一种幽门螺旋杆菌O:6血清型O-抗原糖链的合成方法与流程
本发明具体涉及一种幽门螺旋杆菌o:6血清型o-抗原糖链的合成方法,属于糖化学领域。
背景技术:
自1983年warren.marshall首先从胃炎病人胃内分离出幽门螺杆菌(helicobacterpylori,hp)后,各国学者经过广泛深入的研究发现hp与慢性活动性胃炎、胃十二指肠溃疡、胃粘膜相关性淋巴瘤(malt)及胃癌的发病有密切关系,1994年国际癌症研究中心将其归为i类致癌因子。作为一种革兰氏阴性细菌,hp是一群细长、柔韧、弯曲呈螺旋状、能自由运动的原核细胞微生物,它的特性介于细菌与原虫之间,主要位于人的胃黏膜的深层、胃黏膜上皮细胞,以胃小凹、上皮褶皱及腺腔内为多。世界上大约50%的人口感染了幽门螺杆菌,其中发展中国家高达70%,而发达国家感染率相对较小只有20%-30%。儿童是幽门螺杆菌的易感人群,在发展中国家儿童的感染主要是社会经济地位低下和医疗卫生设施落后造成的,提高个人卫生习惯对hp的传播具有重要影响。
目前,抗hp感染治疗方案是以铋剂或质子泵抑制剂联合应用抗生素的三联或四联疗法。但是这些基于抗生素的治疗有许多缺点,包括长期服用可以使hp对抗生素产生抗性,具有重复感染的危险及抗生素治疗费用的昂贵,因此,迫切需要新的方法来预防和根治幽门螺杆菌的感染。研究表明hp疫苗可能成为控制这一全球性感染的最有效的方法,目前对hp疫苗的配方研究主要是蛋白质成份,而其它成分如多糖的研究相对较少。然而,研究表明发展糖类疫苗抵抗hp的感染是非常合理的,以多糖为基础的共轭疫苗已经成功地用于防止系统性感染和抑制宿主定植。目前对肠道病原体的研究是基于其表面脂多糖(lps)的调查研究,可以作为候选疫苗为人类所使用。lps是幽门螺旋杆菌细胞表面主要的抗原组成成分,结构鉴定研究表明lps是由o-链多糖、核心结构和脂质a三部分组成,lps结构如下所示:
o-chain→core→lipida~cell。
在hp早期的研究中,penner和他的同事根据lps分子抗原性的差异发展了一套血清型系统,根据o-链多糖的结构差异定义了六种不同的血清型(o:1-o:6)。mario和他的同事研究证明以多价的hp脂多糖为基础的糖缀合在物小鼠体内引起免疫产生的抗体可以识别其他血清型hp。o:6血清型hp血清型中的一种,是由非还原端的lewiso-链与庚糖连接组成,具体结构如下所示:
目前,针对幽门螺旋杆菌脂多糖的研究都是通过从灭活细菌中提取,这类方法的不足在于一次提取得到的产物极少,另外受细菌基因表达和修饰的特性,提取得到的脂多糖也存在结构不够专一,并且容易附带结构类似杂质的特点,实验重复性差,对研究存在一定的干扰。由于o:6血清型o-抗原寡糖至今无人合成,为了研究其免疫效果,我们决定通过化学方法合成幽门螺杆菌o:6血清型抗原,并对其进行免疫学研究。然而,在复杂的糖化学合成过程中,糖苷键的构建是糖合成中最基本但是也最为棘手和关键的问题,由于糖类化合物结构的多样性,立体化学的复杂性,因此与其他结构的有机化合物不同,糖的合成的方法学依然是不成熟和不完善的,被认为是有机化学领域中唯一存在众多方法(数十种之多)但又有没有一个具有普适性的方法为大家公认的领域。因为糖模块的结构复杂、顺式糖苷键选择性低,难以实现该结构合成的构建,从而制约了o:6血清型o-抗原寡糖化学合成方法的研究。
技术实现要素:
为了解决上述问题,本发明提供了一种简单、快速有效的制备幽门螺旋杆菌o:6血清型o-抗原糖链片段的化学合成方法。
本发明简便易得的葡萄糖胺,半乳糖,甘露糖和岩藻糖为起始原料,经过一系列的化学反应得到七个糖砌块,然后利用这些糖砌块在相应的活化试剂的作用下,基于邻基参与效应、溶剂效应、添加剂效应等设计,经过一系列的糖苷化反应偶联得到幽门螺旋杆菌o:6血清型o-抗原糖链,同时还原端带有氨基的连接臂,为以后连接蛋白制成糖缀合物疫苗做准备。本发明的第一个目的是提供一种合成幽门螺旋杆菌o:6血清型o-抗原糖链片段的方法,所述方法是利用七个糖砌块构建幽门螺旋杆菌o:6血清型o-抗原糖链片段,所述七个糖砌块分别为式1~7所示化合物:
其中,pg1,pg2,pg3,pg4,pg5,pg6,pg7,pg8,pg9,pg10,pg11,pg12,pg13,pg14,pg15,pg17,pg18,pg19,pg21,pg22,pg23,pg25,pg26,pg27,pg28,pg29和pg30分别独立的选自氢、酰基、2-萘甲基及其衍生物、苄基及其衍生物、烯丙基和硅烷基中任意一种;
pg16和pg24分别独立的选自氢、酰基、烷氧羰基和烷氧羰酰基中任意一种;
pg20选自烷酰基、二甲酰基、苄氧羰基及其衍生物中任意一种;
式1~7结构中的离去基团lg分别独立的选自卤素、亚胺酯基、硫基和膦酸基中任意一种。
在本发明的一种实施方式中,所述pg1,pg9,pg12,pg17,pg21,pg22和pg24为羟基临时保护基,选自氢(h)、乙酰基(ac)、苯甲酰(bz)、新戊酰基(piv)、氯乙酰(clac)、乙酰丙酰基(lev)、9-芴甲氧羰基(fmoc)、烯丙氧羰酰基(alloc)、2-萘甲基(nap)、对甲氧基苄基(pmbn)或者烯丙基(all)中任意一种。
在本发明的一种实施方式中,所述pg2,pg3,pg4,pg6,pg7,pg8,pg11,pg13,pg14,pg18,pg25,pg26,pg29和pg30选自氢(h)、乙酰基(ac)、苯甲酰(bz)、新戊酰基(piv)、氯乙酰(clac)、烯丙氧羰酰基(alloc)、苄基(bn)、2-萘甲基(nap)、对甲氧基苄基(pmbn)或者烯丙基(all)中任意一种。
