一种福瑞德咔唑碱甲类化合物及其制备方法以及应用与流程
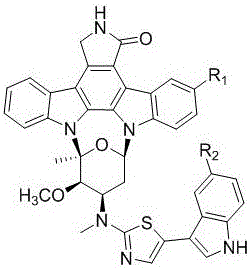
本发明属于药物领域,具体涉及一种福瑞德咔唑碱甲类化合物及其制备方法以及应用。
背景技术:
:癌症是我国各类疾病的死因之首,且发病率和死亡率还在持续增加。据统计,2015年中国有429.2万癌症新发病例、281.4万癌症死亡病例,每天约有1.2万人新患癌症、0.75万人死于癌症;与2010年相比,2015年中国的癌症发病率和死亡率分别增加了约39%和44%,分别占全球癌症新发病例和死亡病例的22%和27%。白血病是我国男性的十种高发癌症之一,且发病率呈上升趋势(w.chen,r.zheng,p.d.baade,etal.cancerstatisticsinchina,2015.ca:cancerj.clin.2016,66:115–132)。急性白血病的发病较快,患者在短期内难以进行骨髓移植,药物治疗是目前最有效的方法。其中,急性骨髓性白血病(acutemyeloidleukemia,aml),是一种骨髓性白细胞异常增殖的血癌。17-34%的aml患者体内的fms样酪氨酸激酶3(fms-liketyrosinekinase-3,flt3)高表达(ayhleung,chman,ylkwong.flt3inhibition:amovingandevolvingtargetinacutemyeloidleukaemia.leukemia.2013,27:260–268)。福瑞德咔唑碱甲(fradcarbazolea)是一种从费氏链霉菌突变株(streptomycesfradiae007m135)的发酵产物中获得的微量吲哚咔唑类生物碱(pfu,ybzhuang,ywang,ppliu,x.qi,k.gu,d.zhang,wmzhu.newindolocarbazolesfromamutantstrainofthemarine-derivedactinomycetestreptomycesfradiae007m135,organicletter.2012,14:6194–6197);其结构的新颖性以及较好的肿瘤细胞毒活性获得研究者关注,申请人通过对福瑞德咔唑碱甲及其3个结构类似物进行了合成,3个类似物均表现出较好的肿瘤细胞毒活性和分子靶向(pkc-β)抑制作用(lpwang,xgmei,cwang,wmzhu.biomimeticsemi-synthesisoffradcarbazoleaanditsanalogues.tetrahedron2015,71:7990–7997)。本发明以十字孢碱为骨架合成新的福瑞德咔唑碱甲类化合物。十字孢碱衍生物米哚妥林(midostaurin)作为治疗aml的成功上市(2017年4月28日被美国fda批准),奠定了此类化合物可以作为抗白血病药物研发的基础。因此以十字孢碱为骨架合成的化合物可能对急性骨髓性细胞有强的抑制活性。技术实现要素:本发明的目的是提供一种福瑞德咔唑碱甲类化合物及其制备方法,以及该化合物用于制备预防或治疗白血病的药物中的应用。本发明的目的及解决其主要技术问题是采用以下技术方案来实现的:一种福瑞德咔唑碱甲类化合物,该化合物以十字孢碱或者卤代十字孢碱为基础制得,其的结构式为(ⅰ)式:(ⅰ);其中,r1为:-h或卤素,r2为:-h、卤素或甲氧基;该化合物制备步骤包括:硫酰化反应、与碘甲烷成盐反应、取代反应和脱水合环反应,具体为:(1)r1选自-h时,制备方法为:将十字孢碱(ia)溶于二氯甲烷,然后加入三乙胺,与n,n′-硫羰基二咪唑进行硫酰化反应;然后在乙腈溶剂中,与碘甲烷成盐得到化合物(ib);在n,n-二甲基甲酰胺溶剂中与吲哚乙胺进行取代反应得到化合物(ic);在二氯甲烷溶剂中,与三氟乙酸酐和乙醇作用下进行脱水合环得到福瑞德咔唑碱甲类化合物(i);(2)r1选自卤素时,制备方法为:将十字孢碱在甲醇或者二氯甲烷溶剂中与卤代丁二酰亚胺进行卤代反应,得卤代十字孢碱;再将卤代十字孢碱(ia)溶于二氯甲烷,然后加入三乙胺,与n,n′-硫羰基二咪唑进行硫酰化反应;然后在乙腈溶剂中,与碘甲烷成盐得到化合物(ib);在n,n-二甲基甲酰胺溶剂中与吲哚乙胺进行取代反应得到化合物(ic);在二氯甲烷溶剂中,与三氟乙酸酐和乙醇作用下进行脱水合环得到福瑞德咔唑碱甲类化合物,所述的合成技术路线见图2所示。其中所述r1选自-h、cl、br,r2选自-h、f、cl、br、-ome。所述的福瑞德咔唑碱甲类化合物在制备预防或治疗白血病的药物中的应用。所述的化合物用作药物时,可以直接使用或者以药物组合物的形式使用,该药物组合物含有0.1–99%的所述化合物,其余为药用载体或赋形剂。本发明与现有技术相比具有明显的优点和有益效果。由以上技术方案可知,本发明化合物对flt3-itd突变的急性骨髓性细胞株mv4-11具有很强的选择性抑制活性;对人外周血单核细胞pbmc抑制作用弱;可被开发为高效低毒的预防和治疗白血病的药物。附图说明图1是福瑞德咔唑碱甲类化合物的结构图示。图2是合成福瑞德咔唑碱甲类化合物路线。图3是化合物1-14的结构式。具体实施方式实施例1化合物1的制备氩气保护下,将十字孢碱(1.86g,4.0mmol)用100ml二氯甲烷溶解,室温下加入10ml三乙胺和n,n’-硫羰基二咪唑(1.3g,8.0mmol),室温过夜反应。反应液倒入100ml冰水中,二氯甲烷萃取,无水硫酸钠干燥有机相后浓缩,硅胶柱色谱分离、二氯甲烷:甲醇=20:1(v/v)洗脱得到3’-n-(1-咪唑硫代甲酰)十字孢碱1.9g,产率82%,esi-msm/z599.2[m+na]+。氩气保护下,将3’-n-(1-咪唑硫代甲酰)十字孢碱(1.9g,3.30mmol)溶于200ml乙腈中,加入碘甲烷(2.0ml,33.0mmol),室温反应24小时。反应液直接浓缩,用200ml石油醚:二氯甲烷=4:1(v/v)的混合溶液洗纯即得化合物3’-n-(1-咪唑硫代甲酰)十字孢碱的咪唑部分的碘甲烷盐1.7g,产率72%,esi-msm/z591.2[m-i]+。氩气保护下,在100ml两口反应瓶中,加入400mg5-氟色胺(2.25mmol)用15ml四氢呋喃溶解,加入0.5ml三乙胺,10℃下加二碳酸二叔丁酯(488mg,2.25mmol),10℃反应1h,加水终止反应,乙酸乙酯萃取,无水硫酸钠干燥后浓缩,硅胶柱色谱分离、石油醚:乙酸乙酯(v/v3:1)洗脱得白色粉末产物n-[2-(3-(5-氟吲哚))乙基]氨基甲酸叔丁酯(486mg,收率83%)。1hnmr(400mhz,dmso-d6)δ10.91(s,1h,nh),7.21-7.33(m,3h,arh),6.87-6.92(m,2h,arh,nh),3.14-3.19(m,2h,ch2),2.76(t,j=7.4hz,2h,ch2),1.37(s,9h,3×ch3);13cnmr(100mhz,dmso-d6)δ156.6(d,1jc-f=230.6hz),155.6,132.9,127.6(d,3jc-f=9.7hz),124.8,112.3(d,3jc-f=9.7hz),112.2(d,4jc-f=4.5hz),109.0(d,2jc-f=25.2hz),103.0(d,2jc-f=25.2hz),77.5,40.8,28.3(3×c),25.4;esi-msm/z301.0[m+na]+。在100ml两口反应瓶中,加入n-[2-(3-(5-氟吲哚))乙基]氨基甲酸叔丁酯(100mg,0.31mmol)用11mlthf/h2o(10:1)溶解,0℃下加入ddq(144mg,0.64mmol),在此温度下反应2小时,反应液倒入100ml乙酸乙酯中,用饱和碳酸氢钠溶液洗至无色,乙酸乙酯层用无水硫酸钠干燥后浓缩,硅胶柱层析分离、石油醚:乙酸乙酯(v/v2:1)洗脱得n-[2-氧亚基-2-(3-(5-氟吲哚))乙基]氨基甲酸叔丁酯(70mg,收率67%)。1hnmr(600mhz,dmso-d6)δ8.47(s,1h,arh),7.81-7.84(m,1h,arh),7.48-7.51(m,1h,arh),7.06-7.10(m,1h,arh),7.03(t,j=5.9hz,1h,nh),4.28(d,j=5.9hz,2h,ch2),1.40(s,9h,3×ch3);13cnmr(150mhz,dmso-d6)δ190.9,158.6(d,1jc-f=235.5hz),156.0,134.9,133.0,126.0(d,3jc-f=10.8hz),114.1(d,4jc-f=4.0hz),113.5(d,3jc-f=10.8hz),111.1(d,2jc-f=26.0hz),106.0(d,2jc-f=26.0hz),77.9,46.8,28.3(3×c);esi-msm/z315.0[m+na]+。将n-[2-氧亚基-2-(3-(5-氟吲哚))乙基]氨基甲酸叔丁酯(300mg,0.95mmol)用5ml三氟乙酸溶解,10℃下反应一小时,加入苯(5ml×3次)共沸除去三氟乙酸即得到n-[2-氧亚基-2-(3-(5-氟吲哚))乙基]三氟乙酸铵185mg,产率72%。3’-n-(1-咪唑硫代甲酰)十字孢碱咪唑部分的碘甲烷盐(121mg,0.17mmol)溶于5mldmf中,加入0.5ml三乙胺和n-[2-氧亚基-2-(3-(5-氟吲哚))乙基]三氟乙酸铵(147mg,0.51mmol),室温反应24小时。反应液用10ml乙酸乙酯稀释,用1n的盐酸洗,无水硫酸钠干燥后浓缩。半制备hplc分离、meoh:h2o=9:1(v/v)洗脱得到3’-n-[n-(2-氧亚基-2-(3-(5-氟吲哚))乙基)氨基硫代甲酰]十字孢碱61mg,产率51%。1hnmr(600mhz,dmso-d6)δ12.15(s,1h,nh),9.29(d,j=7.9hz,1h,arh),8.61(s,1h,nh),8.58(s,1h,arh),8.06(d,j=7.7hz,1h,arh),8.01(d,j=8.5hz,1h,arh),7.94(brs,1h,nh),7.85(dd,j=9.8,2.4hz,1h,arh),7.75(d,j=8.2hz,1h,arh),7.48-7.54(m,3h,arh),7.37(t,j=7.4hz,1h,arh),7.31(t,j=7.4hz,1h,arh),7.08-7.12(m,2h,arh,h-1’),5.92(brs,1h,h-3’),4.95-5.05(m,4h,h-7,h-3’’),4.53(brs,1h,h-4’),2.95(s,3h,3'-nch3),2.87(s,3h,4'-och3),2.71-2.76(m,1h,h-2’a),2.36(s,3h,6'-ch3),2.27-2.33(m,1h,h-2’b);13cnmr(150mhz,dmso-d6)δ190.2,182.5,172.0,158.6(d,1jc-f=233.5hz),139.1,136.4,134.8,133.0,132.8,129.1,126.0(d,3jc-f=10.0hz),125.7,125.4,125.1,125.0,123.8,122.7,121.5,120.4,119.5,119.4,115.3,114.3(d,4jc-f=4.2hz),114.2,113.9,113.5(d,3jc-f=10.0hz),111.1(d,2jc-f=24.0hz),109.2,106.0(d,2jc-f=24.0hz),95.0,82.9,82.4,60.4,54.3,51.9,45.5,32.8,29.6,27.7;esi-msm/z723.2[m+na]+。氩气保护下,在25ml两口反应瓶中,加入3’-n-[n-(2-氧亚基-2-(3-(5-氟吲哚))乙基)氨基硫代甲酰]十字孢碱(55mg,0.079mmol),加入6ml二氯甲烷和200µl乙醇溶解,0℃下加入400µl三氟乙酸酐,反应1小时后加水终止反应,二氯甲烷萃取,无水硫酸钠干燥后浓缩,半制备hplc分离、meoh:h2o=9:1(v/v)洗脱得到5’’’-氟福瑞德咔唑碱甲(化合物1)34mg,收率63%。