.8;ir(kbr):vmax(cm-1)3445,1652,1572,1520,1384,1362,1293,1270,1091,1016,866,822,772,577;hrms(esi-tof+):m/zcalcdforc13h11fno2+[(m+h)+]232.0768;found,232.0763.
6-chloro-3-(cyclopropanecarbonyl)quinolin-4(1h)-one(3c):whitesolid;yield74%;m.p.>300oc;1hnmr(400mhz,dmso-d6,ppm):δ12.63(brs,1h),8.48(s,1h),8.15(d,1h,j=2.40hz),7.77-7.65(m,2h),3.62-3.56(m,1h),1.00-0.97(m,4h);13cnmr(100mhz,dmso-d6,ppm):δ199.3,174.6,144.7,138.1,113.0,130.0,129.6,125.2,121.7,118.2,19.5,11.9;ir(kbr):vmax(cm-1)3445,2338,1649,1552,1399,1350,1299,1076,1014,845,637,557;hrms(esi-tof+):m/zcalcdforc13h11clno2+[(m+h)+]248.0473;found,248.0467.
6-bromo-3-(cyclopropanecarbonyl)quinolin-4(1h)-one(3d):whitesolid;yield70%;m.p.258-260oc;1hnmr(400mhz,dmso-d6,ppm):δ12.56(brs,1h),8.43(s,1h),8.24(d,1h,j=2.32hz),7.81(dd,1h,j=2.36,2.32hz),7.54(d,1h,j=8.76hz),3.56-3.50(m,1h),0.96-0.86(m,4h);13cnmr(100mhz,dmso-d6,ppm):δ199.3,174.5,144.7,138.4,135.6,129.9,128.4,121.9,118.3,118.1,19.2,11.9;ir(kbr):vmax(cm-1)3445,2338,1648,1616,1551,1464,1400,1364,1298,1068,1014,920,841,585;hrms(esi-tof+):m/zcalcdforc13h11brno2+[(m+h)+]291.9968;found,291.9966.
3-(cyclopropanecarbonyl)-6-iodoquinolin-4(1h)-one(3e):whitesolid;yield71%;m.p.>300oc;1hnmr(400mhz,dmso-d6,ppm):δ12.58(brs,1h),8.51(d,1h,j=1.96hz),8.47(s,1h),8.01(dd,1h,j=2.04,2.04hz),7.45(d,1h,j=8.64hz),3.61-3.55(m,1h),1.00-0.96(m,4h);13cnmr(100mhz,dmso-d6,ppm):δ199.3,174.4,144.7,141.0,138.8,134.7,130.1,121.7,118.4,90.3,19.5,11.9;ir(kbr):vmax(cm-1)3446,2329,1646,1616,1550,1460,1401,1343,1299,1080,1014,550;hrms(esi-tof+):m/zcalcdforc13h11ino2+[(m+h)+]339.9829;found,339.9822.
3-(cyclopropanecarbonyl)-6-nitroquinolin-4(1h)-one(3f):gray-whitesolid;yield82%;m.p.>300oc;1hnmr(400mhz,dmso-d6,ppm):δ12.90(brs,1h),8.91(d,1h,j=2.64hz),8.52(s,1h),8.47(dd,1h,j=2.68,2.68hz),7.81(d,1h,j=9.08hz),3.54-3.48(m,1h),1.03-0.98(m,4h);13cnmr(100mhz,dmso-d6,ppm):δ199.1,175.1,145.7,144.2,143.3,127.8,127.1,122.4,121.2,119.1,19.7,12.1;ir(kbr):vmax(cm-1)3445,1648,1556,1505,1401,1342,1301,1068,931,859,794,545;hrms(esi-tof+):m/zcalcdforc13h11n2o4+[(m+h)+]259.0713;found,259.0713.