在本发明的一种实施方式中,所述pg16、pg24选自氢(h)、乙酰基(ac)、苯甲酰(bz)、新戊酰基(piv)、氯乙酰(clac)、乙酰丙酰基(lev)、9-芴甲氧羰基(fmoc)、烯丙氧羰酰基(alloc)中任意一种。
在本发明的一种实施方式中,所述pg5,pg10,pg15,pg19,pg23,pg27和pg28选自氢(h)、乙酰基(ac)、苯甲酰(bz)、新戊酰基(piv)、氯乙酰(clac)、烯丙氧羰酰基(alloc)、苄基(bn)、2-萘甲基(nap)、对甲氧基苄基(pmbn)、烯丙基(all)、叔丁基二甲基硅烷基、叔丁基二苯基硅烷基和三乙基硅烷基中任意一种。
在本发明的一种实施方式中,所述pg20为氨基保护基团,选自三氯乙酰基(tca)、三氯乙酰氧羰基(troc)、邻苯二甲酰基(phth)、苄氧羰基(cbz)中任意一种。
在本发明的一种实施方式中,所述lg为用于糖基化反应的离去基团,选自氟(f)、氯(cl)、溴(br)、碘(i)、三氯乙酰亚胺酯(ccl3c(=nh)o-)、n-苯基三氟乙酰亚胺酯糖苷(cf3c(=nph)o-)、乙硫基(set)、苯硫基(sph)、对甲苯硫基(stol)、二丁基膦酸基(-p(=o)-(obu)2)中任意一种。
在本发明的一种实施方式中,所述方法包括预先合成二糖,然后通过糖苷键的构建,合成幽门螺旋杆菌o:6血清型o-抗原糖链片段。
在本发明的一种实施方式中,所述糖苷键的构建是利用活性试剂偶联糖基供体和受体,实现d-α-d-hep-(1-2)连接。
在本发明的一种实施方式中,所述活性试剂包括nis、nbs、dbu、tmsotf中的一种或多种。
在本发明的一种实施方式中,所述活性试剂优选nis和tmsotf两种混合。
在本发明的一种实施方式中,所述方法包括合成二糖化合物,所述二糖化合物的合成路线如下所示:
在本发明的一种实施方式中,所述二糖是利用糖砌块1为糖基供体,糖砌块8为糖基受体,在有机溶剂中偶联得到d-α-d-hep-(1-2)连接的二糖化合物(化合物9)。
在本发明的一种实施方式中,所述糖砌块1与糖砌块8的摩尔比为(1-2):1
在本发明的一种实施方式中,所述有机溶剂为二氯甲烷、四氢呋喃、氯仿、乙腈中的一种或多种。
在本发明的一种实施方式中,所述有机溶剂优选二氯甲烷。
在本发明的一种实施方式中,所述二糖的合成方法具体包括:将糖砌块1与糖砌块8按照摩尔比溶于有机溶剂中,加入酸洗的分子筛,然后在路易斯酸催化,-10℃搅拌下2-4小时偶联完全,,制备出d-α-d-hep-(1-2)连接的二糖化合物9。
在本发明的一种实施方式中,所述方法还包括合成三糖化合物,所述三糖化合物的合成路线如下所示:
在本发明的一种实施方式中,利用二糖化合物9脱保护得到糖基受体二糖化合物10,然后以化合物2作为糖基供体,偶联得到d-α-d-hep-(1-2)连接的三糖化合物11。
在本发明的一种实施方式中,二糖化合物10与化合物2的摩尔比为1:(1-2)。
在本发明的一种实施方式中,所述三糖化合物11的制备方法具体包括:二糖化合物9选择性的脱去二糖3的2-位保护基得到糖基受体10,再按照摩尔比将糖基供体2与糖基受体10在路易斯酸催化,在-10℃搅拌下偶联得到d-α-d-hep-(1-2)连接的目标三糖化合物11。
在本发明的一种实施方式中,所述方法还包括合成四糖化合物,所述四糖化合物的合成路线如下所示:
在本发明的一种实施方式中,以糖砌块3为糖基供体,三糖化合物12为糖基受体,在有机溶剂中偶联得到四糖化合物13。
在本发明的一种实施方式中,糖砌块3与三糖化合物12的的摩尔比为(1-2):1。
在本发明的一种实施方式中,所述有机溶剂为二氯甲烷。
在本发明的一种实施方式中,所述四糖化合物13的合成方法具体包括:按照摩尔比,将糖砌块3,三糖化合物12溶于干燥的二氯甲烷中,加入酸洗的分子筛,然后在路易斯酸催化,在-10℃搅拌下偶联2-4小时,制备出目标四糖化合物13
在本发明的一种实施方式中,所述方法还包括合成五糖化合物,所述五糖化合物的合成路线如下所示:
在本发明的一种实施方式中,以糖砌块3为糖基供体,三糖化合物14为糖基受体,在有机溶剂中偶联得到四糖化合物15;然后以四糖化合物15为糖基供体,三糖化合物12为糖基受体,在有机溶剂中偶联得到五糖化合物16。
在本发明的一种实施方式中,所述五糖化合物16的制备方法具体包括:
按照摩尔比(1-2):1,将糖砌块3与糖砌块14溶于有机溶剂中,加入分子筛和路易斯酸,-10℃反应2-4小时,得到二糖供体15;然后与1-2倍摩尔当量的三糖化合物12偶联得到五糖化合物16。
在本发明的一种实施方式中,所述方法还包括合成八糖化合物,所述八糖化合物的合成路线如下所示:
在本发明的一种实施方式中,以糖砌块3为糖基供体,二糖化合物17为糖基受体,在有机溶剂中偶联得到三糖化合物18;然后以三糖化合物18为糖基供体,五糖化合物19为糖基受体,在有机溶剂中偶联得到八糖化合物20。
在本发明的一种实施方式中,所述八糖化合物20的制备方法具体包括:
按照摩尔比(1-2):1,将糖砌块3与二糖化合物17溶于有机溶剂中,加入分子筛和路易斯酸,-10℃反应2-4小时,得到三糖供体18;然后与1-2倍摩尔当量的五糖化合物19偶联得到八糖化合物20。
在本发明的一种实施方式中,所述方法还包括合成十三糖化合物,所述十三糖化合物的合成路线如下所示:
在本发明的一种实施方式中,所述十三糖化合物的合成方法包括:
(1)以糖砌块6为糖基供体,糖砌块21为糖基受体,在有机溶剂中偶联得到二糖供体22;然后与糖基受体23偶联得到三糖片段24;
(2)三糖片段2作为,与糖基受体25在路易斯酸催化,在-10℃搅拌下偶联得到十一糖片段26,选择性脱去保护剂pg21和pg24,得到糖基受体27;再以糖砌块7为糖基供体,偶联反应制备得到十三糖化合物28。