1hnmr(600mhz,dmso-d6)δ11.46(s,1h,nh),9.32(d,j=7.8hz,1h,arh),8.62(s,1h,nh),8.06(d,j=8.0hz,1h,arh),8.02(d,j=8.0hz,1h,arh),7.66-7.68(m,2h,arh),7.54-7.58(m,2h,arh),7.44-7.51(m,3h,arh),7.35(t,j=7.2hz,1h,arh),7.31(t,j=7.3hz,1h,arh),7.08(t,j=7.2hz,1h,arh),7.04(t,j=8.4hz,1h,h-1’),4.96-5.01(m,3h,h-7,h-3’),4.49(s,1h,h-4’),2.89(s,3h,3'-nch3),2.85-2.88(m,1h,h-2’a),2.71(s,3h,4'-och3),2.43(s,3h,6'-ch3),2.38-2.42(m,1h,h-2’b);13cnmr(150mhz,dmso-d6)δ171.9,167.5,157.4(d,1jc-f=232.7hz),138.8,136.3,134.1,133.2,132.7,129.3,125.7,125.4,125.1,125.0,124.9(d,3jc-f=9.5hz),124.9,123.8,122.7,121.5,120.4,119.9,119.5,119.4,115.2,114.2,113.6,113.1(d,3jc-f=9.8hz),110.1(d,2jc-f=24.1hz),109.0,107.2(d,4jc-f=4.9hz),104.0(d,2jc-f=24.1hz),94.8,82.5,82.4,60.2,53.2,45.5,34.5,29.3,27.1;hresi-msm/z683.2236[m+h]+(calcdforc39h32n6o3fs,683.2235)。实施例2化合物2的制备氩气保护下,在100ml两口反应瓶中,加入5-氯色胺(300mg,1.55mmol)用15ml四氢呋喃溶解,加入0.5ml三乙胺,10℃下加二碳酸二叔丁酯(349mg,1.60mmol),10℃反应1h,加水终止反应,乙酸乙酯萃取,无水硫酸钠干燥后浓缩,硅胶柱色谱分离、石油醚:乙酸乙酯(v/v3:1)洗脱得白色粉末产物n-[2-(3-(5-氯吲哚))乙基]氨基甲酸叔丁酯(411mg,收率90%)。1hnmr(600mhz,cdcl3)δ8.40(s,1h,nh),7.53(d,j=1.5hz,1h,arh),7.24(d,j=8.6hz,1h,arh),7.12(dd,j=8.6,1.5hz,1h,arh),7.00(s,1h,arh),4.65(brs,1h,nh),3.35-3.44(m,2h,ch2),2.88(t,j=6.4hz,2h,ch2),1.44(s,9h,3×ch3);13cnmr(150mhz,cdcl3)δ156.0,134.7,128.5,125.1,123.4,122.3,118.3,112.9,112.2,79.3,41.0,28.4(3×c),25.6;esi-msm/z317.0[m+na]+。在100ml两口反应瓶中,加入n-[2-(3-(5-氯吲哚))乙基]氨基甲酸叔丁酯(380mg,1.29mmol)用11mlthf/h2o(10:1)溶解,0℃下加入ddq(586mg,2.58mmol),在此温度下反应2小时,反应液倒入100ml乙酸乙酯中,用饱和碳酸氢钠溶液洗至无色,乙酸乙酯层用无水硫酸钠干燥后浓缩,硅胶柱层析分离,石油醚:乙酸乙酯(v/v1:1)洗脱得n-[2-氧亚基-2-(3-(5-氯吲哚))乙基]氨基甲酸叔丁酯(268mg,收率67%)。1hnmr(600mhz,dmso-d6)δ12.16(s,1h,nh),8.47(s,1h,arh),8.14(s,1h,arh),7.51(d,j=8.6hz,1h),7.24(d,j=8.6hz,1h),7.03(t,j=5.9hz,1h,nh),4.28(d,j=5.9hz,2h,ch2),1.40(s,9h,3×ch3);13cnmr(150mhz,dmso-d6)δ190.9,156.0,134.9,134.7,126.5,126.5,122.9,120.2,113.8,113.5,77.9,46.8,28.2(3×c);esi-msm/z331.0[m+na]+。将n-[2-氧亚基-2-(3-(5-氯吲哚))乙基]氨基甲酸叔丁酯(230mg,0.75mmol)用5ml三氟乙酸溶解,10℃下反应一小时,加入苯(5ml×3次)共沸除去三氟乙酸即得到n-[2-氧亚基-2-(3-(5-氯吲哚))乙基]三氟乙酸铵202mg,产率88%。3’-n-(1-咪唑硫代甲酰)十字孢碱咪唑部分的碘甲烷盐(200mg,0.28mmol)溶于5mldmf中,加入三乙胺0.5ml和n-[2-氧亚基-2-(3-(5-氯吲哚))乙基]三氟乙酸铵(85mg,0.28mmol),室温反应24小时。反应液用10ml乙酸乙酯稀释,用1n的盐酸洗,无水硫酸钠干燥后浓缩。半制备hplc分离、meoh:h2o=9:1(v/v)洗脱得到3’-n-[n-(2-氧亚基-2-(3-(5-氯吲哚))乙基)氨基硫代甲酰]十字孢碱81mg,产率40%。1hnmr(600mhz,dmso-d6)δ12.22(s,1h,nh),9.30(d,j=7.9hz,1h,arh),8.61(s,1h,nh),8.59(s,1h,arh),8.17(d,j=2.0hz,1h,arh),8.05(t,j=10.3hz,1h,arh),7.99(t,j=9.6hz,1h,arh),7.95(brs,1h,nh),7.74(d,j=8.3hz,1h,arh),7.54(d,j=8.6hz,1h,arh),7.47-7.50(m,2h,arh),7.36(t,j=7.5hz,1h,arh),7.31(t,j=7.5hz,1h,arh),7.26(dd,j=8.6,2.0hz,1h,arh),7.09(t,j=7.7hz,1h,h-1’),5.83(brs,1h,h-3’),4.94-5.07(m,4h,h-7,h-3’’),4.53(brs,1h,h-4’),2.95(s,3h,3'-nch3),2.87(s,3h,4'-och3),2.71-2.75(m,1h,h-2’a),2.36(s,3h,6'-ch3),2.27-2.33(m,1h,h-2’b);13cnmr(150mhz,dmso-d6)δ190.2,182.5,171.9,139.1,136.3,134.9,134.6,132.7,129.1,126.6,126.5,125.7,125.4,125.0,124.9,123.8,122.9,122.7,121.4,120.3,120.3,119.5,119.4,115.3,115.2,114.2,113.9,113.8,109.1,95.0,82.9,82.3,60.4,54.3,51.9,45.5,32.9,29.5,27.7;esi-msm/z739.2[m+na]+。氩气保护下,在25ml两口反应瓶中,加入3’-n-[n-(2-氧亚基-2-(3-(5-氯吲哚))乙基)氨基硫代甲酰]十字孢碱(60mg,0.084mmol),加入6ml二氯甲烷和200µl乙醇溶解,0℃下加入400µl三氟乙酸酐,反应1小时后加水终止反应,二氯甲烷萃取,无水硫酸钠干燥后浓缩,半制备hplc分离、meoh:h2o=9:1(v/v)洗脱得到5’’’-氯福瑞德咔唑碱甲(化合物2)29mg,收率49%。1hnmr(600mhz,dmso-d6)δ11.57(s,1h,nh),9.32(d,j=7.8hz,1h,arh),8.63(s,1h,nh),8.07(d,j=7.8hz,1h,arh),8.03(d,j=8.5hz,1h,arh),7.80(s,1h,arh),7.68(brs,1h,arh),7.58(brs,1h,arh),7.47-7.51(m,3h,arh),7.36(t,j=7.4hz,1h,arh),7.32(t,j=7.5hz,1h,arh),7.18-7.20(m,1h,arh),7.09(t,j=7.4hz,1h,h-1’),4.97-5.01(m,3h,h-7,h-3’),4.50(s,1h,h-4’),2.91(s,3h,3'-nch3),2.85-2.90(m,1h,h-2’a),2.71(s,3h,4'-och3),2.43(s,3h,6'-ch3),2.39-2.43(m,1h,h-2’b);13cnmr(150mhz,dmso-d6)δ171.9,167.7,138.7,136.3,135.0,134.5,132.7,129.2,125.9,125.7,125.4,125.1,125.0,124.6,124.3,123.8,122.7,121.9,121.5,120.3,119.5(2×c),119.4,118.2,115.2,114.1,113.6(2×c),109.0,106.8,94.8,82.5,82.4,60.2,53.2,45.4,34.5,29.2,27.1;hresi-msm/z699.1940[m+h]+(calcdforc39h32n6o3cls,699.1940)。实施例3:化合物3的制备氩气保护下,在100ml两口反应瓶中,加入5-溴色胺(300mg,1.26mmol)用15ml四氢呋喃溶解,加入0.5ml三乙胺,10℃下加二碳酸二叔丁酯(285mg,1.31mmol),10℃反应1h,加水终止反应,乙酸乙酯萃取,无水硫酸钠干燥后浓缩,硅胶柱色谱分离、石油醚:乙酸乙酯(v/v3:1)洗脱得白色粉末产物n-[2-(3-(5-溴吲哚))乙基]氨基甲酸叔丁酯(359mg,收率84%)。1hnmr(600mhz,cdcl3)δ8.44(s,1h,nh),7.73(d,j=1.5hz,1h,arh),7.27-7.30(m,1h,arh),7.25(d,j=8.6hz,1h,arh),7.02(s,1h,arh),4.69(brs,1h,nh),3.37-3.47(m,2h,ch2),2.92(t,j=6.6hz,2h,ch2),1.48(s,9h,3×ch3);13cnmr(150mhz,cdcl3)δ156.0,135.0,129.2,124.8,123.3,121.4,112.9,112.6(2×c),79.3,41.0,28.4(3×c),25.6;esi-msm/z361.0[m+na]+。在100ml两口反应瓶中,加入n-[2-(3-(5-溴吲哚))乙基]氨基甲酸叔丁酯(100mg,0.296mmol)用11mlthf/h2o(10:1)溶解,0℃下加入ddq(134mg,0.592mmol),在此温度下反应2小时,反应液倒入100ml乙酸乙酯中,用饱和碳酸氢钠溶液洗至无色,乙酸乙酯层用无水硫酸钠干燥后浓缩,硅胶柱层析分离,石油醚:乙酸乙酯(v/v1:1)洗脱得n-[2-氧亚基-2-(3-(5-溴吲哚))乙基]氨基甲酸叔丁酯(61mg,收率58%)。1hnmr(600mhz,dmso-d6)δ8.45(s,1h,arh),8.30(d,j=1.5hz,1h,arh),7.46(d,j=8.6hz,1h),7.35(dd,j=8.6,1.5hz,1h),7.03(t,j=5.9hz,1h,nh),4.29(d,j=5.9hz,2h,ch2),1.40(s,9h,3×ch3);13cnmr(150mhz,dmso-d6)δ190.9,156.0,135.1,134.5,127.2,125.4,123.3,114.6,114.3,113.4,77.9,46.8,28.2(3×c);esi-msm/z375.0[m+na]+。将n-[2-氧亚基-2-(3-(5-溴吲哚))乙基]氨基甲酸叔丁酯(61mg,0.17mmol)用5ml三氟乙酸溶解,10℃下反应一小时,加入苯(5ml×3次)共沸除去三氟乙酸即得到n-[2-氧亚基-2-(3-(5-溴吲哚))乙基]三氟乙酸铵56mg,产率94%。3’-n-(1-咪唑硫代甲酰)十字孢碱咪唑部分的碘甲烷盐(185mg,0.26mmol)溶于5mldmf中,加入三乙胺0.5ml和n-[2-氧亚基-2-(3-(5-溴吲哚))乙基]三氟乙酸铵(180mg,0.52mmol,),室温反应24小时。反应液用10ml乙酸乙酯稀释,用1n的盐酸洗,无水硫酸钠干燥后浓缩。半制备hplc分离、meoh:h2o=9:1(v/v)洗脱得到3’-n-[n-(2-氧亚基-2-(3-(5-溴吲哚))乙基)氨基硫代甲酰]十字孢碱91mg,产率46%。1hnmr(600mhz,dmso-d6)δ12.22(s,1h,nh),9.29(d,j=7.9hz,1h,arh),8.61(s,1h,nh),8.56(s,1h,arh),8.31(s,1h,arh),8.05(d,j=7.7hz,1h,arh),8.00(d,j=8.5hz,1h,arh),7.95(brs,1h,nh),7.74(d,j=8.3hz,1h,arh),7.48-7.50(m,3h,arh),7.35-7.38(m,2h,arh),7.30(t,j=7.5hz,1h,arh),7.08(t,j=7.7hz,1h,h-1’),5.90(brs,1h,h-3’),4.92-5.06(m,4h,h-7,h-3’’),4.52(brs,1h,h-4’),2.94(s,3h,3'-nch3),2.87(s,3h,4'-och3),2.70-2.74(m,1h,h-2’a),2.35(s,3h,6'-ch3),2.26-2.32(m,1h,h-2’b);13cnmr(150mhz,dmso-d6)δ190.3,182.5,172.0,139.1,136.4,135.2,134.5,132.8,129.1,127.3,125.7,125.5,125.4,125.1,124.9,123.8,123.3,122.7,121.5,120.4,119.5,119.4,115.3,114.6,114.4,114.2,113.9,113.7,109.2,95.0,82.9,82.4,60.5,54.3,51.9,45.5,32.9,29.7,27.7;esi-msm/z783.2[m+na]+。