3-(cyclopropanecarbonyl)-6-(trifluoromethyl)quinolin-4(1h)-one(3g):whitesolid;yield80%;m.p.>300oc;1hnmr(400mhz,dmso-d6,ppm):δ12.77(brs,1h),8.54(s,1h),8.47(s,1h)8.03(dd,1h,j=1.60,1.84hz),7.83(d,1h,j=8.64hz),3.59-3.53(m,1h),1.00-0.94(m,4h);13cnmr(100mhz,domso-d6,ppm):δ199.3,175.1,145.5,141.8,129.0,127.9,125.8,125.6,125.2,123.7,123.1,121.0,119.0,19.6,12.0;ir(kbr):vmax(cm-1)3445,1650,1561,1525,1407,1332,1297,1070,1013,546;hrms(esi-tof+):m/zcalcdforc14h11f3no2+[(m+h)+]282.0736;found,282.0731.
6-acetyl-3-(cyclopropanecarbonyl)quinolin-4(1h)-one(3h):brownsolid;yield76%;m.p.>300oc;1hnmr(400mhz,dmso-d6,ppm):δ12.70(brs,1h),8.79(d,1h,j=2.00hz),8.50(s,1h),8.23(dd,1h,j=2.04,2.08hz),7.72(d,1h,j=8.60hz),3.62-3.56(m,1h),2.66(s,3h)1.02-0.99(m,4h);13cnmr(100mhz,dmso-d6,ppm):δ199.4,197.2,175.8,145.1,142.4,133.3,131.7,127.7,127.5,119.9,119.0,27.1,19.6,11.9;ir(kbr):vmax(cm-1)3446,3071,1684,1649,1557,1526,1481,1391,1356,1297,1046,1018,980,916,829,781,608,515;hrms(esi-tof+):m/zcalcdforc15h14no3+[(m+h)+]256.0968;found,256.0963.
3-(cyclopropanecarbonyl)-6-methylquinolin-4(1h)-one(3i):gray-whitesolid;yield65%;m.p.277-279oc;1hnmr(400mhz,dmso-d6,ppm):δ12.43(brd,1h,j=5.66hz),8.43(d,1h,j=6.76hz),8.03(s,1h),7.55-7.51(m,2h),3.70-3.64(m,1h),3.34(s,3h)0.99-0.90(m,4h);13cnmr(100mhz,dmso-d6,ppm):δ199.6,175.7,143.9,137.4,134.8,134.2,128.4,125.6,119.2,117.8,21.2,19.3,11.7;ir(kbr):vmax(cm-1)3437,2360,1651,1556,1523,1489,1359,1259,1194,1049,1015,580;hrms(esi-tof+):m/zcalcdforc14h14no2+[(m+h)+]228.1019;found,228.1017.
3-(cyclopropanecarbonyl)-6-ethylquinolin-4(1h)-one(3j):whitesolid;yield69%;m.p.162-164oc;1hnmr(400mhz,dmso-d6,ppm):δ12.44(brs,1h),8.43(d,1h,j=5.32hz),8.06(d,1h,j=1.24hz),7.60-7.54(m,2h),3.70-3.64(m,1h),3.33(s,1h)2.76-2.71(m,2h),1.23(t,3h,j=7.56hz),1.00-0.90(m,4h);13cnmr(100mhz,dmso-d6,ppm):δ199.6,175.8,143.9,141.1,137.6,133.2,128.4,124.3,119.3,117.8,28.3,19.3,15.9,11.7;ir(kbr):vmax(cm-1)3447,1651,1555,1524,1490,1358,1292,1213,1065,1014,828,783,595;hrms(esi-tof+):m/zcalcdforc15h16no2+[(m+h)+]242.1176;found,242.1174.
6-(tert-butyl)-3-(cyclopropanecarbonyl)quinolin-4(1h)-one(3k):whitesolid;yield54%;m.p.239-241oc;1hnmr(400mhz,dmso-d6,ppm):δ12.44(brs,1h),8.43(s,1h),8.23(d,1h,j=2.96hz),7.83(dd,1h,j=2.28,2.28hz),7.59(d,1h,j=8.64hz),3.70-3.64(m,1h),1.34(s,9h),1.00-0.90(m,4h);13cnmr(100mhz,dmso-d6,ppm):δ199.6,175.9,147.9,143.9,137.4,131.0,127.9,121.4,119.1,117.9,35.0,31.4,19.4,11.6;ir(kbr):vmax(cm-1)3445,3205,3078,2961,2361,1655,1565,1522,1388,1358,1265,1088,1043,1012,924,831,598,571;hrms(esi-tof+):m/zcalcdforc17h20no2+[(m+h)+]270.1489;found,270.1489.