在本发明的一种实施方式中,所述十三糖化合物28的制备方法具体包括:
(1)以1.5摩尔的糖砌块6为糖基供体,以1摩尔的糖砌块21为糖基受体,将糖基供体与糖基受体溶于干燥的二氯甲烷中,加入酸洗的
(2)然后再使等摩尔比的糖基供体24与糖基受体25在路易斯酸催化,在-10℃搅拌下偶联得到十一糖片段26,选择性脱去两个临时保护剂pg21和pg24,得到糖基受体27;以4摩尔的糖砌块7为糖基供体,以1摩尔的十一糖27为糖基受体,将糖基供体与糖基受体溶于干燥的二氯甲烷/乙醚(1:1)中,加入酸洗的
本发明的第二个目的是利用上述方法合成得到一种组装有连接臂的幽门螺旋杆菌o:6血清型o-抗原寡类糖化合物,所述化合物的结构如式i所示:
其中,x为1、2或3;y为1、2或3;z为1、2或3;n1,n2,n3,n4,n5为0~5之间的整数,其中n1,n2,n3不同时为零;n;n6,n7为0或1;
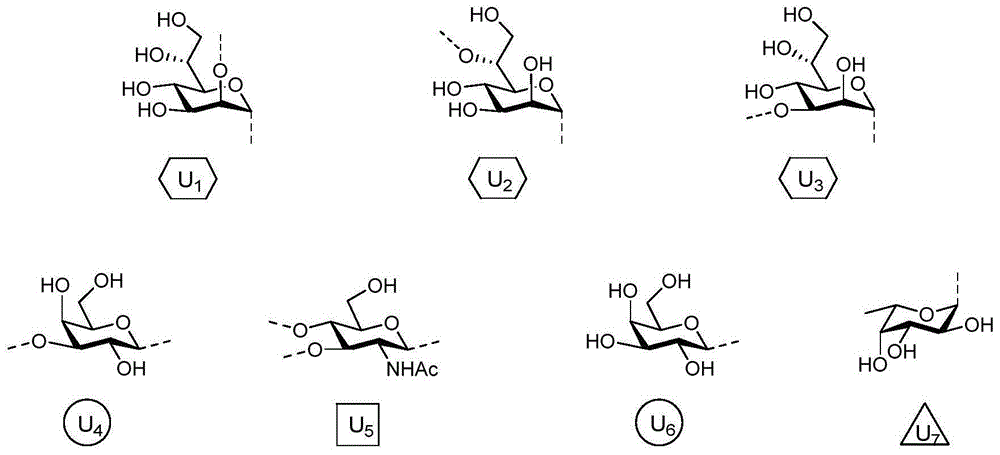
其中,u1、u2、u3、u4、u5、u6、u7的结构式如下所示:
连接臂linker包括氨基连接臂[-(ch2)n-n-y1y2];n代表连接臂可以为不同的碳链长度,n=2~40;y1和y2为氨基的保护基,其中,y1为h或者为卞基(bn),y2为h或者卞甲氧羰基(cbz)。
在本发明的一种实施方式,连接臂linker可以全部或部分氟取代。
在本发明的一种实施方式,连接臂linker可以包含一个三、四、五或六元饱和碳环;也可以包含一个五元不饱和碳环(非芳香环);也可以包含四、五或六元饱和氧杂环;也可以包含一个四、五或六元饱和氮杂环;也可以包含一个六元芳香碳环。
在本发明的一种实施方式,连接臂linker也可以包含酰胺键和/或脲基。
在本发明的一种实施方式,连接臂linker可以含有一个或多个取代基团,这些取代基可以包括:-f,-cl,-ch3,-c2h5,-c3h7,-c5h9,-c6h13,-och3,-oc2h5,-ch2f,-chf2,-cf3,-c(o)-nh2,-sch3,-sc2h5,-nhc(o)ch3,-n(ch3)2和-n(c2h5)2。
本发明的第三个目的是提供一种糖-蛋白缀合物的制备方法,所述方法是利用上述的组装有连接臂的幽门螺旋杆菌o:6血清型o-抗原寡类糖化合物。
本发明的第四个目的是将上述的组装有连接臂的幽门螺旋杆菌o:6血清型o-抗原寡类糖化合物应用于在开发或制备幽门螺旋杆菌疫苗或者治疗幽门螺旋杆菌感染导致的疾病的药物中。
本发明的有益效果:
本发明方法步骤简单、省时、省力且成本低廉。本发明是通过化学合成得到幽门螺旋杆菌o:6血清型o-抗原二糖,三糖、五糖、八塘、十三糖。本发明通过保护剂策略,温度效应,溶剂效应和添加剂效应,发现了一条选择性构建各糖砌块连接的合成路线方法,并且将此方法应用在幽门螺旋杆菌o:6血清型o-抗原二糖,三糖、五糖、八塘、十三糖的合成之中。合成得到的幽门螺旋杆菌o:6血清型o-抗原糖链片段的还原端均组装有氨基连接臂,可以与载体蛋白制成糖缀合物,用于免疫学研究,对发展预防和治疗幽门螺旋杆菌具有重要作用。
附图说明
图1:通式i中u1,u2,u3,u4,u5,u6,u7所示化合物;
图2:单糖砌块1,2,3,4,5,6和7所示化合物;
图3:糖砌块6*和8*的合成;
图4:糖砌块11*和13*的合成;
图5:还原端三糖的合成;
图6:重复二糖和三糖的合成;
图7:还原端五糖和八糖的合成;
图8:幽门螺旋杆菌o:6血清型o-抗原十三糖的合成。
具体实施方式
下面将结合实施例对本发明的实施方案进行详细描述,但是本领域技术人员将会理解,下列实施例仅用于说明本发明,而不应视为限定本发明的范围。实施例中未注明具体条件者,按照常规条件或制造商建议的条件进行。所用试剂或仪器未注明生产厂商者,均为可以通过市售购得的常规产品。
所有试剂除特殊说明外均为分析纯,且除特殊说明外未经进一步纯化。所有溶剂使用之前采用通用方法干燥和再蒸馏。所有的反应除另注明,都是利用磁力搅拌在烘干的玻璃器皿中惰性气体的保护下进行。薄层分析(tlc)所用硅胶薄板型号gf254,青岛海洋化工有限公司生产;tlc板通过紫外光(uv)和hanessian溶液(硫酸铈和钼酸铵溶于硫酸溶液中)或5%的硫酸-乙醇溶液染色,可直观的进行检测。柱层析硅胶为青岛海洋化工公司生产,柱层析硅胶(300-400目)。1hnmr,13cnmr,1h-13chsqc和1h-1hcosy谱由nvanceiii400-mhz,600-mhz和700-mhz核磁共振仪测量,除特别指明外,均为cdcl3作溶剂,tms作内标,环境温度下测定。峰型的表示方法:单峰(s),宽的单峰(brs),双峰(d),四重峰(dd),三重锋(t),多重峰(m)。所有nmr的化学位移(δ)单位记为ppm,耦合常数(j)单位记为hz。质谱通过thermoscientifictsqquantumultra仪器测得,高分辨率质谱通过ionspecultra仪器测得。
实施例1糖砌块8*的合成:
合成路线如图3所示。
以2,3-o-丙叉基-4-o-卞基甘露乙硫糖苷为起始原料,经过swern氧化,6-位羟基被氧化成醛得到化合物1*。然后利用wittig反应延长6位的碳链,得到6位脱氧的烯烃化合物2*。烯烃化合物在锇酸钾(k2oso4)、铁氰酸钾(k3fe(cn)6)和碳酸钾(k2co3)的共同作用下进行双羟基化,得到6,7-二-羟基化合物3*。在氢化钠(nah)的作用下对6,7-二-羟基进行bn保护得到化合物4*。在80%的醋酸作用下脱去丙叉基后得到化合物5*,然后在d(+)-10-樟脑磺酸(csa)的作用下,对2,3-位羟基进行成环保护,在弱酸条件下开环,得到2-obz保护的化合物6*。对2-oh用lev保护后得到化合物7*,然后利用n-碘代丁二酰亚胺(nis)和三氟甲磺酸(tfoh)对端基位乙硫基进行水解,最后与三氯乙腈反应得到三氯乙酰亚胺酯糖基供体8*。