氩气保护下,在25ml两口反应瓶中,加入3’-n-[n-(2-氧亚基-2-(3-(5-溴吲哚))乙基)氨基硫代甲酰]十字孢碱(42mg,0.055mmol),加入6ml二氯甲烷和200µl乙醇溶解,0℃下加入400µl三氟乙酸酐,反应1小时后加水终止反应,二氯甲烷萃取,无水硫酸钠干燥后浓缩,半制备hplc分离、meoh:h2o=9:1(v/v)洗脱得到5’’’-溴福瑞德咔唑碱甲(化合物3)28mg,收率69%。1hnmr(600mhz,dmso-d6)δ11.58(s,1h,nh),9.32(d,j=3.0hz,1h,arh),8.62(s,1h,nh),8.06(d,j=7.4hz,1h,arh),8.02(d,j=7.9hz,1h,arh),7.93(s,1h,arh),7.65-7.68(m,2h,arh),7.56(s,1h,arh),7.46-7.51(m,2h,arh),7.43(d,j=8.5hz,1h,arh),7.35(t,j=6.3hz,1h,arh),7.29-7.32(m,2h,arh),7.09(brs,1h,h-1’),4.98-5.01(m,3h,h-7,h-3’),4.49(s,1h,h-4’),2.91(s,3h,3'-nch3),2.85-2.90(m,1h,h-2’a),2.71(s,3h,4'-och3),2.43(s,3h,6'-ch3),2.38-2.43(m,1h,h-2’b);13cnmr(150mhz,dmso-d6)δ171.9,167.8,138.7,136.3,135.2,134.5,132.7,129.2,126.6,125.7,125.4,125.1,125.0,124.5,124.4,123.8,122.7,121.5,121.2,120.3,119.5,119.4(2×c),115.2,114.2,114.0,113.5,112.3,109.0,106.6,94.8,82.5,82.4,60.2,53.2,45.4,34.5,29.2,27.1;hresi-msm/z743.1432[m+h]+(calcdforc39h32n6o3brs,743.1434)。实施例4化合物4的制备氩气保护下,在100ml两口反应瓶中,加入5-甲氧基色胺(1.0g,5.26mmol)用15ml四氢呋喃溶解,加入1.5ml三乙胺,10℃下加二碳酸二叔丁酯(1.37g,6.32mmol),10℃反应1h,加水终止反应,乙酸乙酯萃取,无水硫酸钠干燥后浓缩,硅胶柱色谱分离、石油醚:乙酸乙酯(v/v5:1)洗脱得白色粉末产物n-[2-(3-(5-甲氧基吲哚))乙基]氨基甲酸叔丁酯(1.51g,收率99%)。1hnmr(600mhz,cdcl3)δ8.18(s,1h,nh),7.27(d,j=8.8hz,1h,arh),7.06(s,1h,arh),7.01(s,1h,arh),6.89(dd,j=8.8,1.5hz,1h,arh),4.61(brs,1h,nh),3.89(s,3h,ome),3.46-3.50(m,2h,ch2),2.94(t,j=6.4hz,2h,ch2),1.46(s,9h,3×ch3);13cnmr(150mhz,cdcl3)δ156.0,154.0,131.5,127.7,122.8,112.8,112.3,111.9,100.6,79.1,55.9,40.7,28.4(3×c),25.8;esi-msm/z313.1[m+na]+。在100ml两口反应瓶中,加入n-[2-(3-(5-甲氧基吲哚))乙基]氨基甲酸叔丁酯(1.3g,4.48mmol)用55mlthf/h2o(10:1)溶解,0℃下加入ddq(2.0g,8.96mmol),在此温度下反应2小时,反应液倒入100ml乙酸乙酯中,用饱和碳酸氢钠溶液洗至无色,乙酸乙酯层用无水硫酸钠干燥后浓缩,硅胶柱层析分离,石油醚:乙酸乙酯(v/v1:1)洗脱得n-[2-氧亚基-2-(3-(5-甲氧基吲哚))乙基]氨基甲酸叔丁酯(1.1g,收率81%)。1hnmr(600mhz,dmso-d6)δ11.86(brs,1h,nh),8.33(d,j=3.1hz,1h,arh),7.67(d,j=2.5hz,1h,arh),7.37(d,j=8.8hz,1h),6.97(t,j=5.9hz,1h,nh),6.85(dd,j=8.8,2.5hz,1h),4.26(d,j=5.9hz,2h,ch2),3.78(s,3h,ome),1.41(s,9h,3×ch3);13cnmr(150mhz,dmso-d6)δ190.7,156.0,155.4,133.4,131.2,126.2,113.8,112.9,112.7,102.9,77.8,55.2,46.7,28.3(3×c);esi-msm/z327.1[m+na]+。将n-[2-氧亚基-2-(3-(5-甲氧基吲哚))乙基]氨基甲酸叔丁酯(1.1g,3.62mmol)用10ml三氟乙酸溶解,10℃下反应一小时,加入苯(5ml×3次)共沸除去三氟乙酸即得到n-[2-氧亚基-2-(3-(5-甲氧基吲哚))乙基]三氟乙酸铵0.9g,产率83%。3’-n-(1-咪唑硫代甲酰)十字孢碱咪唑部分的碘甲烷盐(200mg,0.28mmol)溶于5mldmf中,加入三乙胺0.5ml和n-[2-氧亚基-2-(3-(5-甲氧基吲哚))乙基]三氟乙酸铵(169mg,0.56mmol,),室温反应24小时。反应液用10ml乙酸乙酯稀释,用1n的盐酸洗,无水硫酸钠干燥后浓缩。半制备hplc分离、meoh:h2o=9:1(v/v)洗脱得到3’-n-[n-(2-氧亚基-2-(3-(5-甲氧基吲哚))乙基)氨基硫代甲酰]十字孢碱96mg,产率48%。1hnmr(600mhz,dmso-d6)δ11.93(d,j=3.0hz,1h,nh),9.30(d,j=8.0hz,1h,arh),8.63(s,1h,nh),8.45(d,j=3.0hz,1h,arh),8.06(d,j=7.8hz,1h,arh),8.01(d,j=8.5hz,1h,arh),7.90(brs,1h,nh),7.74(d,j=8.3hz,1h,arh),7.70(d,j=2.5hz,1h,arh),7.46-7.51(m,2h,arh),7.41(d,j=8.8hz,1h,arh),7.36(t,j=7.4hz,1h,arh),7.31(t,j=7.5hz,1h,arh),7.10(t,j=7.6hz,1h,h-1’),6.87(dd,j=8.8,2.5hz,1h,arh),5.94(brs,1h,h-3’),4.94-5.05(m,4h,h-7,h-3’’),4.52(brs,1h,h-4’),3.79(s,3h,5’’’-ome),2.96(s,3h,3'-nch3),2.85(s,3h,4'-och3),2.71-2.76(m,1h,h-2’a),2.37(s,3h,6’-ch3),2.27-2.30(m,1h,h-2’b);13cnmr(150mhz,dmso-d6)δ190.0,182.4,172.0,155.5,139.0,136.4,133.5,132.8,131.3,129.2,126.3,125.7,125.4,125.0,125.0,123.8,122.7,121.5,120.4,119.5,119.4,115.3,114.2,114.0,113.9,113.0,112.7,109.2,102.9,95.0,83.0,82.4,60.4,55.3,54.3,51.9,45.5,32.8,29.5,27.7;esi-msm/z735.2[m+na]+。氩气保护下,在25ml两口反应瓶中,加入3’-n-[n-(2-氧亚基-2-(3-(5-甲氧基吲哚))乙基)氨基硫代甲酰]十字孢碱(38mg,0.053mmol),加入6ml二氯甲烷和200µl乙醇溶解,0℃下加入400µl三氟乙酸酐,反应1小时后加水终止反应,二氯甲烷萃取,无水硫酸钠干燥后浓缩,半制备hplc分离、meoh:h2o=9:1(v/v)洗脱得到5’’-甲氧基福瑞德咔唑碱甲(化合物4)30mg,收率79%。1hnmr(600mhz,dmso-d6)δ11.23(s,1h,nh),9.31(d,j=8.0hz,1h,arh),8.65(s,1h,nh),8.06(d,j=7.7hz,1h,arh),8.02(d,j=8.5hz,1h,arh),7.67(d,j=8.2hz,1h,arh),7.57(s,1h,arh),7.53(d,j=2.0hz,1h,arh),7.45-7.51(m,2h,arh),7.30-7.36(m,3h,arh),7.26(brs,1h,arh),7.07-7.10(m,1h,h-1’),6.84(dd,j=8.8,2.0hz,1h,arh),5.02(s,2h,h-7),4.97(d,j=12.2hz,1h,h-3’),4.50(brs,1h,h-4’),3.84(s,3h,5’’’-ome),2.91(s,3h,3'-nch3,),2.84-2.89(m,1h,h-2’a),2.70(s,3h,4’-ome),2.44(s,3h,6’-ch3),2.38-2.42(m,1h,h-2’b);13cnmr(150mhz,dmso-d6)δ172.0,167.3,154.0,138.8,136.3,133.7,132.7,131.6,129.3,125.9,125.8,125.4,125.2,125.0,123.8,123.6,122.7,121.5,120.7,120.4,119.6,119.5,115.2,114.2,113.6,112.8,112.1,109.1,106.7,100.8,94.8,82.5,82.4,60.2,55.4,53.3,45.5,34.5,29.3,27.1;hresi-msm/z695.2433[m+h]+(calcdforc40h35n6o4s,695.2435)。实施例5化合物5的制备氩气保护下,在100ml两口反应瓶中,加入色胺(200mg,1.25mmol)用15ml四氢呋喃溶解,加入0.5ml三乙胺,10℃下加二碳酸二叔丁酯(301mg,1.38mmol),10℃反应1h,加水终止反应,乙酸乙酯萃取,无水硫酸钠干燥后浓缩,硅胶柱色谱分离、石油醚:乙酸乙酯(v/v3:1)洗脱得白色粉末产物n-[2-(3-吲哚)乙基]氨基甲酸叔丁酯(250mg,收率77%),esi-msm/z261.3[m+h]+。在100ml两口反应瓶中,加入n-[2-(3-吲哚)乙基]氨基甲酸叔丁酯(250mg,0.96mmol)用11mlthf/h2o(10:1)溶解,0℃下加入ddq(436mg,1.92mmol),在此温度下反应2小时,反应液倒入100ml乙酸乙酯中,用饱和碳酸氢钠溶液洗至无色,乙酸乙酯层用无水硫酸钠干燥后浓缩,硅胶柱层析分离,石油醚:乙酸乙酯(v/v1:1)洗脱得n-[2-氧亚基-2-(3-吲哚)乙基]氨基甲酸叔丁酯(170mg,收率64%),esi-msm/z297.0[m+na]+。将n-[2-氧亚基-2-(3-溴吲哚)乙基]氨基甲酸叔丁酯(170mg,0.62mmol)用5ml三氟乙酸溶解,10℃下反应一小时,加入苯(5ml×3次)共沸除去三氟乙酸即得到n-[2-氧亚基-2-(3-吲哚)乙基]三氟乙酸铵158mg,产率93%。氩气保护下,在100ml两口反应瓶中,加入2.33g十字孢碱(5.0mmol)用50ml四氢呋喃溶解,加入2ml三乙胺,0℃下加入1.3g二碳酸二叔丁酯(6.0mmol),0℃反应30min,加水终止反应,二氯甲烷萃取,无水硫酸钠干燥后浓缩,硅胶柱色谱分离、二氯甲烷:甲醇(v/v30:1)洗脱得到3'-n-叔丁氧羰基十字孢碱2.18g,收率76%。1hnmr(600mhz,dmso-d6)δ9.32(d,j=7.9hz,1h,arh),8.62(s,1h,nh),7.99-8.08(m,2h,arh),7.64(d,j=8.2hz,1h,arh),7.46-7.51(m,2h,arh),7.29-7.37(m,2h,arh),7.00(t,j=7.6hz,1h,h-1’),5.01(s,2h,h-7),4.48-4.65(m,1h,h-3’),4.28(s,1h,h-4’),2.72-2.83(m,4h,3'-nch3,h-2’a),2.64(s,3h,4'-och3),2.34(s,3h,6'-ch3),2.14-2.21(m,1h,h-2’b),1.57/1.46(s,9h,3×4’’-ch3);13cnmr(125mhz,dmso-d6)δ171.9,154.9/154.2,139.0/138.8,136.2,132.7,129.1,125.7,125.3,124.9(2×c),123.7,122.6,121.4,120.3,119.5,119.4,115.2,114.1,113.7,108.9,94.6,84.0/83.3,82.2,79.6/79.3,60.5,50.5/49.7,45.4,30.1,29.5,28.1(3×c),27.0;esi-msm/z589.2[m+na]+。