3-(cyclopropanecarbonyl)-6-methoxyquinolin-4(1h)-one(3l):whitesolid;yield62%;m.p.254-256oc;1hnmr(400mhz,dmso-d6,ppm):δ12.47(brd,1h,j=5.64hz),8.41(d,1h,j=6.80hz),7.66(dd,2h,j=2.88,8.96hz),7.38-7.32(m,1h),3.85(s,3h),3.72-3.66(m,1h),0.99-0.90(m,4h);13cnmr(100mhz,dmso-d6,ppm):δ199.6,175.2,157.1,143.1,133.8,129.7,122.7,121.0,117.1,106.2,55.9,19.3,11.6;ir(kbr):vmax(cm-1)3445,2326,1651,1584,1532,1491,1384,1303,1071,1013,817,567;hrms(esi-tof+):m/zcalcdforc14h14no3+[(m+h)+]244.0968;found,244.0965.
8-bromo-3-(cyclopropanecarbonyl)quinolin-4(1h)-one(3m):whitesolid;yield78%;m.p.261-263oc;1hnmr(400mhz,dmso-d6,ppm):δ11.80(brs,1h),8.38(s,1h),8.27(dd,1h,j=1.36,1.32hz),8.07(dd,1h,j=1.32,1.32hz),7.38(t,1h,j=7.84hz),3.62-3.56(m,1h),1.02-0.94(m,4h);13cnmr(100mhz,dmso-d6,ppm):δ199.2,175.3,145.1,137.1,136.5,130.1,126.2,126.2,118.3,112.3,19.5,12.0;ir(kbr):vmax(cm-1)3447,1657,1601,1543,1436,1389,1328,1273,1206,1068,1008,907,788,756,545;hrms(esi-tof+):m/zcalcdforc13h11brno2+[(m+h)+]291.9968;found,291.9965.
3-(cyclopropanecarbonyl)-8-methylquinolin-4(1h)-one(3n):whitesolid;yield64%;m.p.242-244oc;1hnmr(400mhz,dmso-d6,ppm):δ11.80(brd,1h,j=5.64hz),8.34(d,1h,j=6.96hz),8.12(d,1h,j=7.80hz),7.58-7.56(m,1h)7.33(t,1h,j=7.64hz),3.71-3.64(m,1h),2.51(s,3h)1.01-0.92(m,4h);13cnmr(100mhz,dmso-d6,ppm):δ199.5,176.1,144.0,137.9,133.8,128.6,127.5,124.9,124.2,117.8,19.4,17.3,11.8;ir(kbr):vmax(cm-1)3446,2324,1652,1553,1453,1388,1282,1213,1052,905,766,558;hrms(esi-tof+):m/zcalcdforc14h14no2+[(m+h)+]228.1019;found,228.1016.
3-(cyclopropanecarbonyl)-8-isopropylquinolin-4(1h)-one(3o):whitesolid;yield52%;m.p.235-237oc;1hnmr(400mhz,dmso-d6,ppm):δ11.81(brs,1h),8.35(d,1h,j=5.28hz),8.16(dd,1h,j=1.20,1.20hz),7.66(dd,1h,j=0.96,1.00hz),7.40(t,1h,j=7.67hz),3.71-3.65(m,1h),3.48-3.42(m,1h),1.29(d,6h,j=6.76hz)1.01-0.92(m,4h);13cnmr(100mhz,dmso-d6,ppm):δ199.5,176.1,144.1,137.7,136.5,129.1,128.9,125.2,124.1,117.5,26.8,23.3,19.4,11.8;ir(kbr):vmax(cm-1)3444,1659,1611,1547,1447,1390,1357,1269,1208,1045,1014,905,853,760,583;hrms(esi-tof+):m/zcalcdforc16h18no2+[(m+h)+]256.1332;found,256.1324.