具体试验操作和步骤:
化合物2*:将草酰氯(3.6ml,42.3mmol)溶于二氯甲烷(22ml)中,在-78℃条件下,将溶有dmso(6.0ml,84.6mmol)的二氯甲烷溶液逐滴加入,搅拌15min之后,利用恒压滴液漏斗将溶有化合物2,3-o-丙叉基-4-o-卞基甘露乙硫糖苷(10.0g,28.2mmol)的二氯甲烷(115ml)溶液加入上述反应液中,在-78℃条件下反应1h之后,将et3n(15.7ml,112.8mmol)加入到上述溶液,反应温度升至室温,然后在室温下反应4h。tlc显示反应完成之后,加水将反应淬灭,反应液用二氯甲烷萃取,有机相依次用水和饱和食盐水洗涤,然后用无水na2so4干燥,浓缩有机相,真空干燥得到粗品醛,未经纯化直接用于下一步反应。在0℃条件下,将甲基三苯基溴化磷(24.2g,67.7mmol)溶于thf(90ml)中,然后加入n-buli(23.5ml,56.4mmol,2.5minhexane),反应搅拌1h,后将反应温度降到-78℃,将溶有上述粗醛产物的thf(28ml)逐滴加入,反应温度升至室温,继续反应12h。tlc检测反应完全之后,加入饱和的nh4cl淬灭反应,乙酸乙酯萃取(5×100ml),无水na2so4干燥,浓缩有机相后利用柱层析进行纯化(石油醚/乙酸乙酯:100/1→50/1)得到化合物2*(5.5g,56%)。rf=0.32,petroleumether/etoac=15:1.[α]25d=+129.3(c1.0,ch3cl).1hnmr(400mhz,chloroform-d)δ7.40–7.23(m,5h,arom.h),5.99(ddd,j=16.7,10.6,5.5hz,1h,6-h),5.58(s,1h,1-h),5.41(dt,j=17.3,1.7hz,1h,7-h),5.25(dt,j=10.7,1.6hz,1h,7-h’),4.85(d,j=11.5hz,1h,ph-ch2),4.63(d,j=11.5hz,1h,ph-ch2),4.42(dd,j=10.0,5.5hz,1h,5-h),4.29(dd,j=7.2,5.7hz,1h,3-h),4.19(d,j=5.6hz,1h,2-h),3.38(dd,j=10.0,7.2hz,1h,4-h),2.58(ddq,j=52.5,13.1,7.4hz,2h,sch2),1.49(s,3h,ch3),1.36(s,3h,ch3),1.28(t,j=7.4hz,3h,ch3).13cnmr(101mhz,chloroform-d)δ138.2,135.0,128.2,128.0,127.6,117.3,109.4,80.1,79.6,78.5,76.7,73.2,69.5,28.0,26.4,24.4,14.6.ir(film):ν=2985,2931,1454,1380,1242,1219,1162,1124,1090,1066,996,872,748,697cm-1.hrms(esi)m/zcalcdforc19h26o4sna[m+na]+373.1449,found373.1445.
化合物3*:将铁氰化钾(k3fe(cn)6,46.2mmol,15.2g),二水合锇酸钾(k2oso4·2h2o,0.385mmol,142mg)和碳酸钾(k2co3,50.8mmol,7.0g)加入到叔丁醇(77ml)和水(77ml)溶液中,然后在0℃下,将溶有化合物2*(5.4g,15.4mmol)的甲苯(30ml)溶液逐滴加入到反应溶液中,反应混合液在0℃下反应36h。tlc检测反应完全之后,加入亚硫酸钠(na2so3,25g)淬灭反应,搅拌15min之后,乙酸乙酯萃取,有机相用1mkoh洗涤,无水na2so4干燥,过滤浓缩。柱层析分离纯化(石油醚/乙酸乙酯:5/1→4/1)得到化合物3*(4.3g,73%)。rf=0.36,petroleumether/etoac=1:1.[α]25d=+173.6(c1.0,ch3cl).1hnmr(400mhz,chloroform-d)δ7.41–7.28(m,5h,arom.h),5.55(s,1h,1-h),4.98(d,j=11.3hz,1h,ph-ch2),4.64(d,j=11.3hz,1h,ph-ch2),4.32(dd,j=6.9,5.7hz,1h,3-h),4.20(dd,j=5.7,0.7hz,1h,2-h),4.06(dd,j=9.9,6.3hz,1h,5-h),3.89(ddt,j=6.8,4.8,2.2hz,1h,6-h),3.69(dd,j=10.0,7.0hz,1h,4-h),3.66–3.63(m,2h,7-h/7-h’),3.54(d,j=2.8hz,1h,oh),2.63(ddq,j=51.1,12.9,7.4hz,2h,sch2),1.54(s,3h,ch3),1.37(s,3h,ch3),1.29(t,j=7.4hz,3h,ch3).13cnmr(101mhz,chloroform-d)δ137.2,109.6,79.7,79.5,78.3,76.5,73.2,72.8,67.7,62.8,28.1,26.4,24.1,14.2.ir(film):ν=3446,2984,2931,1454,1380,1242,1219,1162,1066,872,750,699cm-1.hrms(esi)m/zcalcdforc19h28o6sna[m+na]+407.1504,found407.1507.