氩气保护下,在100ml两口反应瓶中,3'-n-叔丁氧羰基十字孢碱(2.0g,3.54mmol),用30ml二氯甲烷/甲醇(1:1)溶解,室温下加入氯代丁二酰亚胺(745mg,5.6mmol),此温度下反应6小时,加水终止反应,二氯甲烷萃取,并用无水na2so4干燥,真空蒸干,硅胶柱层析分离,二氯甲烷:甲醇(v/v30:1)得到3-氯-3′-n-叔丁氧羰基十字孢碱(1.03g,收率48%)。1hnmr(400mhz,dmso-d6)δ9.32(d,j=2.0hz,1h,arh),8.70(s,1h,nh),8.01-8.10(m,2h,arh),7.70(d,j=8.0hz,1h,arh),7.47-7.53(m,2h,arh),7.35(t,j=8.0hz,1h,arh),7.01(t,j=7.6hz,1h,h-1’),5.01(s,2h,h-7),4.44-4.62(m,1h,h-3’),4.27(brs,1h,h-4’),2.76(s,3h,3'-nch3),2.60-2.68(m,4h,4'-och3,h-2’a),2.33(s,3h,6'-ch3),2.11-2.20(m,1h,h-2’b),1.56/1.45(m,9h,3×4’’-ch3);13cnmr(100mhz,dmso-d6)δ171.8,154.9/154.2,138.8,134.6,133.2,128.9,125.7,125.2,125.1,124.6,123.8,123.7,123.6,121.6,120.5,119.4,114.6,114.2,113.9/113.8,110.7,94.7,83.9/83.2,82.3,79.7/79.4,60.5,50.4/49.6,45.6,30.2,29.5,28.1(3×c),27.0;esi-msm/z623.3[m+na]+。在100ml两口反应瓶中,加入3-氯-3′-n-叔丁氧羰基十字孢碱(1.03g,1.72mmol),用10ml二氯甲烷溶解。0℃加入8ml三氟乙酸,此温度下反应1小时,浓缩后加入50ml饱和碳酸氢钠水溶液搅拌0.5小时,后用二氯甲烷萃取,无水硫酸钠干燥后浓缩,硅胶柱色谱分离,二氯甲烷:乙酸乙酯(v/v5:1)洗脱得到3-氯十字孢碱(800mg,收率93%)。1hnmr(600mhz,dmso-d6)δ9.32(d,j=2.1hz,1h,arh),8.61(s,1h,nh),7.96-7.99(m,2h,arh),7.64(d,j=8.0hz,1h,arh),7.46(dd,j=8.0,2.0hz,1h,arh),7.42(t,j=8.0hz,1h,arh),7.28(t,j=8.0hz,1h,arh),6.69-6.71(m,1h,h-1’),4.96(s,2h,h-7),4.05(d,j=3.5hz,1h,h-3’),3.85-3.87(m,1h,h-4’),3.34(s,3h,4'-och3),3.23-3.26(m,1h,h-3’),2.47-2.52(m,1h,h-2’a),2.28-2.32(m,4h,h-2’b,3'-nch3),1.42(s,3h,6’-ch3);13cnmr(150mhz,dmso-d6)δ172.1,139.5,134.7,132.5,129.9,127.6,124.5(2×c),124.4,123.7,123.5,123.3,120.9,119.8,118.7,115.4,113.9,113.0,110.0,91.1,82.7,80.0,57.2,49.8,45.5,33.2,29.7,29.3;esi-msm/z523.2[m+na]+。将3-氯十字孢碱(470mg,0.94mmol)用20ml二氯甲烷溶解,室温下加入2ml三乙胺和n,n′-硫羰基二咪唑(502mg,2.82mmol),室温过夜反应。反应液倒入20ml冰水中,二氯甲烷萃取,无水硫酸钠干燥有机相后浓缩,硅胶柱色谱分离、二氯甲烷:甲醇=30:1(v/v)洗脱得到3-氯-3’-n-(1-咪唑硫代甲酰)十字孢碱410mg,产率71%。1hnmr(600mhz,dmso-d6)δ9.34(s,1h,arh),8.71(s,1h,nh),8.11(s,1h,arh),8.07(d,j=7.8hz,1h,arh),8.03(d,j=8.3hz,1h,arh),7.51-7.68(m,4h,arh),7.38(t,j=7.4hz,1h,arh),7.14(brs,1h,arh),7.05(brs,1h,h-1’),5.46(brs,1h,h-3’),5.01(s,2h,h-7),4.78(brs,1h,h-4’),3.03-3.07(m,4h,3’-nch3,h-2’a),2.70(s,3h,4’-ome),2.39-2.46(m,4h,6’-ch3,h-2’b);13cnmr(150mhz,dmso-d6)δ179.4,171.8,138.8,137.7,134.6,133.1,129.1,129.0,126.0,125.5,125.2,124.7,123.9,123.8,123.7,121.7,120.7,119.9,119.6,114.8,114.3,113.5,110.6,94.9,82.0,81.7,60.4,58.2,45.6,38.2,29.4,27.0;esi-msm/z633.0[m+na]+。将3-氯-3’-n-(1-咪唑硫代甲酰)十字孢碱(360mg,0.59mmol)溶于10ml乙腈中,加入碘甲烷0.5ml,室温反应24小时。反应液直接浓缩,用50ml石油醚:二氯甲烷=4:1(v/v)的混合溶液洗纯即得3-氯-3’-n-(1-咪唑硫代甲酰)十字孢碱咪唑部分的碘甲烷盐300mg,产率68%。1hnmr(600mhz,dmso-d6)δ9.69(s,1h,arh),9.35(d,j=1.8hz,1h,arh),8.73(s,1h,nh),8.14(brs,1h,arh),8.10(d,j=7.7hz,1h,arh),8.07(d,j=8.3hz,1h,arh),7.87(brs,1h,arh),7.69(d,j=8.0hz,1h,arh),7.52-7.55(m,2h,arh),7.39(t,j=7.5hz,1h,arh),7.19(brs,1h,h-1’),5.45(d,j=11.3hz,1h,h-3’),5.02(s,2h,h-7),4.75(s,1h,h-4’),3.90(s,3h,5’’-nch3),3.05-3.11(m,4h,3’-nch3,h-2’a),2.69(s,3h,4’-ome),2.43-2.48(m,4h,6’-ch3,h-2’b);13cnmr(150mhz,dmso-d6)δ174.3,171.7,138.7,138.0,134.6,133.1,129.1,126.0,125.5,125.2,124.7,123.9,123.8,123.7(2×c),121.8,121.2,120.7,119.6,114.8,114.3,113.5,110.6,94.7,81.9,81.2,60.5,59.1,45.6,38.5,36.5,29.2,26.7;esi-msm/z625.3[m-i]+。3-氯-3’-n-(1-咪唑硫代甲酰)十字孢碱咪唑部分的碘甲烷盐(280mg,0.37mmol)溶于10mldmf中,加入三乙胺1.0ml和n-[2-氧亚基-2-(3-吲哚)乙基]三氟乙酸铵(200mg,0.74mmol,),室温反应24小时。反应液用20ml乙酸乙酯稀释,用1n的盐酸洗,无水硫酸钠干燥后浓缩。硅胶柱色谱分离、石油醚:乙酸乙酯=1:1(v/v)洗脱得到3-氯-3’-n-[n-(2-氧亚基-2-(3-吲哚)乙基)氨基硫代甲酰]十字孢碱130mg,产率49%。1hnmr(600mhz,dmso-d6)δ12.03(s,1h,nh),9.33(d,j=2.0hz,1h,arh),8.70(s,1h,nh),8.50(d,j=1.7hz,1h,arh),8.18(d,j=7.3hz,1h,arh),8.07(d,j=7.8hz,1h,arh),8.02(d,j=8.5hz,1h,arh),7.92(brs,1h,nh),7.79(d,j=8.7hz,1h,arh),7.49-7.53(m,3h,arh),7.37(t,j=7.4hz,1h,arh),7.20-7.25(m,2h,arh),7.10(t,j=7.7hz,1h,h-1’),5.92(brs,1h,h-3’),4.97-5.08(m,4h,h-7,h-3’’),4.52(brs,1h,h-4’),2.96(s,3h,3'-nch3),2.85(s,3h,4'-och3),2.71-2.76(m,1h,h-2’a),2.36(s,3h,6'-ch3),2.26-2.32(m,1h,h-2’b);13cnmr(150mhz,dmso-d6)δ190.0,182.4,171.8,139.1,136.4,134.7,133.3,133.2,128.9,125.8,125.4,125.3,125.1,124.5,123.8,123.7(2×c),122.9,121.8,121.5,121.2,120.5,119.4,114.7,114.2,114.1,113.9,112.2,110.9,95.1,82.8,82.5,60.4,54.2,51.9,45.6,32.8,29.5,27.6;esi-msm/z739.2[m+na]+。氩气保护下,在25ml两口反应瓶中,加入3-氯-3’-n-[n-(2-氧亚基-2-(3-吲哚)乙基)氨基硫代甲酰]十字孢碱(61mg,0.085mmol),加入6ml二氯甲烷和200µl乙醇溶解,0℃下加入400µl三氟乙酸酐,反应1小时后加水终止反应,二氯甲烷萃取,无水硫酸钠干燥后浓缩,半制备hplc分离、meoh:h2o=9:1(v/v)洗脱得到3-氯福瑞德咔唑碱甲(化合物5)43mg,收率73%。1hnmr(600mhz,dmso-d6)δ11.36(s,1h,nh),9.35(s,1h,arh),8.71(s,1h,nh),8.07(d,j=7.8hz,1h,arh),8.04(d,j=8.6hz,1h,arh),7.82(d,j=7.8hz,1h,arh),7.2(d,j=7.8hz,1h,arh),7.58(brs,1h,arh),7.55(brs,1h,arh),7.52(dd,j=8.7,2.1hz,1h,arh),7.49(t,j=7.8hz,1h,arh),7.45(d,j=8.1hz,1h,arh),7.37(t,j=7.4hz,1h,arh),7.18(t,j=7.5hz,1h,arh),7.08-7.13(m,2h,arh,h-1’),5.02(s,2h,h-7),4.97(d,j=12.0hz,1h,h-3’),4.49(brs,1h,h-4’),2.91(s,3h,3'-nch3),2.84-2.90(m,1h,h-2’a),2.70(s,3h,4'-och3),2.43(s,3h,6'-ch3),2.37-2.42(m,1h,h-2’b);13cnmr(150mhz,dmso-d6)δ171.8,167.4,138.8,136.5,134.7,133.9,133.1,129.1,125.9,125.3,125.1,124.8,124.6,123.8,123.7(2×c),122.8,121.8,121.6,120.6,120.5,119.7,119.4,119.2,114.7,114.2,113.6,112.0,110.8,106.9,94.9,82.5(2×c),60.2,53.1,45.6,34.5,29.2,27.0;hresi-msm/z699.1938[m+h]+(calcdforc39h32n6o3cls,699.1940)。实施例6化合物6的制备3-氯-3’-n-(1-咪唑硫代甲酰)十字孢碱咪唑部分的碘甲烷盐(300mg,0.40mmol)溶于10mldmf中,加入三乙胺1.0ml和n-[2-氧亚基-2-(3-(5-氟吲哚))乙基]三氟乙酸铵(116mg,0.40mmol),室温反应24小时。反应液用20ml乙酸乙酯稀释,用1n的盐酸洗,无水硫酸钠干燥后浓缩。半制备hplc分离、meoh:h2o=9:1(v/v)洗脱得到3-氯-3’-n-[n-(2-氧亚基-2-(3-(5-氟吲哚))乙基)氨基硫代甲酰]十字孢碱150mg,产率50%。