3-(cyclopropanecarbonyl)-8-methoxyquinolin-4(1h)-one(3p):whitesolid;yield58%;m.p.245-247oc;1hnmr(400mhz,dmso-d6,ppm):δ12.07(brs,1h),8.28(s,1h),7.80(dd,1h,j=1.44,1.48hz),7.38-7.31(m,2h),4.00(s,3h),3.70-3.63(m,1h),1.00-0.91(m,4h);13cnmr(100mhz,dmso-d6,ppm):δ199.6,175.7,149.2,143.5,129.8,129.4,125.2,118.2,117.4,112.8,56.8,19.4,11.8;ir(kbr):vmax(cm-1)3444,3190,1654,1573,1533,1466,1392,1287,1264,1219,1077,1008,932,806,755,587,529;hrms(esi-tof+):m/zcalcdforc14h14no3+[(m+h)+]244.0968;found,244.0966.
7-bromo-3-(cyclopropanecarbonyl)quinolin-4(1h)-one(3q):whitesolid;yield67%;m.p.289-291oc;1hnmr(400mhz,dmso-d6,ppm):δ12.41(brs,1h),8.42(s,1h),8.08(d,1h,j=8.64hz),7.76(d,1h,j=1.76hz),7.53(dd,1h,j=1.84,1.80hz),3.55-3.49(m,1h),0.94-0.86(m,4h);13cnmr(100mhz,dmso-d6,ppm):δ199.4,175.3,145.0,140.4,128.5,128.3,127.3,126.3,121.6,118.6,19.4,11.9;ir(kbr):vmax(cm-1)3448,1650,1524,1457,1393,1295,1077,1013,778,548;hrms(esi-tof+):m/zcalcdforc13h11brno2+[(m+h)+]293.9947;found,293.9941.
3-(cyclopropanecarbonyl)-7-methylquinolin-4(1h)-one(3r):pale-yellowsolid;yield44%;m.p.261-263oc;1hnmr(400mhz,dmso-d6,ppm):δ12.37(brs,1h),8.41(s,1h),8.13(d,1h,j=8.24hz),7.38(s,1h),7.27(dd,1h,j=1.08,1.08hz),3.70-3.63(m,1h),2.43(s,3h),0.99-0.90(m,4h);13cnmr(100mhz,dmso-d6,ppm):δ199.6,175.7,144.2,143.3,139.5,126.9,126.3,126.2,118.6,118.0,21.6,19.4,11.7;ir(kbr):vmax(cm-1)3445,1648,1559,1528,1477,1385,1355,1284,1226,1046,1011,909,849,788,592,513;hrms(esi-tof+):m/zcalcdforc14h14no2+[(m+h)+]228.1019;found,228.1015.
3-(cyclopropanecarbonyl)-7-methoxyquinolin-4(1h)-one(3s):pale-brownsolid;yield49%;m.p.271-273oc;1hnmr(400mhz,dmso-d6,ppm):δ12.26(brs,1h),8.40(s,1h),8.15(dd,1h,j=1.16,1.20hz),7.03-7.01(m,2h),3.86(s,3h),3.68-3.63(m,1h),0.99-0.89(m,4h);13cnmr(100mhz,dmso-d6,ppm):δ199.6,175.4,162.8,144.3,141.2,128.1,122.4,118.0,114.7,100.6,56.0,19.3,11.7;ir(kbr):vmax(cm-1);3442,1648,1573,1532,1481,1386,1267,1179,1024,853,783,591,512;hrms(esi-tof+):m/zcalcdforc14h14no3+[(m+h)+]244.0968;found,244.0964.
7,8-dichloro-3-(cyclopropanecarbonyl)quinolin-4(1h)-one(3t):whitesolid;yield67%;m.p.>300oc;1hnmr(400mhz,dmso-d6,ppm):δ12.16(brs,1h),8.34(d,1h,j=3.12hz),8.19(d,1h,j=8.72hz),7.66(d,1h,j=8.76hz),3.58-3.51(m,1h),1.01-0.96(m,4h);13cnmr(100mhz,dmso-d6,ppm):δ199.1,174.9,145.3,137.4,136.3,128.3,126.4,126.2,121.0,118.7,19.6,12.2;ir(kbr):vmax(cm-1)3445,2390,1658,1604,1520,1437,1386,1295,1179,1067,1037,1007,842,778,538;hrms(esi-tof+):m/zcalcdforc13h10cl2no2+[(m+h)+]282.0080;found,282.0078.