化合物4*:将化合物3*(1.7g,4.4mmol)溶于dmf(22ml))中,加入氢化钠(0.7g,17.7mmol)(60%分散在矿物油中),将反应温度降至0℃,然后加入bnbr(2.1ml,17.7mmol),反应在室温下搅拌3h,tlc检测反应完全之后,加入适量的甲醇淬灭,二氯甲烷萃取,有机相依次用水、饱和食盐水洗涤,无水na2so4干燥之后减压浓缩。粗品利用柱层析分离纯化(石油醚/乙酸乙酯:100/1→50/1)得到化合物4*(2.2g,89%)。rf=0.27,petroleumether/etoac=20:1.[α]25d=+117.7(c1.0,ch3cl).1hnmr(400mhz,chloroform-d)δ7.40–7.17(m,15h,arom.h),5.54(s,1h,1-h),4.83(d,j=11.4hz,1h,)ph-ch2,4.72(d,j=11.8hz,1h,ph-ch2),4.67(d,j=11.8hz,1h,ph-ch2),4.53(d,j=11.4hz,1h,ph-ch2),4.49(d,j=12.0hz,1h,ph-ch2),4.43(d,j=12.0hz,1h,ph-ch2),4.32–4.24(m,2h,3-h/5-h),4.15(d,j=5.7hz,1h,2-h),4.04(td,j=5.7,1.5hz,1h,6-h),3.68(d,j=5.7hz,2h,7-h/7-h’),3.68(dd,j=10.0,7.0hz,1h,4-h),2.59(ddq,j=67.5,12.8,7.4hz,2h,sch2),1.46(s,3h,ch3),1.35(s,3h,ch3),1.23(t,j=7.4hz,3h,ch3).13cnmr(101mhz,chloroform-d)δ138.7,138.5,138.2,128.3,128.2,128.2,128.0,127.7,127.5,127.5,127.4,127.3,109.3,79.5,79.0,77.9,76.4,76.3,73.2,72.7,72.5,70.5,69.3,28.0,26.5,23.8,14.2.ir(film):ν=2929,1453,1380,1218,1093,1066,1027,870,734,696cm-1.hrms(esi)m/zcalcdforc33h40o6sna[m+na]+587.2443,found587.2429.
化合物5*:将化合物4*(2.2g,3.8mmol)溶于80%醋酸溶液(40ml)中,反应混合物在60℃下反应5h,tlc检测反应完全之后,旋转蒸发浓缩反应液,加入适量dcm溶解,然后依次用饱和nahco3和饱和食盐水洗涤,无水na2so4干燥,过滤浓缩,柱层析分离纯化(石油醚/乙酸乙酯:4/1)得到化合物5*(2g,quan.)。rf=0.33,petroleumether/etoac=2:1.[α]25d=+119.6(c1.0,ch3cl).1hnmr(400mhz,chloroform-d)δ7.45–7.14(m,15h,arom.h),5.25(d,j=1.8hz,1h,1-h),4.74(d,j=11.9hz,1h,ph-ch2),4.69(d,j=11.8hz,1h,ph-ch2),4.68(d,j=11.7hz,1h,ph-ch2),4.63(d,j=11.5hz,1h,ph-ch2),4.53(d,j=12.0hz,1h,ph-ch2),4.48(d,j=11.9hz,1h,ph-ch2),4.28(dd,j=9.5,1.6hz,1h,5-h),4.00(ddd,j=6.7,5.1,1.6hz,1h,6-h),3.93(ddd,j=7.4,3.9,2.2hz,1h,2-h),3.89(dq,j=5.7,3.4,2.8hz,1h,3-h),3.81(d,j=5.2hz,0h),3.79(d,j=10.0,5.2hz,1h,7-h),3.77(dd,j=10.0,7.0hz,1h,4-h),3.70(dd,j=10.2,6.7hz,1h,7-h’),2.71–2.50(m,2h,sch2),2.47(d,j=4.6hz,1h,2-oh),2.28(d,j=5.7hz,1h,3-oh),1.25(t,j=7.4hz,3h,ch3).13cnmr(101mhz,chloroform-d)δ138.6,138.3,138.2,128.6,128.4,128.3,127.9,127.9,127.8,127.7,127.6,127.5,83.4,77.8,76.4,74.2,73.4,72.6,72.5,72.2,71.7,70.6,24.7,14.7.ir(film):ν=3420,2924,1453,1075,1027,792,733,696cm-1.hrms(esi)m/zcalcdforc30h36o6sna[m+na]+547.2130,found547.2118.
化合物6*:将化合物5*(1.06g,2.0mmol)溶于无水的dcm(20ml)中,加入原苯甲酸三乙酯(0.7ml,3.0mmol)和csa(23mg,0.1mmol),在室温下搅拌反应1h,tlc检测原料完全转化为中间体后,加入水(70μl,~4.0mmol),在室温下搅拌反应1h,tlc检测反应完全后,加入适量dcm稀释,用饱和的nahco3洗涤,水层用dcm萃取一次,合并有机相并用饱和的食盐水洗,无水na2so4干燥,过滤浓缩后柱层析分离纯化(石油醚/乙酸乙酯:15/1→10/1)得到化合物6*(1.0g,80%)。rf=0.43,petroleumether/etoac=4:1.[α]25d=+47.5(c1.0,ch3cl).1hnmr(400mhz,chloroform-d)δ8.31–7.12(m,20h,arom.h),5.42(dd,j=3.3,1.7hz,1h,2-h),5.39(d,j=1.6hz,1h,1-h),4.82(t,j=11.4hz,2h,ph-ch2),4.71(dd,j=12.8,11.5hz,2h,,ph-ch2),4.53(s,2h,ph-ch2),4.38–4.32(m,1h,5-h),4.20(ddd,j=8.9,5.4,3.3hz,1h,3-h),4.04(t,j=9.4hz,1h,4-h),4.04(m,1h,6-h),3.83(dd,j=10.3,5.0hz,1h,7-h),3.74(dd,j=10.3,6.8hz,1h,7-h’),2.76–2.54(m,2h,sch2),2.08(d,j=5.4hz,1h,3-oh),1.27(t,j=7.4hz,3h,ch3).13cnmr(101mhz,chloroform-d)δ166.1,138.8,138.4,138.2,133.3,129.8,129.7,128.5,128.3,128.3,128.0,127.8,127.5,127.5,127.4,82.1,78.8,76.4,74.8,74.7,73.3,72.7,72.2,71.8,71.1,25.4,14.9.ir(film):ν=3435,3030,2870,1719,1452,1267,1089,1026,902,735,711,697cm-1.hrms(esi)m/zcalcdforc37h40o7sna[m+na]+651.2392,found651.2383.