1hnmr(600mhz,dmso-d6)δ12.16(s,1h,nh),9.33(d,j=2.1hz,1h,arh),8.71(s,1h,nh),8.58(s,1h,arh),8.07(d,j=7.7hz,1h,arh),8.02(d,j=8.5hz,1h,arh),7.95(brs,1h,nh),7.85(dd,j=9.8,2.1hz,1h,arh),7.80(d,j=8.7hz,1h,arh),7.50-7.54(m,3h,arh),7.38(t,j=7.4hz,1h,arh),7.09-7.12(m,2h,arh,h-1’),5.92(brs,1h,h-3’),4.95-5.05(m,4h,h-7,h-3’’),4.52(brs,1h,h-4’),2.95(s,3h,3'-nch3),2.86(s,3h,4'-och3),2.72-2.76(m,1h,h-2’a),2.36(s,3h,6'-ch3),2.26-2.32(m,1h,h-2’b);13cnmr(150mhz,dmso-d6)δ190.1,182.5,171.8,158.6(d,1jc-f=234.1hz),139.1,134.8(2×c),133.2,133.0,128.9,126.0(d,3jc-f=10.0hz),125.8,125.3,125.1,124.5,123.8,123.7(2×c),121.6,120.5,119.4,114.7,114.3(d,4jc-f=4.0hz),114.3,114.0,113.5(d,3jc-f=10.0hz),111.1(d,2jc-f=23.8hz),110.9,106.0(d,2jc-f=23.8hz),95.1,82.8,82.5,60.4,54.3,51.9,45.6,32.8,29.5,27.6;esi-msm/z757.2[m+na]+。氩气保护下,在25ml两口反应瓶中,加入3-氯-3’-n-[n-(2-氧亚基-2-(3-(5-氟吲哚))乙基)氨基硫代甲酰]十字孢碱(50mg,0.068mmol),加入6ml二氯甲烷和200µl乙醇溶解,0℃下加入400µl三氟乙酸酐,反应1小时后加水终止反应,二氯甲烷萃取,无水硫酸钠干燥后浓缩,半制备hplc分离、meoh:h2o=9:1(v/v)洗脱得到3-氯-5’’’-氟福瑞德咔唑碱甲(化合物6)30mg,收率62%。1hnmr(600mhz,dmso-d6)δ11.47(s,1h,nh),9.34(s,1h,arh),8.71(s,1h,nh),8.02-8.08(m,2h,arh),7.72(t,j=7.9hz,1h,arh),7.65(brs,1h,arh),7.43-7.56(m,5h,arh),7.35-7.38(m,1h,arh),7.07-7.10(m,1h,arh),7.03(t,j=9.0hz,1h,h-1’),5.02(s,2h,h-7),4.96(d,j=12.7hz,1h,h-3’),4.49(brs,1h,h-4’),2.90(s,3h,3'-nch3),2.85-2.89(m,1h,h-2’a),2.70(s,3h,4'-och3),2.43(s,3h,6'-ch3),2.37-2.41(m,1h,h-2’b);13cnmr(150mhz,dmso-d6)δ171.8,167.6,157.4(d,1jc-f=232.8hz),138.8,134.7,134.1,133.2,133.1,129.1,126.0,125.3,125.2,124.9,124.9(d,3jc-f=9.7hz),124.6,123.8(2×c),123.7,121.6,120.6,120.0,119.4,114.7,114.2,113.7,113.1(d,3jc-f=9.7hz),110.8,110.1(d,2jc-f=26.2hz),107.2(d,4jc-f=4.4hz),104.0(d,2jc-f=26.2hz),94.9,82.5,82.5,60.2,53.2,45.6,34.5,29.2,27.0;hresi-msm/z717.1843[m+h]+(calcdforc39h31n6o3clfs,717.1845)。实施例7化合物7的制备3-氯-3’-n-(1-咪唑硫代甲酰)十字孢碱咪唑部分的碘甲烷盐(110mg,0.15mmol)溶于10mldmf中,加入三乙胺1.0ml和n-[2-氧亚基-2-(3-(5-氯吲哚))乙基]三氟乙酸铵(46mg,0.15mmol,),室温反应24小时。反应液用20ml乙酸乙酯稀释,用1n的盐酸洗,无水硫酸钠干燥后浓缩。半制备hplc分离、meoh:h2o=9:1(v/v)洗脱得到3-氯-3’-n-[n-(2-氧亚基-2-(3-(5-氯吲哚))乙基)氨基硫代甲酰]十字孢碱50mg,产率45%。1hnmr(600mhz,dmso-d6)δ9.32(d,j=2.0hz,1h,arh),8.71(s,1h,nh),8.58(s,1h,arh),8.16(d,j=2.0hz,1h,arh),8.07(d,j=7.8hz,1h,arh),8.02(d,j=8.5hz,1h,arh),7.96(brs,1h,nh),7.79(d,j=8.7hz,1h,arh),7.49-7.55(m,3h,arh),7.37(t,j=7.4hz,1h,arh),7.26(dd,j=8.6,2.1hz,1h,arh),7.10(t,j=7.7hz,1h,h-1’),5.91(brs,1h,h-3’),4.94-5.07(m,4h,h-7,h-3’’),4.52(brs,1h,h-4’),2.95(s,3h,3'-nch3),2.85(s,3h,4'-och3),2.72-2.76(m,1h,h-2’a),2.36(s,3h,6'-ch3),2.26-2.32(m,1h,h-2’b);13cnmr(150mhz,dmso-d6)δ190.2,182.5,171.8,139.1,135.0,134.7,133.2,128.9,126.6,126.5,125.7,125.3,125.1,124.5,123.8,123.7(2×c),122.9,121.7,121.5,120.5,120.3,119.4,114.7,114.2,114.0,113.9,113.8,110.9,95.1,82.8,82.5,60.4,54.2,51.9,45.6,32.8,29.5,27.6;esi-msm/z773.0[m+na]+。氩气保护下,在25ml两口反应瓶中,加入3-氯-3’-n-[n-(2-氧亚基-2-(3-(5-氯吲哚))乙基)氨基硫代甲酰]十字孢碱(27mg,0.036mmol),加入6ml二氯甲烷和200µl乙醇溶解,0℃下加入400µl三氟乙酸酐,反应1小时后加水终止反应,二氯甲烷萃取,无水硫酸钠干燥后浓缩,半制备hplc分离、meoh:h2o=9:1(v/v)洗脱得到3,5’’’-二氯福瑞德咔唑碱甲(化合物7)14mg,收率53%。1hnmr(600mhz,dmso-d6)δ11.70(s,1h,nh),9.35(s,1h,arh),8.71(s,1h,nh),8.08(d,j=7.7hz,1h,arh),8.05(d,j=8.4hz,1h,arh),7.78(brs,1h,arh),7.73(d,j=8.6hz,1h,arh),7.66(brs,1h,arh),7.45-7.56(m,4h,arh),7.37(t,j=7.0hz,1h,arh),7.18(d,j=8.6hz,1h,arh),7.10(t,j=7.0hz,1h,h-1’),5.03(s,2h,h-7),4.98(d,j=13.0hz,1h,h-3’),4.49(brs,1h,h-4’),2.91(s,3h,3'-nch3),2.86-2.90(m,1h,h-2’a),2.70(s,3h,4'-och3),2.42(s,3h,6'-ch3),2.38-2.42(m,1h,h-2’b);13cnmr(150mhz,dmso-d6)δ171.8,167.7,138.8,135.0,134.7,134.4,133.1,129.1,128.5,125.9(2×c),125.3,125.1,124.7,124.6,124.3,123.8,123.7,123.6,121.8,121.6,120.5,119.6,119.4,118.2,114.7,114.2,113.6(2×c),106.7,94.9,82.5,82.4,60.2,53.1,45.6,34.5,29.2,27.0;hresi-msm/z755.1368[m+h]+(calcdforc39h30n6o3cl2nas,755.1369)。实施例8化合物8的制备3-氯-3’-n-(1-咪唑硫代甲酰)十字孢碱咪唑部分的碘甲烷盐(110mg,0.15mmol)溶于10mldmf中,加入三乙胺1.0ml和n-[2-氧亚基-2-(3-(5-溴吲哚))乙基]三氟乙酸铵(52mg,0.15mmol,),室温反应24小时。反应液用20ml乙酸乙酯稀释,用1n的盐酸洗,无水硫酸钠干燥后浓缩。半制备hplc分离、meoh:h2o=9:1(v/v)洗脱得到3-氯-3’-n-[n-(2-氧亚基-2-(3-(5-溴吲哚))乙基)氨基硫代甲酰]十字孢碱45mg,产率38%。1hnmr(600mhz,dmso-d6)δ12.22(s,1h,nh),9.33(s,1h,arh),8.70(s,1h,nh),8.57(s,1h,arh),8.31(s,1h,arh),8.07(d,j=8.0hz,1h,arh),8.02(d,j=8.6hz,1h,arh),7.95(brs,1h,nh),7.79(d,j=8.6hz,1h,arh),7.36-7.52(m,5h,arh),7.10(t,j=7.5hz,1h,h-1’),5.90(brs,1h,h-3’),4.92-5.05(m,4h,h-7,h-3’’),4.52(brs,1h,h-4’),2.95(s,3h,3'-nch3),2.86(s,3h,4'-och3),2.71-2.74(m,1h,h-2’a),2.35(s,3h,6'-ch3),2.26-2.31(m,1h,h-2’b);13cnmr(150mhz,dmso-d6)δ190.2,182.5,171.8,139.1,135.1,134.7,134.5,133.2,128.9,127.2,125.7,125.5,125.3,125.1,124.5,123.8,123.7(2×c),123.3,121.5,120.5,119.4,114.7,114.6,114.3,114.2,114.0,113.6,110.9,95.1,82.8,82.5,60.4,54.2,51.9,45.6,32.8,29.5,27.6;esi-msm/z817.0[m+na]+。氩气保护下,在25ml两口反应瓶中,加入3-氯-3’-n-[n-(2-氧亚基-2-(3-(5-溴吲哚))乙基)氨基硫代甲酰]十字孢碱(30mg,0.038mmol),加入6ml二氯甲烷和200µl乙醇溶解,0℃下加入400µl三氟乙酸酐,反应1小时后加水终止反应,二氯甲烷萃取,无水硫酸钠干燥后浓缩,半制备hplc分离、meoh:h2o=9:1(v/v)洗脱得到3-氯-5’’’-溴福瑞德咔唑碱甲(化合物8)14mg,收率47%。1hnmr(600mhz,dmso-d6)δ11.58(s,1h,nh),9.35(d,j=1.8hz,1h,arh),8.71(s,1h,nh),8.08(d,j=7.8hz,1h,arh),8.04(d,j=8.5hz,1h,arh),7.92(brs,1h,arh),7.73(d,j=8.7hz,1h,arh),7.65(d,j=2.4hz,1h,arh),7.56(brs,1h,arh),7.48-7.54(m,2h,arh),7.42(d,j=8.6hz,1h,arh),7.37(t,j=7.4hz,1h,arh),7.29(dd,j=8.6,1.8hz,1h,arh),7.09-7.11(m,1h,h-1’),5.03(s,2h,h-7),4.96-4.99(m,1h,h-3’),4.49(s,1h,h-4’),2.91(s,3h,3'-nch3),2.86-2.90(m,1h,h-2’a),2.70(s,3h,4'-och3),2.43(s,3h,6'-ch3),2.38-2.42(m,1h,h-2’b);13cnmr(150mhz,dmso-d6)δ171.8,167.8,138.8,135.2,134.7,134.5,133.1,129.1,126.6,125.9,125.3,125.1,124.5(2×c),124.4,123.8,123.7,123.6,121.6,121.2,120.5,119.5,119.4,114.7,114.2,114.0,113.6,112.2,110.8,106.6,94.9,82.5,82.4,60.2,53.1,45.6,34.5,29.2,27.0;hresi-msm/z777.1042[m+h]+(calcdforc39h31n6o3brcls,777.1045)。