6-benzyl-3-(cyclopropanecarbonyl)quinolin-4(1h)-one(3u):whitesolid;yield78%;m.p.228-230oc;1hnmr(400mhz,dmso-d6,ppm):δ12.49(brd,1h,j=6.12hz),8.44(d,1h,j=6.68hz),8.08(s,1h),7.61-7.55(m,2h),7.32-7.24(m,4h),7.20(t,1h,j=7.04hz),4.08(s,2h),3.68-3.64(m,1h),1.00-0.90(m,4h);13cnmr(100mhz,dmso-d6,ppm):δ199.6,175.8,144.0,141.3,138.7,137.8,133.8,129.2,128.9,128.4,126.5,125.5,119.5,117.9,19.4,11.7;ir(kbr):vmax(cm-1)3421,1645,1571,1512,1488,1391,1357,1292,1216,1072,1015,959,853,796,619,562;hrms(esi-tof+):m/zcalcdforc20h18no2+[(m+h)+]304.1332;found,304.1331.
6-(4-chlorophenoxy)-3-(cyclopropanecarbonyl)quinolin-4(1h)-one(3v):pale-brownsolid;yield73%;m.p.286-288oc;1hnmr(400mhz,dmso-d6,ppm):δ12.62(brs,1h),8.47(s,1h),7.73-7.65(m,2h),7.53-7.48(m,3h),7.14(d,2h,j=7.63hz),3.61(s,1h),0.97-0.92(m,4h),13cnmr(100mhz,dmso-d6,ppm):δ199.8,175.5,142.9,136.5,135.0,129.3,129.2,127.7,125.7,125.5,123.8,122.4,122.3,119.9,19.6,12.0;ir(kbr):vmax(cm-1)3447,2025,1652,1563,1478,1390,1273,1238,1086,1013,830,569;hrms(esi-tof+):m/zcalcdforc19h15clno3+[(m+h)+]340.0735;found,340.0734.
3-(cyclopropanecarbonyl)benzo[h]quinolin-4(1h)-one(3w):pale-brownsolid;yield69%;m.p.>300oc;1hnmr(400mhz,f3ccood,ppm):δ9.41(s,1h),8.57(d,1h,j=8.20hz),8.26(d,1h,j=9.04hz),8.02(t,2h,j=9.84hz),7.87-7.78(m,2h),2.67-2.68(m,1h),1.48-1.33(m,4h);13cnmr(100mhz,dmso-d6,ppm):δ207.4,175.3,145.4,141.1,139.0,134.9,133.5,131.9,124.0,123.3,121.5,120.3,114.7,19.4,16.9;ir(kbr):vmax(cm-1)3446,2327,1656,1574,1524,1435,1389,1272,1072,1010,756,536;hrms(esi-tof+):m/zcalcdforc17h14no2+[(m+h)+]264.0109;found,264.0107.
实施例4
实施例反应式如下:
该制备方法包括以下步骤:
第一步,搅拌下,以丙二酸二乙酯、原甲酸三乙酯、苯胺为原料,三组分一起投入反应器中,升温到130℃保温反应24h,继续加入二苯基醚回流,待有大量固体析出后停止反应得到喹诺酮化合物。减压抽滤,用石油醚、乙酸乙酯洗涤和甲醇洗涤,干燥得到喹诺酮化合物。
实施例中胺类化合物变化制备得到喹诺酮类化合物的得率情况如下:
上述化合物数据如下:
ethyl4-oxo-1,4-dihydroquinoline-3-carboxylate(4a):whitesolid;yield69%;m.p.279-281oc;1hnmr(400mhz,dmso-d6,ppm):δ12.32(brd,1h,j=4.92hz),8.55(d,1h,j=6.64hz),8.16(dd,1h,j=0.88,0.84hz),7.70-7.68(m,1h),7.62(d,1h,j=8.00hz),7.43-7.39(m,1h),4.23(q,2h,j=7.20hz),1.27(t,3h,j=7.12hz);13cnmr(100mhz,dmso-d6,ppm):δ173.9,165.2,145.3,139.4,132.8,127.7,126.0,125.1,119.2,110.2,60.0,14.7;hrms(esi-tof+):m/zcalcdforc12h12no3+[(m+h)+]218.0812;found,218.0808.