化合物7*:将化合物6*(1.6g,2.5mmol)溶于无水dcm(20ml)中,然后依次加入乙酰丙酸(0.4ml,3.8mmol),n,n-二环己基二酰亚胺(0.79g,3.8mmol)和4-二甲氨基吡啶(0.47g,3.8mmol),反应在室温下搅拌1h。tlc检测反应完全后,加入适量dcm稀释,有机层依次用饱和nahco3和饱和食盐水洗,无水na2so4干燥,过滤浓缩后,柱层析分离纯化(石油醚/乙酸乙酯:10/1→8/1)得到化合物7*(1.5g,82%)。rf=0.43,petroleumether/etoac=3:1.[α]25d=+27.9(c1.0,ch3cl).1hnmr(400mhz,chloroform-d)δ8.15–7.10(m,20h,arom.h),5.58(dd,j=3.2,1.7hz,1h,2-h),5.41(dd,j=9.6,3.2hz,1h,3-h),5.37(d,j=1.6hz,1h,1-h),4.87(d,j=12.0hz,1h,ph-ch2),4.75(d,j=11.9hz,1h,ph-ch2),4.68(d,j=11.1hz,1h,ph-ch2),4.62(d,j=11.1hz,1h,ph-ch2),4.51(s,2h,ph-ch2),4.45(d,j=9.9hz,1h,5-h),4.24(t,j=9.7hz,1h,4-h),4.04(ddd,j=6.4,5.0,1.2hz,1h,6-h),3.80(dd,j=10.3,5.0hz,1h,7-h),3.72(dd,j=10.3,6.8hz,1h,7-h’),2.77–2.53(m,4h,sch2/ch2),2.51–2.33(m,2h,ch2),2.07(s,3h,ac),1.27(t,j=7.4hz,3h,sch2ch3).13cnmr(101mhz,chloroform-d)δ206.1,171.7,165.4,138.7,138.3,138.0,133.4,129.8,129.6,128.5,128.3,128.3,127.8,127.6,127.5,127.5,127.4,127.4,82.0,78.9,74.6,73.5,73.4,73.3,72.8,72.4,72.1,71.1,37.8,29.7,27.9,25.2,14.8.ir(film):ν=2928,1719,1452,1265,1151,1089,1026,736,711,697cm-1.hrms(esi)m/zcalcdforc42h46o9sna[m+na]+749.2760,found749.2763.
化合物8*:将化合物7*(1.34g,1.84mmol)溶于ch2cl2(18ml)中,然后加入水(0.33ml,18.4mmol)搅拌,在0℃条件下加入nis(0.62g,2.76mmol)和tfoh(36μl,0.41mmol),然后在0℃下搅拌1.5h,tlc检测反应完全后加入et3n终止反应,加适量dcm稀释,后用10%的na2s2o3和饱和食盐水洗涤,无水na2so4干燥,过滤浓缩后柱层析分离纯化(石油醚/乙酸乙酯:3/1→2/1)得到相应的半缩醛(1.33g,quan.)。rf=0.36,petroleumether/etoac=1:1.
将上面得到的半缩醛(238mg,0.35mmol)溶于ch2cl2(4ml)中,在0℃下加入ccl3cn(107μl,1.07mmol)和dbu(7μl,0.046mmol),反应在室温下搅拌45min。tlc检测反应完全之后,在30℃下浓缩反应液,后经硅胶柱层析分离纯化(petroleumether/etoac:6/1→4/1)得到化合物8*(266mg,92%)。rf=0.33,petroleumether/etoac=3:1.1hnmr(400mhz,chloroform-d)δ8.72(s,1h,arom.h),8.13–7.93(m,2h,arom.h),7.71–7.53(m,1h,arom.h),7.44–7.14(m,16h,arom.h),6.40(d,j=2.1hz,1h,1-h),5.70(dd,j=3.3,2.1hz,1h,2-h),5.54(dd,j=9.4,3.3hz,1h,3-h),4.89(d,j=11.9hz,1h,ph-ch2),4.76(d,j=11.9hz,1h,ph-ch2),4.72(d,j=10.9hz,1h,ph-ch2),4.64(d,j=10.9hz,1h,ph-ch2),4.47(d,j=1.8hz,2h,ph-ch2),4.37(t,j=9.7hz,1h,4-h),4.30(d,j=10.0hz,1h,5-h),4.10(t,j=6.4hz,1h,6-h),3.76(dd,j=10.2,5.7hz,1h,7-h),3.71(dd,j=10.1,6.8hz,1h,7’-h),2.74(dt,j=18.5,7.2hz,1h,ch2),2.62(dt,j=18.5,6.4hz,1h,ch2),2.54–2.36(m,2h,ch2),2.09(s,3h,ch3co).13cnmr(101mhz,chloroform-d)δ206.0,171.8,165.2,160.1,138.7,138.2,137.7,133.6,129.8,129.2,128.6,128.3,128.3,128.0,127.7,127.6,127.5,127.4,127.4,94.9,90.7,78.8,74.9,74.7,73.3,73.1,72.6,72.6,70.8,68.7,37.8,29.7,27.9.
实施例2糖砌块13*的合成:
合成路线如图4所示。
如图2,以化合物3*为起始原料,利用二丁基氧化锡(bu2sno)选择性的7-oh进行bn保护得到化合物9*,然后6-oh用lev保护得到化合物10*。在80%的醋酸作用下脱去化合物10*的丙叉基后,然后对2,3-oh进行乙酰基保护得到糖砌块11*。
糖砌块13*的合成,首先利用先前制备的中间体化合物3,4位起始原料,在二丁基氧化锡(bu2sno)的作用下,选择性对化合物5*的3-oh进行bn保护得到化合物12*,最后对2-oh进行乙酰基保护得到庚糖砌块13*。
具体试验操作和步骤:
化合物9*:将化合物3*(0.77g,2mmol)和bu2sno(0.75g,3mmol)溶于干燥的甲苯(10ml)中,反应回流4h,在此过程中,用dean-stark装置除去甲苯-水共沸混合物(~5ml),然后将反应体系降至室温,浓缩并利用真空干燥。将上述残余物溶于ch3cn(5ml)中,然后加入csf(456mg,3mmol)和bnbr(360μl,3mmol),反应在70℃下搅拌10h,tlc检测反应完全后,用硅藻土过滤反应混合物并浓缩,粗品利用硅胶柱层析分离纯化(石油醚/乙酸乙酯:8/1)得到化合物9*(0.63g,66%)。rf=0.56,petroleumether/etoac=3:1.[α]25d=+124.6(c1.0,ch3cl).1hnmr(400mhz,chloroform-d)δ7.48–7.21(m,10h,arom.h),5.52(s,1h,1-h),4.92(d,j=11.4hz,1h,ph-ch2),4.59(d,j=11.3hz,1h,ph-ch2),4.53(d,j=12.0hz,1h,ph-ch2),4.49(d,j=12.0hz,1h,ph-ch2),4.34–4.27(m,1h,3-h),4.18(dd,j=5.7,0.8hz,1h,2-h),4.13–4.04(m,2h,6-h/5-h),3.69(dd,j=9.4,6.9hz,1h,4-h),3.58(dd,j=10.3,6.5hz,1h,7-h),3.54(dd,j=10.4,3.5hz,1h,7-h’),3.00(d,j=2.7hz,1h,6-oh),2.57(ddq,j=57.7,12.8,7.4hz,2h,sch2),1.52(s,3h,ch3),1.36(s,3h,ch3),1.24(t,j=7.4hz,3h,ch3).13cnmr(101mhz,chloroform-d)δ138.2,137.7,128.4,128.3,128.2,127.8,127.7,127.6,109.5,79.6,78.6,77.9,76.5,73.5,72.8,72.3,70.9,68.5,28.0,26.4,24.1,14.2.ir(film):ν=3482,2984,2930,1454,1380,1241,1219,1162,1068,1027,870,736,698cm-1.hrms(esi)m/zcalcdforc26h34o6sna[m+na]+497.1974,found497.1969.