实施例9化合物9的制备3-氯-3’-n-(1-咪唑硫代甲酰)十字孢碱咪唑部分的碘甲烷盐(280mg,0.37mmol)溶于10mldmf中,加入三乙胺1.0ml和n-[2-氧亚基-2-(3-(5-甲氧基吲哚))乙基]三氟乙酸铵(111mg,0.37mmol,),室温反应24小时。反应液用20ml乙酸乙酯稀释,用1n的盐酸洗,无水硫酸钠干燥后浓缩。半制备hplc分离、meoh:h2o=9:1(v/v)洗脱得到3-氯-3’-n-[n-(2-氧亚基-2-(3-(5-甲氧基吲哚))乙基)氨基硫代甲酰]十字孢碱119mg,产率43%。1hnmr(600mhz,dmso-d6)δ11.92(s,1h,nh),9.33(s,1h,arh),8.70(s,1h,nh),8.43(s,1h,arh),8.07(d,j=7.8hz,1h,arh),8.02(d,j=8.5hz,1h,arh),7.90(brs,1h,nh),7.79(d,j=8.7hz,1h,arh),7.69(s,1h,arh),7.48-7.52(m,2h,arh),7.36-7.40(m,2h,arh),7.10(t,j=7.6hz,1h,h-1’),6.87(dd,j=8.7,2.4hz,1h,arh),5.93(brs,1h,h-3’),4.95-5.03(m,4h,h-7,h-3’’),4.50(s,1h,h-4’),3.78(s,3h,5'''-och3),2.95(s,3h,3'-nch3),2.83(s,3h,4'-och3),2.71-2.75(m,1h,h-2’a),2.36(s,3h,6'-ch3),2.26-2.32(m,1h,h-2’b);13cnmr(150mhz,dmso-d6)δ189.9,182.4,171.8,155.5,139.1,134.8,133.5,133.2,131.3,129.0,126.3,125.8,125.3,125.1,124.5,123.8,123.7(2×c),121.6,120.5,119.4,114.7,114.3,114.0(2×c),113.0,112.7,110.9,102.9,95.1,82.9,82.5,60.4,55.3,54.2,51.8,45.6,32.7,29.4,27.6;esi-msm/z769.2[m+na]+。氩气保护下,在25ml两口反应瓶中,加入3-氯-3’-n-[n-(2-氧亚基-2-(3-(5-甲氧基吲哚))乙基)氨基硫代甲酰]十字孢碱(35mg,0.047mmol),加入6ml二氯甲烷和200µl乙醇溶解,0℃下加入400µl三氟乙酸酐,反应1小时后加水终止反应,二氯甲烷萃取,无水硫酸钠干燥后浓缩,半制备hplc分离、meoh:h2o=9:1(v/v)洗脱得到3-氯-5’’’-甲氧基福瑞德咔唑碱甲(化合物9)16mg,收率47%。1hnmr(600mhz,dmso-d6)δ11.20(s,1h,nh),9.34(s,1h,arh),8.71(s,1h,nh),8.08(d,j=7.8hz,1h,arh),8.05(d,j=8.6hz,1h,arh),7.72(dd,j=8.7,2.0hz,1h,arh),7.55(s,1h,arh),7.48-7.53(m,3h,arh),7.3-7.38(m,2h,arh),7.24(brs,1h,arh),7.08-7.11(m,1h,h-1’),6.83(dd,j=8.8,2.0hz,1h,arh),5.02(s,2h,h-7),4.97(d,j=12.0hz,1h,h-3’),4.50(s,1h,h-4’),3.83(s,3h,5'''-och3),2.90(s,3h,3'-nch3),2.86-2.89(m,1h,h-2’a),2.67(s,3h,4'-och3),2.43(s,3h,6'-ch3),2.38-2.42(m,1h,h-2’b);13cnmr(150mhz,dmso-d6)δ171.8,167.3,153.9,138.8,134.7,133.7,133.1,131.6,129.1,125.9,125.3,125.1(2×c),124.6,123.8,123.7,123.6,123.5,121.6,120.7,120.5,119.4,114.6,114.2,113.6,112.7,112.0,110.8,106.7,100.8,94.9,82.5(2×c),60.2,55.4,53.1,45.6,34.5,29.2,27.0;hresi-msm/z729.2040[m+h]+(calcdforc40h34n6o4cls,729.2045)。实施例10化合物10的制备氩气保护下,在25ml两口反应瓶中,加入3'-n-叔丁氧羰基十字孢碱(1.0g,1.77mmol),用30ml二氯甲烷/甲醇(1:1)溶解,0℃下加入溴代丁二酰亚胺(347mg,1.95mmol),此温度下反应0.5小时,加水终止反应,二氯甲烷萃取,并用无水na2so4干燥,真空蒸干,硅胶柱层析分离,石油醚:乙酸乙酯(v/v1:1)得到3-溴-3′-n-叔丁氧羰基十字孢碱1.07g,收率94%。1hnmr(400mhz,dmso-d6)δ9.48(s,1h,arh),8.71(s,1h,nh),8.00-8.10(m,2h,arh),7.60-7.66(m,2h,arh),7.50(brs,1h),7.36(t,j=7.4hz,1h,arh),7.01(t,j=7.5hz,1h,h-1’),5.01(s,2h,h-7),4.44-4.63(m,1h,h-3’),4.28(brs,1h,h-4’),2.76(s,3h,3'-nch3),2.60-2.67(m,4h,4'-och3,h-2’a),2.33(s,3h,6'-ch3),2.11-2.20(m,1h,h-2’b),1.56/1.46(m,9h,3×4’’-ch3);13cnmr(100mhz,dmso-d6)δ171.8,154.8/154.2,138.8,134.9,133.2,128.8,127.6,127.5,125.5,125.2,124.3,123.6,121.6,120.4,119.4,114.6,114.0,113.8/113.7,111.6,111.1,94.7,83.9/83.2,82.3,79.7/79.3,60.5,50.4/49.6,45.6,30.1,29.5,28.1(3×c),26.9;esi-msm/z667.2[m+na]+。在100ml两口反应瓶中,加入3-溴-3′-n-叔丁氧羰基十字孢碱(1.07g,1.66mmol),用10ml二氯甲烷溶解。0℃加入8ml三氟乙酸,此温度下反应1小时,浓缩后加入50ml饱和碳酸氢钠水溶液搅拌0.5小时,后用二氯甲烷萃取,无水硫酸钠干燥后浓缩,硅胶柱色谱分离,二氯甲烷:乙酸乙酯(v/v5:1)洗脱得到3-溴十字孢碱(450mg,收率50%)。1hnmr(400mhz,cdcl3)δ9.56(d,j=2.0hz,1h,arh),7.92(d,j=8.0hz,1h,arh),7.89(d,j=8.0hz,1h,arh),7.54(d,j=8.0,2.0hz,1h,arh),7.41-7.44(m,1h,arh),7.31-7.34(m,1h,arh),7.16(d,j=8.0hz,1h,arh),6.49-6.51(m,1h,h-1’),6.45(s,1h,nh),5.01(s,2h,h-7),3.85-3.87(m,1h,h-4’),3.42(s,3h,4'-och3),3.33-3.35(m,1h,h-3’),2.69-2.72(m,1h,h-2’a),2.34-2.39(m,4h,h-2’b,3'-nch3),1.50(s,3h,6’-ch3);13cnmr(100mhz,cdcl3)δ173.3,139.8,135.1,132.5,130.6,128.9,127.7,127.7,124.0,124.4,124.3,120.6,120.1,118.4,115.3,114.4,114.3,112.6,108.3,91.0,84.1,80.1,57.2,50.1,45.9,33.3,30.1,29.7;esi-msm/z567.1[m+na]+。将3-溴十字孢碱(600mg,1.1mmol)用30ml二氯甲烷溶解,室温下加入3ml三乙胺和n,n′-硫羰基二咪唑(587mg,3.3mmol),室温过夜反应。反应液倒入30ml冰水中,二氯甲烷萃取,无水硫酸钠干燥有机相后浓缩,硅胶柱色谱分离、二氯甲烷:甲醇=30:1(v/v)洗脱得到3-溴-3’-n-(1-咪唑硫代甲酰)十字孢碱631mg,产率88%。1hnmr(600mhz,dmso-d6)δ9.45(s,1h,arh),8.71(s,1h,nh),8.12(brs,1h,arh),8.08(d,j=7.8hz,1h,arh),8.04(d,j=7.8hz,1h,arh),7.64(brs,1h,arh),7.62-7.66(m,2h,arh),7.53(t,j=7.8hz,1h,arh),7.38(t,j=7.8hz,1h,arh),7.15(brs,1h,arh),7.05(brs,1h,h-1’),5.46(brs,1h,h-3’),5.03(s,2h,h-7),4.78(brs,1h,h-4’),3.00-3.08(m,4h,3’-nch3,h-2’a),2.76(s,3h,4’-ome),2.38-2.46(m,4h,6’-ch3,h-2’b);13cnmr(150mhz,dmso-d6)δ179.4,171.7,138.9,137.7,134.9,133.2,129.1,129.0,127.7,127.6,125.8,125.5,124.4,123.7,121.7,120.7,119.9,119.6,114.8,114.2,113.6,111.8,111.1,94.9,82.0,81.7,60.4,58.1,45.6,38.2,29.4,26.9;esi-msm/z677.2[m+na]+。将3-溴-3’-n-(1-咪唑硫代甲酰)十字孢碱(583mg,0.89mmol)溶于45ml乙腈中,加入碘甲烷1ml,室温反应24小时。反应液直接浓缩,用50ml石油醚:二氯甲烷=4:1(v/v)的混合溶液洗纯即得3-溴-3’-n-(1-咪唑硫代甲酰)十字孢碱咪唑部分的碘甲烷盐521mg,产率74%。1hnmr(600mhz,dmso-d6)δ9.69(s,1h,arh),9.50(s,1h,arh),8.71(s,1h,nh),8.14(brs,1h,arh),8.09(d,j=7.7hz,1h,arh),8.06(d,j=8.4hz,1h,arh),7.86(brs,1h,arh),7.63-7.67(m,2h,arh),7.54(t,j=7.6hz,1h,arh),7.39(t,j=7.6hz,1h,arh),7.17-7.20(m,1h),5.39(d,j=12.4hz,1h,h-3’),5.02(s,2h,h-7),4.75(s,1h,h-4’),3.92(s,3h,5’’-nch3),3.06-3.11(m,4h,3’-nch3,h-2’a),2.71(s,3h,4’-ome),2.43-2.47(m,4h,6’-ch3,h-2’b);13cnmr(150mhz,dmso-d6)δ174.3,171.7,138.7,138.0,134.9,133.1,129.1,127.7,127.6,125.8,125.5,124.4,123.7,123.1,121.8,121.2,120.8,119.6,114.9,114.2,113.5,111.8,111.1,94.7,81.9,81.2,60.5,59.1,45.6,38.5,36.5,29.2,26.7;esi-msm/z669.2[m-i]+。3-溴-3’-n-(1-咪唑硫代甲酰)十字孢碱咪唑部分的碘甲烷盐(110mg,0.14mmol)溶于10mldmf中,加入三乙胺1.0ml和n-[2-氧亚基-2-(3-吲哚)乙基]三氟乙酸铵(38mg,0.14mmol,),室温反应24小时。反应液用20ml乙酸乙酯稀释,用1n的盐酸洗,无水硫酸钠干燥后浓缩。半制备hplc分离、meoh:h2o=9:1(v/v)洗脱得到3-溴-3’-n-[n-(2-氧亚基-2-(3-吲哚)乙基)氨基硫代甲酰]十字孢碱52mg,产率49%。