ethyl6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate(4b):whitesolid;yield76%;m.p.217-219oc;1hnmr(400mhz,f3ccood,ppm):δ9.11(s,1h),8.07-8.00(m,2h),7.78-7.73(m,1h),4.51(q,2h,j=7.20hz),1.33(t,3h,j=7.16hz);13cnmr(100mhz,f3ccood,ppm):δ175.4,175.3,169.8,147.1,138.7,130.1,129.8,125.4,125.3,67.2,14.5.
ethyl6-chloro-4-oxo-1,4-dihydroquinoline-3-carboxylate(4c):pale-yellowsolid;yield74%;m.p.>300oc;1hnmr(400mhz,f3ccood,ppm):δ9.15(s,1h),8.45(d,1h,j=1.76hz),8.00-7.96(m,2h),4.54(q,2h),1.36(t,3h,j=7.16hz);13cnmr(100mhz,f3ccood,ppm):δ174.9,169.6,147.4,140.7,140.0,140.0,126.0,123.8,123.2,107.7,67.1,14.4.
ethyl6-bromo-4-oxo-1,4-dihydroquinoline-3-carboxylate(4d):whitesolid;yield72%;m.p.>300oc;1hnmr(400mhz,f3ccood,ppm):δ9.19(s,1h),8.67(s,1h),8.17(d,1h,j=8.88hz),7.92(d,1h,j=8.84hz),4.56(t,2h,j=4.80hz),1.40(t,3h,j=5.44hz);13cnmr(100mhz,f3ccood,ppm):δ174.6,169.4,147.2,143.3,140.1,129.2,127.1,123.4,123.2,107.5,66.9,14.2.
ethyl6-iodo-4-oxo-1,4-dihydroquinoline-3-carboxylate(4e):pale-yellowsolid;yield70%;m.p.>300oc;1hnmr(400mhz,f3ccood,ppm):δ9.16(s,1h),8.88(s,1h),8.32(d,1h,j=8.88hz),7.72(d,1h,j=8.92hz),4.55(q,2h,j=7.20hz),1.37(t,3h,j=7.12hz);13cnmr(100mhz,f3ccood,ppm):δ174.6,169.6,149.1,147.5,140.8,136.1,123.5,123.1,107.8,97.2,67.1,14.4.
ethyl4-oxo-6-(trifluoromethyl)-1,4-dihydroquinoline-3-carboxylate(4f):whitesolid;yield76%;m.p.>300oc;1hnmr(400mhz,f3ccood,ppm):δ9.24(s,1h),8.76(s,1h),8.20-8.21(m,2h),4.51(q,2h,j=7.20hz),1.32(t,3h,j=7.61hz);13cnmr(100mhz,f3ccood,ppm):δ176.4,169.5,149.3,143.0,135.9,135.5,135.2,125.0,125.0,123.9,122.1,108.3,67.3,14.4.
ethyl6-methyl-4-oxo-1,4-dihydroquinoline-3-carboxylate(4g):pale-yellowsolid;yield55%;m.p.289-291oc;1hnmr(400mhz,f3ccood,ppm):δ9.08(s,1h),8.31(s,1h),7.93-7.86(m,2h),4.55(q,2h,j=6.40hz),2.54(s,3h),1.40(t,3h,j=5.36hz);13cnmr(100mhz,f3ccood,ppm):δ175.1,169.8,146.0,144.7,142.0,139.9,125.7,122.3,121.7,106.8,66.7,22.0,14.3.
ethyl6-ethyl-4-oxo-1,4-dihydroquinoline-3-carboxylate(4h):whitesolid;yield56%;m.p.271-273oc;1hnmr(400mhz,f3ccood,ppm):δ9.04(s,1h),8.29(s,1h),7.93(dd,2h,j=8.76,8.68hz),4.51(q,2h,j=6.80hz),2.84(q,2h,j=7.60hz),1.36-1.20(m,6h);13cnmr(100mhz,f3ccood,ppm):δ175.3,169.9,150.9,146.1,141.2,140.1,124.5,122.5,121.9,106.9,66.8,30.5,15.3,14.3.