化合物10*:将化合物9*(567mg,1.2mmol)溶于干燥的ch2cl2(23ml),然后加入levoh(185μl,1.8mmol),dcc(370mg,1.8mmol)和dmap(220mg,1.86mmol),反应在室温下搅拌1h。tlc检测反应完全后加入适量dcm稀释,反应液用饱和的nahco3和饱和的食盐水洗涤,无水na2so4干燥,过滤浓缩,硅胶柱层析纯化(石油醚/乙酸乙酯:8/1→4/1)得到化合物10*(707mg,quan.)。rf=0.32,petroleumether/etoac=4:1.[α]25d=+105.8(c1.0,ch3cl).1hnmr(400mhz,chloroform-d)δ7.45–7.16(m,10h,arom.h),5.57–5.52(m,1h,6-h),5.52(s,1h,1-h),4.87(d,j=11.6hz,1h,ph-ch2),4.55(d,j=11.6hz,1h,ph-ch2),4.47(d,j=12.0hz,1h,ph-ch2),4.43(d,j=12.0hz,1h,ph-ch2),4.26(t,j=6.3hz,1h,3-h),4.19(dd,j=10.3,2.2hz,1h,5-h),4.13(dd,j=5.7,0.7hz,1h,2-h),3.68–3.58(m,3h,4-h/7-h/7-h’),2.75–2.63(m,3h,ch2),2.62–2.44(m,3h,sch2/ch2),2.16(s,3h,oac),1.46(s,3h,ch3),1.34(s,3h,ch3),1.28(t,j=7.4hz,3h,ch3).13cnmr(101mhz,chloroform-d)δ206.4,171.8,138.1,138.1,128.3,128.0,127.6,127.5,127.5,109.4,79.5,78.8,76.4,76.2,73.0,72.5,71.6,68.9,68.0,37.9,29.9,28.0,28.0,26.4,23.9,14.4.ir(film):ν=2984,2931,1739,1719,1361,1218,1159,1096,1066,871,748,698cm-1.hrms(esi)m/zcalcdforc31h40o8sna[m+na]+595.2342,found595.2331.
化合物11*:将化合物10*(652mg,1.14mmol)溶于80%醋酸溶液(11ml)中,反应混合物在60℃下反应5h,tlc检测反应完全之后,旋转蒸发浓缩反应液,加入适量dcm溶解,然后依次用饱和nahco3和饱和食盐水洗涤,无水na2so4干燥,过滤浓缩,真空干燥。将上述残余物溶于吡啶(4ml)中,然后加入ac2o(1.1ml,11.4mmol)和dmap(cat.),反应在室温下搅拌3h,tlc检测反应完全后,蒸干反应混合物,加入适量dcm稀释,并分别用1mhcl(aq)、饱和nahco3和饱和食盐水洗涤,无水na2so4干燥,浓缩后利用硅胶柱层析分离纯化(石油醚/乙酸乙酯:10/1→6/1)得到化合物11*(605mg,86%)。rf=0.35,petroleumether/etoac=3:1.[α]25d=+79.9(c1.0,ch3cl).1hnmr(400mhz,chloroform-d)δ7.37–7.20(m,10h,arom.h),5.51(td,j=6.2,2.1hz,1h,6-h),5.30(dd,j=3.3,1.8hz,1h,2-h),5.25(dd,j=9.3,3.3hz,1h,3-h),5.21(d,j=1.7hz,1h,1-h),4.64(s,2h,ph-ch2),4.51(d,j=2.6hz,2h,ph-ch2),4.33(dd,j=9.9,2.1hz,1h,5-h),4.01(t,j=9.6hz,1h,4-h),3.77(dd,j=10.3,5.8hz,1h,7-h),3.62(dd,j=10.2,6.5hz,1h,7-h’),2.74(t,j=6.7hz,2h,ch2),2.69–2.52(m,4h,sch2/ch2),2.17(s,3h,oac),2.11(s,3h,oac),1.92(s,3h,oac),1.27(t,j=7.4hz,3h,ch3).13cnmr(101mhz,chloroform-d)δ206.3,171.7,169.9,169.6,138.0,137.9,128.4,128.4,127.7,127.6,127.6,127.5,81.8,74.3,73.8,73.2,72.3,71.6,71.5,68.3,37.9,29.8,28.0,25.1,20.9,20.8,14.8.ir(film):ν=2922,1744,1719,1365,1236,1157,1093,914,734,698cm-1.hrms(esi)m/zcalcdforc32h40o10sna[m+na]+639.2240,found639.2229.