1hnmr(600mhz,dmso-d6)δ12.03(d,j=2.4hz,1h,nh),9.48(d,j=2.0hz,1h,arh),8.70(s,1h,nh),8.50(d,j=3.0hz,1h,arh),8.18(d,j=7.5hz,1h,arh),8.07(d,j=7.8hz,1h,arh),8.02(d,j=8.5hz,1h,arh),7.92(brs,1h,nh),7.75(d,j=8.7hz,1h,arh),7.63(dd,j=8.6,2.0hz,1h,arh),7.49-7.52(m,2h,arh),7.37(t,j=7.4hz,1h,arh),7.19-7.25(m,2h,arh),7.09(t,j=8.0hz,1h,h-1’),5.92(brs,1h,h-3’),4.97-5.07(m,4h,h-7,h-3’),4.52(s,1h,h-4’),2.95(s,3h,3'-nch3),2.85(s,3h,4'-och3),2.71-2.75(m,1h,h-2’a),2.36(s,3h,6'-ch3),2.26-2.31(m,1h,h-2’b);13cnmr(150mhz,dmso-d6)δ190.0,182.4,171.8,139.1,136.4,135.0,133.3,133.2,128.9,127.7,127.5,125.6,125.4,125.2,124.3,123.6,122.9,121.8,121.6,121.2,120.5,119.4,114.7,114.1(2×c),114.0,112.2,111.6,111.3,95.1,82.8,82.5,60.4,54.2,51.9,45.6,32.6,29.5,27.5;esi-msm/z783.2[m+na]+。氩气保护下,在25ml两口反应瓶中,加入3-溴-3’-n-[n-(2-氧亚基-2-(3-吲哚)乙基)氨基硫代甲酰]十字孢碱(36mg,0.047mmol),加入6ml二氯甲烷和200µl乙醇溶解,0℃下加入400µl三氟乙酸酐,反应1小时后加水终止反应,二氯甲烷萃取,无水硫酸钠干燥后浓缩,半制备hplc分离、meoh:h2o=9:1(v/v)洗脱得到3-溴福瑞德咔唑碱甲(化合物10)19mg,收率55%。1hnmr(600mhz,dmso-d6)δ11.38(s,1h,nh),9.50(s,1h,arh),8.71(s,1h,nh),8.07(d,j=7.6hz,1h),8.04(d,j=8.3hz,1h),7.82(d,j=7.7hz,1h,arh),7.62-7.69(m,2h,arh),7.58(s,1h,arh),7.55(s,1h,arh),7.49(t,j=7.7hz,1h,arh),7.45(d,j=8.0hz,1h,arh),7.37(t,j=7.2hz,1h,arh),7.18(t,j=7.4hz,1h,arh),7.08-7.13(m,2h,arh,h-1’),5.03(s,2h,h-7),4.97(d,j=12.8hz,1h),4.49(s,1h,h-4’),2.91(s,3h,3'-nch3),2.85-2.89(m,1h,h-2’a),2.70(s,3h,4'-och3),2.43(s,3h,6'-ch3),2.37-2.42(m,1h,h-2’b);13cnmr(150mhz,dmso-d6)δ171.8,167.4,138.8,136.5,135.0,133.9,133.1,129.0,127.7,127.6,125.8,125.3,124.8,124.3,123.6,122.8,121.8,121.6,120.6,120.5,119.6,119.4,119.1,114.7,114.0,113.6,112.0,111.6,111.2,106.9,94.9,82.5,82.5,60.2,53.1,45.6,34.5,29.2,27.0;hresi-msm/z743.1431[m+h]+(calcdforc39h32n6o3brs,743.1434)。实施例11化合物11的制备3-溴-3’-n-(1-咪唑硫代甲酰)十字孢碱咪唑部分的碘甲烷盐(228mg,0.29mmol)溶于10mldmf中,加入三乙胺1.0ml和n-[2-氧亚基-2-(3-(5-氟吲哚))乙基]三氟乙酸铵(84mg,0.29mmol,),室温反应24小时。反应液用20ml乙酸乙酯稀释,用1n的盐酸洗,无水硫酸钠干燥后浓缩。半制备hplc分离、meoh:h2o=9:1(v/v)洗脱得到3-溴-3’-n-[n-(2-氧亚基-2-(3-(5-氟吲哚))乙基)氨基硫代甲酰]十字孢碱101mg,产率45%。1hnmr(600mhz,dmso-d6)δ12.16(s,1h,nh),9.48(s,1h,arh),8.70(s,1h,nh),8.57(s,1h,arh),8.07(d,j=7.8hz,1h,arh),8.01(d,j=8.5hz,1h,arh),7.94(s,1h,nh),7.85(d,j=9.8hz,1h,arh),7.74(d,j=8.7hz,1h,arh),7.62(dd,j=8.6,2.0hz,1h,arh),7.48-7.54(m,2h,arh),7.37(t,j=7.4hz,1h,arh),7.07-7.11(m,2h,arh,h-1’),5.91(brs,1h,h-3’),4.95-5.05(m,4h,h-7,h-3’’),4.51(brs,1h,h-4’),2.95(s,3h,3'-nch3),2.84(s,3h,4'-och3),2.71-2.75(m,1h,h-2’a),2.36(s,3h,6'-ch3),2.26-2.33(m,1h,h-2’b);13cnmr(150mhz,dmso-d6)δ190.2,182.5,171.8,158.6(d,1jc-f=234.8hz),139.1,135.0,134.9,133.3,133.1,128.9,127.7,127.6,126.1(d,3jc-f=10.8hz),125.6,125.3,124.4,123.7,121.6,120.5,119.4,114.8,114.3(d,4jc-f=4.2hz),114.2,114.0,113.5(d,3jc-f=10.8hz),111.7,111.4,111.1(d,2jc-f=24.0hz),106.0(d,2jc-f=24.0hz),95.1,82.9,82.5,60.4,54.3,51.9,45.7,32.8,29.5,27.6;esi-msm/z801.2[m+na]+。氩气保护下,在25ml两口反应瓶中,加入3-溴-3’-n-[n-(2-氧亚基-2-(3-(5-氟吲哚))乙基)氨基硫代甲酰]十字孢碱(50mg,0.064mmol),加入6ml二氯甲烷和200µl乙醇溶解,0℃下加入400µl三氟乙酸酐,反应1小时后加水终止反应,二氯甲烷萃取,无水硫酸钠干燥后浓缩,半制备hplc分离、meoh:h2o=9:1(v/v)洗脱得到3-溴-5’’’-氟福瑞德咔唑碱甲(化合物11)24mg,收率50%。1hnmr(600mhz,dmso-d6)δ11.49(s,1h,nh),9.49(s,1h,arh),8.71(s,1h,nh),8.07(d,j=7.8hz,1h,arh),8.03(d,j=8.5hz,1h,arh),7.62-7.68(m,3h,arh),7.53-7.56(m,2h,arh),7.48(t,j=7.8hz,1h,arh),7.44-7.46(m,1h,arh),7.36(t,j=7.4hz,1h,arh),7.01-7.10(m,2h,arh,h-1’),5.02(s,2h,h-7),4.96(d,j=12.0hz,1h,h-3’),4.48(brs,1h,h-4’),2.88(s,3h,3'-nch3),2.84-2.88(m,1h,h-2’a),2.69(s,3h,4'-och3),2.43(s,3h,6'-ch3),2.37-2.41(m,1h,h-2’b);13cnmr(150mhz,dmso-d6)δ171.8,167.5,157.4(d,1jc-f=231.8hz),138.8,135.0,134.1,133.2,133.1,129.0,127.7,127.6,125.8,125.3,124.9(d,3jc-f=9.8hz),124.9,124.3,123.6,121.6,120.5,120.0,119.4,114.7,114.1,113.6,113.1(d,3jc-f=9.8hz),111.7,111.2,110.1(d,2jc-f=25.4hz),107.2(d,4jc-f=4.5hz),103.9(d,2jc-f=25.4hz),94.9,82.5,82.4,60.2,53.1,45.6,34.5,29.2,27.0;hresi-msm/z761.1337[m+h]+(calcdforc39h31n6o3brfs,761.1340)。实施例12化合物12的制备3-溴-3’-n-(1-咪唑硫代甲酰)十字孢碱咪唑部分的碘甲烷盐(200mg,0.25mmol)溶于10mldmf中,加入三乙胺1.0ml和n-[2-氧亚基-2-(3-(5-氯吲哚))乙基]三氟乙酸铵(76mg,0.25mmol,),室温反应24小时。反应液用20ml乙酸乙酯稀释,用1n的盐酸洗,无水硫酸钠干燥后浓缩。半制备hplc分离、meoh:h2o=9:1(v/v)洗脱得到3-溴-3’-n-[n-(2-氧亚基-2-(3-(5-氯吲哚))乙基)氨基硫代甲酰]十字孢碱90mg,产率44%。1hnmr(600mhz,dmso-d6)δ12.21(s,1h,nh),9.47(d,j=2.0hz,1h,arh),8.70(s,1h,nh),8.58(s,1h,arh),8.16(d,j=2.0hz,1h,arh),8.07(d,j=7.8hz,1h,arh),8.02(d,j=8.5hz,1h,arh),7.95(brs,1h,nh),7.75(d,j=8.7hz,1h,arh),7.63(dd,j=8.6,2.0hz,1h,arh),7.54(d,j=8.6hz,1h),7.51(t,j=7.7hz,1h),7.37(t,j=7.4hz,1h,arh),7.26(dd,j=8.6,2.1hz,1h,arh),7.09(t,j=7.7hz,1h,h-1’),5.90(brs,1h,h-3’),4.93-5.05(m,4h,h-7,h-3’),4.52(s,1h,h-4’),2.95(s,3h,3'-nch3),2.86(s,3h,4'-och3),2.71-2.75(m,1h,h-2’a),2.35(s,3h,6'-ch3),2.26-2.32(m,1h,h-2’b);13cnmr(150mhz,dmso-d6)δ190.2,182.5,171.8,139.1,135.0,134.9,134.7,133.2,128.9,127.7,127.5,126.6,126.5,125.6,125.3,124.3,123.7,122.9,121.6,120.5,120.3,119.4,114.7,114.1,114.0,113.9,113.8,111.6,111.3,95.1,82.8,82.5,60.4,54.2,51.9,45.6,32.8,29.5,27.5;esi-msm/z817.0[m+na]+。氩气保护下,在25ml两口反应瓶中,加入3-溴-3’-n-[n-(2-氧亚基-2-(3-(5-氯吲哚))乙基)氨基硫代甲酰]十字孢碱(38mg,0.048mmol),加入6ml二氯甲烷和200µl乙醇溶解,0℃下加入400µl三氟乙酸酐,反应1小时后加水终止反应,二氯甲烷萃取,无水硫酸钠干燥后浓缩,半制备hplc分离、meoh:h2o=9:1(v/v)洗脱得到3-溴-5’’’-氯福瑞德咔唑碱甲(化合物12)18mg,收率48%。1hnmr(600mhz,dmso-d6)δ11.58(s,1h,nh),9.50(s,1h,arh),8.71(s,1h,nh),8.06(t,j=7.5hz,1h,arh),8.03(d,j=8.4hz,1h,arh),7.79(brs,1h,arh),7.62-7.68(m,3h,arh),7.56(s,1h,arh),7.46-7.50(m,2h,arh),7.36(t,j=7.5hz,1h,arh),7.18(dd,j=8.4,1.9hz,1h,arh),7.09(brs,1h,h-1’),5.02(s,2h,h-7),4.95-4.99(m,1h,h-3’),4.48(s,1h,h-4’),2.90(s,3h,3'-nch3),2.85-2.89(m,1h,h-2’a),2.69(s,3h,4'-och3),2.43(s,3h,6'-ch3),2.37-2.42(m,1h,h-2’b);13cnmr(150mhz,dmso-d6)δ171.8,167.7,138.8,134.9(2×c),134.5,133.1,129.0,127.7,127.6,125.9,125.8,125.3,124.7,124.3(2×c),123.6,121.9,121.6,120.5,119.6,119.4,118.2,114.7,114.0,113.6(2×c),111.7,111.2,106.8,94.9,82.5,82.4,60.2,53.1,45.6,34.5,29.2,27.0;hresi-msm/z777.1044[m+h]+(calcdforc39h31n6o3brcls,777.1045)。