ethyl6-isopropyl-4-oxo-1,4-dihydroquinoline-3-carboxylate(4i):whitesolid;yield64%;m.p.247-249oc;1hnmr(400mhz,f3ccood,ppm):δ9.07(s,1h),8.35(d,1h,j=1.52hz),8.02(dd,2h,j=1.80,1.80hz),7.91(d,1h,j=8.80hz),4.54(q,2h,j=7.20hz),3.14-3.07(m,1h),1.37(t,3h,j=7.16hz),1.27(d,6h,j=6.92hz);13cnmr(100mhz,f3ccood,ppm):δ175.1,169.8,155.3,145.9,140.0,139.9,106.7,66.6,36.4,23.8,14.1.
ethyl6-(tert-butyl)-4-oxo-1,4-dihydroquinoline-3-carboxylate(4j):whitesolid;yield71%;m.p.271-273oc;1hnmr(400mhz,f3ccood,ppm):δ9.08(s,1h),8.51(d,1h,j=1.92hz),8.23(dd,1h,j=2.04,2.04hz),7.92(d,1h,j=9.00hz),4.54(q,2h,j=5.36hz),1.39-1.34(m,12h);13cnmr(100mhz,f3ccood,ppm);δ175.3,169.8,157.8,146.0,139.8,139.1,122.0,121.9,121.5,106.7,66.6,37.3,31.3,14.2.
ethyl6-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylate(4k):brownsolid;yield69%;m.p.282-284oc;1hnmr(400mhz,f3ccood,ppm):δ8.94(s,1h),7.85(d,1h,j=9.28hz),7.70-7.62(m,1h),4.49(q,2h,j=7.20hz),3.87(s,3h),1.34(t,3h,j=7.16hz);13cnmr(100mhz,f3ccood,ppm):δ174.2,170.1,144.4,137.2,132.5,124.3,123.8,107.1,104.7,66.9,57.7,14.5.
ethyl8-methyl-4-oxo-1,4-dihydroquinoline-3-carboxylate(4l):brownsolid;yield70%;m.p.255-257oc;1hnmr(400mhz,dmso-d6,ppm):δ11.92(brs,1h),8.34(s,1h),7.72(dd,1h,j=1.24,1.36hz),7.37-7.31(m,2h),4.24(q,2h,j=7.20hz),4.00(s,1h),1.27(t,3h,j=7.08hz);13cnmr(100mhz,f3ccood,ppm):δ173.6,165.1,149.1,144.4,129.8,128.6,125.0,117.2,112.6,110.4,60.0,56.8,14.7.
ethyl7-bromo-4-oxo-1,4-dihydroquinoline-3-carboxylate(4m):pale-pinksolid;yield62%;m.p.>300oc;1hnmr(400mhz,f3ccood,ppm):δ9.12(s,1h),8.33(d,1h,j=8.80hz),8.18(s,1h),7.91(d,1h,j=8.80hz),4.52(q,2h,j=7.20hz),1.37(t,3h,j=7.20hz);13cnmr(100mhz,f3ccood,ppm):δ175.8,169.6,148.0,142.0,136.4,136.3,127.9,125.0,120.9,107.4,67.0,14.3.
ethyl7-nitro-4-oxo-1,4-dihydroquinoline-3-carboxylate(4n):yellowsolid;yield66%;m.p.>300oc;1hnmr(400mhz,f3ccood,ppm):δ9.45(s,1h),9.03(s,1h),8.81(d,1h,j=9.20hz),8.62(t,1h,j=1.60hz),4.64(q,2h,j=7.20hz),1.46(q,3h,j=3.20hz);13cnmr(100mhz,f3ccood,ppm):δ175.7,169.8,151.4,145.9,132.9,132.8,123.1,117.6,107.5,66.9,58.2,14.3.
ethyl7-methyl-4-oxo-1,4-dihydroquinoline-3-carboxylate(4o):pale-brownsolid;yield50%;m.p.281-282oc;1hnmr(400mhz,f3ccood,ppm):δ8.98(s,1h),8.30(d,1h,j=8.48hz),7.80-7.48(m,2h),4.44(q,2h,j=6.40hz),2.46(s,3h),1.27(t,3h,j=6.96hz);13cnmr(100mhz,f3ccood,ppm):δ175.5,170.1,154.3,147.1,142.2,134.6,126.9,121.4,121.1,120.3,106.9,67.1,66.9,23.2,14.6.
ethyl7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylate(4p):whitesolid;yield41%;m.p.290-292oc;1hnmr(400mhz,f3ccood,ppm):δ8.93(s,1h),8.28(d,1h,j=9.24hz),7.28(t,2h,j=9.12hz),4.43(q,2h,j=6.80hz),3.84(s,3h),1.28(t,3h,j=6.88hz);13cnmr(100mhz,f3ccood,ppm):δ174.4,170.4,170.1,147.0,144.9,128.8,124.4,116.5,106.3,101.7,66.7,57.9,14.5.
ethyl6,7-dichloro-4-oxo-1,4-dihydroquinoline-3-carboxylate(4q):pale-yellowsolid;yield76%;m.p.>300oc;1hnmr(400mhz,f3ccood,ppm):δ9.17(s,1h),8.56(s,1h),8.17(s,1h),4.53(d,2h,j=7.20hz),1.39(d,3h,j=6.88hz);13cnmr(100mhz,f3ccood,ppm):δ174.9,169.6,148.3,146.9,140.6,140.2,139.2,127.8,123.8,121.5,121.4,107.8,67.3,14.4.
ethyl6,7-dimethyl-4-oxo-1,4-dihydroquinoline-3-carboxylate(4r):pale-yellowsolid;yield63%;m.p.199-201oc;1hnmr(400mhz,dmso-d6,ppm):δ11.21(brd,1h,j=6.44hz),8.27(d,1h,j=6.88hz),7.36(d,1h,j=7.52hz),7.00(d,1h,j=7.48hz),4.21(q,2h,j=6.80hz),2.74(s,3h),2.41(s,3h),1.27(t,3h,j=7.08hz);13cnmr(100mhz,f3ccood,ppm):δ176.7,165.3,143.7,139.2,138.0,132.8,127.3,126.0,124.7,111.7,59.9,23.8,17.5,14.7.
ethyl6-benzyl-4-oxo-1,4-dihydroquinoline-3-carboxylate(4s):whitesolid;yield79%;m.p.264-265oc;1hnmr(400mhz,f3ccood,ppm):δ9.09(s,1h),8.31(s,1h),7.89(s,1h),7.16(d,5h,j=32.80hz),4.52(d,2h,j=2.60hz),4.13(d,2h,j=33.2hz),1.38(s,3h);13cnmr(100mhz,f3ccood,ppm):δ175.3,169.8,148.0,146.4,141.6,140.4,140.3,130.8,130.8,128.9,125.7,122.4,122.1,107.0,66.8,43.3,14.3.
ethyl6-(4-chlorophenoxy)-4-oxo-1,4-dihydroquinoline-3-carboxylate(4t):whitesolid;yield81%;m.p.289-290oc;1hnmr(400mhz,f3ccood,ppm):δ8.66(s,1h),7.61-7.25(m,3h),6.89-6.57(m,4h),4.12(d,2h,j=4.40hz),0.97(s,3h);13cnmr(100mhz,f3ccood,ppm):175.2,170.5,155.9,146.2,138.3,134.4,133.3,132.7,125.2,124.7,67.6,15.2;ir(kbr):vmax(cm-1)3452,1692,1619,1559,1478,1381,1298,1237,800,746,609,547;hrms(esi-tof+):m/zcalcdforc12h11no3+[(m+h)+]344.0684;found,344.0677.
ethyl4-oxo-1,4-dihydrobenzo[h]quinoline-3-carboxylate(4w):pale-brownsolid;yield68%;m.p.267-270oc;1hnmr(400mhz,f3ccood,ppm):δ8.83(s,1h),8.20(d,1h,j=8.24hz),7.92(d,1h,j=9.12hz),7.73(q,2h,j=5.36hz),7.53-7.45(m,2h),4.21(q,2h,j=7.20hz),1.02(t,3h,j=7.16hz);13cnmr(100mhz,f3ccood,ppm):δ174.2,169.5,144.9,141.3,138.7,134.8,133.6,131.8,131.8,123.8,123.0,120.6,108.8,66.9,14.2,14.1。
- 还没有人留言评论。精彩留言会获得点赞!