实施例13化合物13的制备3-溴-3’-n-(1-咪唑硫代甲酰)十字孢碱咪唑部分的碘甲烷盐(100mg,0.13mmol)溶于10mldmf中,加入三乙胺1.0ml和n-[2-氧亚基-2-(3-(5-氯吲哚))乙基]三氟乙酸铵(45mg,0.13mmol),室温反应24小时。反应液用20ml乙酸乙酯稀释,用1n的盐酸洗,无水硫酸钠干燥后浓缩。半制备hplc分离、meoh:h2o=9:1(v/v)洗脱得到3-溴-3’-n-[n-(2-氧亚基-2-(3-(5-溴吲哚))乙基)氨基硫代甲酰]十字孢碱45mg,产率42%。1hnmr(600mhz,dmso-d6)δ9.48(d,j=1.8hz,1h,arh),8.71(s,1h,nh),8.56(s,1h,arh),8.31(d,j=1.6hz,1h,arh),8.07(d,j=7.8hz,1h,arh),8.02(d,j=8.5hz,1h,arh),7.97(brs,1h,nh),7.75(d,j=8.7hz,1h,arh),7.63(dd,j=8.6,1.8hz,1h,arh),7.49-7.52(m,2h,arh),7.36-7.38(m,2h,arh),7.09(t,j=7.7hz,1h,h-1’),5.90(s,1h,h-3’),4.92-5.07(m,4h,h-7,h-3’),4.52(s,1h,h-4’),2.95(s,3h,3'-nch3),2.86(s,3h,4'-och3),2.71-2.74(m,1h,h-2’a),2.35(s,3h,6'-ch3),2.26-2.31(m,1h,h-2’b);13cnmr(150mhz,dmso-d6)δ190.2,182.5,171.8,139.1,135.3,135.0,134.6,133.2,128.9,127.7,127.5,127.3,125.6,125.4,125.3,124.3,123.7,123.3,121.6,120.5,119.4,114.7,114.5,114.4,114.1,114.0,113.6,111.6,111.3,95.1,82.8,82.5,60.4,54.2,51.9,45.6,32.8,29.5,27.5;esi-msm/z861.2[m+na]+。氩气保护下,在25ml两口反应瓶中,加入3-溴-3’-n-[n-(2-氧亚基-2-(3-(5-溴吲哚))乙基)氨基硫代甲酰]十字孢碱(50mg,0.060mmol),加入6ml二氯甲烷和200µl乙醇溶解,0℃下加入400µl三氟乙酸酐,反应1小时后加水终止反应,二氯甲烷萃取,无水硫酸钠干燥后浓缩,半制备hplc分离、meoh:h2o=9:1(v/v)洗脱得到3,5’’’-二溴福瑞德咔唑碱甲(化合物13)26mg,收率53%。1hnmr(600mhz,dmso-d6)δ11.66(s,1h,nh),9.49(s,1h,arh),8.71(s,1h,nh),8.07(d,j=7.7hz,1h,arh),8.04(d,j=8.5hz,1h,arh),7.92(s,1h,arh),7.62-7.69(m,3h,arh),7.55(s,1h,arh),7.49(d,j=8.0hz,1h,arh),7.43(d,j=8.5hz,1h,arh),7.37(t,j=7.4hz,1h,arh),7.28-7.30(m,1h,arh),7.08-7.11(m,1h,h-1’),5.04(s,2h,h-7),4.97(d,j=12.7hz,1h,h-3’),4.49(s,1h,h-4’),2.91(s,3h,3'-nch3),2.85-2.89(m,1h,h-2’a),2.69(s,3h,4'-och3),2.43(s,3h,6'-ch3),2.36-2.41(m,1h,h-2’b);13cnmr(150mhz,dmso-d6)δ171.8,167.8,138.8,135.2,135.0,134.5,133.1,129.1,127.7,127.6,126.6,125.8,125.3,124.5,124.4,124.3,123.6,121.6,121.2,120.5,119.5,119.4,114.7,114.1(2×c),113.6,112.2,111.7,111.2,106.6,94.9,82.5,82.4,60.2,53.1,45.6,34.5,29.2,27.0;hresi-msm/z821.0539[m+h]+(calcdforc39h31n6o3br2s,821.0539)。实施例14化合物14的制备3-溴-3’-n-(1-咪唑硫代甲酰)十字孢碱咪唑部分的碘甲烷盐(200mg,0.25mmol)溶于10mldmf中,加入三乙胺1.0ml和n-[2-氧亚基-2-(3-(5-甲氧基吲哚))乙基]三氟乙酸铵(75mg,0.25mmol),室温反应24小时。反应液用20ml乙酸乙酯稀释,用1n的盐酸洗,无水硫酸钠干燥后浓缩。半制备hplc分离、meoh:h2o=9:1(v/v)洗脱得到3-溴-3’-n-[n-(2-氧亚基-2-(3-(5-甲氧基吲哚))乙基)氨基硫代甲酰]十字孢碱109mg,产率56%。1hnmr(600mhz,dmso-d6)δ11.92(s,1h,nh),9.47(s,1h,arh),8.70(s,1h,nh),8.43(d,j=2.0hz,1h,arh),8.07(d,j=7.6hz,1h,arh),8.02(d,j=8.4hz,1h,arh),7.89(brs,1h,nh),7.74(d,j=8.6hz,1h,arh),7.69(s,1h,arh),7.63(d,j=8.6hz,1h,arh),7.50(t,j=7.6hz,1h,arh),7.36-7.41(m,2h,arh),7.09(t,j=7.6hz,1h,h-1’),6.87(dd,j=8.6,2.1hz,1h,arh),5.93(brs,1h,h-3’),4.93-5.03(m,4h,h-7,h-3’’),4.50(s,1h,h-4’),3.78(s,3h,5'''-och3),2.95(s,3h,3'-nch3),2.82(s,3h,4'-och3),2.71-2.75(m,1h,h-2’a),2.36(s,3h,6'-ch3),2.26-2.32(m,1h,h-2’b);13cnmr(150mhz,dmso-d6)δ189.9,182.4,171.8,155.5,139.1,135.0,133.4,133.2,131.3,129.0,127.7,127.5,126.3,125.6,125.3,124.3,123.7,121.6,120.5,119.4,114.7,114.1,113.9(2×c),113.0,112.7,111.6,111.4,102.9,95.1,82.9,82.5,60.4,55.3,54.2,51.8,45.6,32.7,29.4,27.6;esi-msm/z813.2[m+na]+。氩气保护下,在25ml两口反应瓶中,加入3-溴-3’-n-[n-(2-氧亚基-2-(3-(5-甲氧基吲哚))乙基)氨基硫代甲酰]十字孢碱(56mg,0.071mmol),加入6ml二氯甲烷和200µl乙醇溶解,0℃下加入400µl三氟乙酸酐,反应1小时后加水终止反应,二氯甲烷萃取,无水硫酸钠干燥后浓缩,半制备hplc分离、meoh:h2o=9:1(v/v)洗脱得到3-溴-5’’’-甲氧基福瑞德咔唑碱甲(化合物14)32mg,收率59%。1hnmr(600mhz,dmso-d6)δ11.21(s,1h,nh),9.50(s,1h,arh),8.71(s,1h,nh),8.07(d,j=7.8hz,1h,arh),8.04(d,j=8.5hz,1h,arh),7.62-7.68(m,2h,arh),7.55(s,1h,arh),7.51(s,1h,arh),7.49(t,j=7.8hz,1h,arh),7.34-7.37(m,2h,arh),7.24(s,1h,arh),7.09(t,j=7.6hz,1h,h-1’),6.83(dd,j=8.5,1.6hz,1h),5.04(s,2h,h-7),4.96(d,j=12.0hz,1h,h-3’),4.49(s,1h,h-4’),3.83(s,3h,5'''-och3),2.90(s,3h,3'-nch3),2.85-2.89(m,1h,h-2’a),2.68(s,3h,4'-och3),2.43(s,3h,6'-ch3),2.37-2.43(m,1h,h-2’b);13cnmr(150mhz,dmso-d6)δ171.8,167.3,153.9,138.8,134.9,133.7,133.1,131.6,129.1,127.7,127.6,125.8,125.3,125.1,124.4,123.6,123.5,121.6,120.7,120.5,119.4,114.7,114.1,113.6,112.7,112.0,111.7,111.2,106.7,100.8,94.9,82.5,82.5,60.2,55.4,53.1,45.6,34.5,29.1,27.0;hresi-msm/z773.1536[m+h]+(calcdforc40h34n6o4brs,773.1540)。为了进一步验证本发明合成的化合物的有益效果,通过对实施例1-14方案中合成的化合物进行抗肿瘤活性测试,具体实验如下:1、实验方法被测样品溶液的配制:测试样品为上述实施例1~14中合成的化合物1~14。准确称取适量样品,用dmso配制成所需浓度的溶液,供活性测试。细胞系及细胞的继代培养:活性测试采用k562、mv-4-11、hl-60和pbmc细胞。各种细胞均用含10%fbs的rpmi-1640培养基,在37℃通入5%二氧化碳的培养箱中继代培养。本实验采用celltiterglo(ctg)法检测不同药物对k562、mv-4-11、hl-60和pbmc细胞的生长抑制作用。ctg法:腺嘌呤核苷三磷酸(简称三磷酸腺苷,atp)参与生物体内多种酶促反应,是活细胞新陈代谢的一个指标,其含量直接反应了细胞的数量及细胞状态。实验过程中向细胞培养基加入等体积ctg试剂,测量发光值,在光信号和体系中,发光值与atp量成正比,而atp又和活细胞数正相关,因此可通过检测atp含量得细胞活力。活性测试时,取对数生长期的k562、mv-4-11、hl-60和pbmc细胞,用新鲜的imdm培养基配制成密度为每毫升2×104个细胞的细胞悬液,细胞经计数后接种于96孔培养板,2000细胞/100μl/孔,每孔加入90μl培养液(含血清)培养过夜,再加入10μl药物溶液,继续培养48小时。然后加入100μlctg,室温静置10min,测定发光值,0.5ms。按照下式计算每个浓度下的细胞增殖率(%):ir%=(对照组化学发光值-实验组化学发光值)/对照组化学发光值×100%。应用graphpad软件,计算ic50。pkc-412为阳性对照组。2、实验结果实验结果如表1所示。表1.福瑞德咔唑碱甲衍生物1~14对人白血病细胞增殖抑制活性(ic50)化合物mv4-11(μm)k562(μm)hl-60(μm)pbmc(μm)10.513>10>10>1020.422>10>10>1030.413>10>10>1040.364>10>10>1050.564>10>10>1060.321>10>10>1070.589>10>10>1080.426>10>10>1090.441>10>10>10100.355>10>10>10110.964>10>10>10120.698>10>10>10130.394>10>10>10140.512>10>10>10pkc-4120.037>10>10>10由表1可知,(1)化合物1~14对人白血病细胞mv4-11细胞株均具有很强的抑制活性,ic50低于1μm。(2)化合物1~14对人外周血单核细胞pbmc抑制作用均较弱,ic50均大于10μm。表明本发明所有化合物对人白血病细胞mv4-11细胞株具有高选择性的抑制作用(对其它白血病细胞株hl-60和k562的活性较弱,ic50均大于10μm)。3、实验结论通过上述实验可知,本发明合成的福瑞德咔唑碱甲类化合物选择性地对人白血病细胞mv4-11细胞株具有很强的抑制活性,而对人外周血单核细胞pbmc细胞株抑制作用较弱,这表明其可被开发为高效低毒的预防和治疗白血病的药物。上述实施例仅仅是为清楚地说明所作的举例,而并非对实施方式的限定。对于所属领域的普通技术人员来说,在上述说明的基础上还可以做出其它不同形式的变化或变动。这里无需也无法对所有的实施方式予以穷举。而由此所引伸出的显而易见的变化或变动仍处于本发明创造的保护范围之中。当前第1页12
相关技术
网友询问留言
已有0条留言
- 还没有人留言评论。精彩留言会获得点赞!
1