化合物12*:将化合物5*(600mg,1.14mmol)和bu2sno(426mg,1.71mmol)溶于干燥的甲苯(5.7ml)中,反应回流4h,在此过程中,用dean-stark装置除去甲苯-水共沸混合物(~3ml),然后将反应体系降至室温,浓缩并利用真空干燥。将上述残余物溶于ch3cn(3ml)中,然后加入csf(260mg,1.71mmol)和bnbr(200μl,1.71mmol),反应在70℃下搅拌10h,tlc检测反应完全后,用硅藻土过滤反应混合物并浓缩,粗品利用硅胶柱层析分离纯化(石油醚/乙酸乙酯:6/1)得到化合物12*(540mg,77%)。rf=0.38,petroleumether/etoac=3:1.[α]25d=+95.5(c1.0,ch3cl).1hnmr(400mhz,chloroform-d)δ7.43–7.12(m,20h,arom.h),5.33(d,j=1.5hz,1h,1-h),4.79(d,j=10.9hz,1h,ph-ch2),4.75(d,j=12.0hz,1h,ph-ch2),4.68(d,j=12.0hz,1h,ph-ch2),4.65–4.63(m,2h,ph-ch2),4.59(d,j=10.9hz,1h,ph-ch2),4.50(d,j=12.0hz,1h,ph-ch2),4.45(d,j=12.1hz,1h,ph-ch2),4.30(dd,j=9.5,1.3hz,1h,5-h),4.05(dt,j=3.4,1.7hz,1h,2-h),3.99(ddd,j=6.4,4.7,1.5hz,1h,6-h),3.90(t,j=9.2hz,1h,4-h),3.85(dd,j=8.8,3.1hz,1h,3-h),3.76(dd,j=10.4,4.6hz,1h,7-h),3.69(dd,j=10.5,6.9hz,1h,7-h’),2.72–2.47(m,2h,sch2),2.58(d,j=2.2hz,1h,2-oh),1.24(t,j=7.4hz,3h,ch3).13cnmr(101mhz,chloroform-d)δ138.7,138.4,138.3,137.6,128.6,128.3,128.3,128.2,128.1,128.0,127.8,127.7,127.6,127.6,127.4,127.3,83.0,80.9,78.1,74.7,74.6,73.2,72.3,72.1,71.9,70.8,69.6,29.7,24.6,14.7.ir(film):ν=2917,2849,1453,1088,1027,790,733,696cm-1.hrms(esi)m/zcalcdforc37h42o6sna[m+na]+637.2600,found637.2585.
化合物13*:将化合物12*(1.2g,2mmol)溶于吡啶(6ml)中,然后加入ac2o(1.1ml,11.4mmol)和dmap(cat.),反应在室温下搅拌3h,tlc检测反应完全后,蒸干反应混合物,加入适量dcm稀释,并分别用1mhcl(aq)、饱和nahco3和饱和食盐水洗涤,无水na2so4干燥,浓缩后利用硅胶柱层析分离纯化(石油醚/乙酸乙酯:10/1→5/1)得到化合物13*(1.2g,93%)。rf=0.36,petroleumether/etoac=3:1.[α]25d=+67.4(c1.0,ch3cl).1hnmr(400mhz,chloroform-d)δ7.48–7.11(m,20h,arom.h),5.39(dd,j=3.0,1.7hz,1h,2-h),5.25(d,j=1.7hz,1h,1-h),5.25(d,j=1.7hz,1h,1-h,ph-ch2),4.84(d,j=10.8hz,1h,ph-ch2),4.77(d,j=11.9hz,1h,ph-ch2),4.68(d,j=11.90hz,1h,ph-ch2),4.66(d,j=11.19hz,1h,ph-ch2),4.57(d,j=10.8hz,1h,ph-ch2),4.50(d,j=11.8hz,1h,ph-ch2),4.50(d,j=11.1hz,1h,ph-ch2),4.46(d,j=12.1hz,1h,ph-ch2),4.29(dd,j=9.3,1.2hz,1h,5-h),3.99(ddd,j=6.4,4.7,1.5hz,1h,6-h),3.97(t,j=9.3hz,1h,4-h),3.92(dd,j=9.1,3.0hz,1h,3-h),3.75(dd,j=10.4,4.6hz,1h,7-h),3.68(dd,j=10.4,6.9hz,1h,7-h’),2.72–2.50(m,2h,sch2),2.10(s,3h,oac),1.25(t,j=7.4hz,3h,ch3).13cnmr(101mhz,chloroform-d)δ170.2,138.8,138.8,138.4,137.6,128.4,128.3,128.2,128.2,127.8,127.8,127.5,127.5,127.4,82.1,79.0,78.6,74.8,74.6,73.3,72.4,72.3,71.8,71.1,70.4,25.3,21.1,14.8.ir(film):ν=3029,2869,1742,1453,1369,1230,1091,1027,734,696cm-1.hrms(esi)m/zcalcdforc39h44o7sna[m+na]+679.2705,found679.2694.
实施例3还原端三糖的合成:
还原端三糖的合成路线如图5所示。
3.1对图5所示的还原端三糖中糖基化反应条件进行优化(表1),确定较优的糖基化反应的条件如下:糖基供体和受体在甲苯中共蒸三次;加入无水的dcm,反应浓度为0.1m,活化的
表1糖基化反应条件优化
3.2脱去乙酰基条件为:将起始原料溶解在meoh/thf(v/v,1:1)中,反应浓度为0.05m,加入0.5当量meona(5minmeoh)。反应温度为室温,tlc检测反应结束后用amerliteir120(h+)树脂中和反应液ph达到7。过滤,浓缩后利用硅胶柱层析分离纯化。
具体试验操作和步骤:
化合物14*:根据反应条件3.1,糖基供体13*(980mg,1.49mmol)和糖基受体linker(1.04g,3.43mmol)反应得到14*(1.13g,85%)。rf=0.33,petroleumether/etoac=4:1.[α]25d=+14.8(c1.0,ch3cl).1hnmr(400mhz,chloroform-d)δ7.46–7.11(m,30h,arom.h),5.27(s,1h,2-h),5.17(s,2h,ph-ch2),4.84(d,j=10.7hz,1h,ph-ch2),4.78–4.59(m,3h,ph-ch2),4.68(s,1h,1-h),4.56(d,j=10.8hz,1h,ph-ch2),4.53–4.37(m,5h),3.98(t,j=5.8hz,1h,6-h),3.94–3.78(m,3h,3-h/4-h/-5-h),3.73(dd,j=10.4,4.7hz,1h,7-h),3.67(dd,j=10.4,6.7hz,1h,7-h’),3.74–3.56(m,1h,ch2),3.44–3.20(m,3h,ch2),2.09(s,3h,ch3co),1.89–1.64(m,2h,ch2).13cnmr(101mhz,chloroform-d)δ170.2,156.4(d,j=52.1hz),138.7,138.4(d,j=3.1hz),137.8,137.8,136.8,128.6,128.4,128.3,128.2,128.1,127.9,127.9,127.7,127.5,127.4,97.5,78.5,78.4,74.8,74.3,73.3,72.5,72.2,71.8,70.8,68.7,67.2,65.3(d,j=22.4hz),50.7(d,j=24.7hz),44.1(d,j=91.4hz),27.9(d,j=48.9hz),21.0.ir(film):ν=3030,2919,1744,1698,1453,1368,12321090,1027,734,696cm-1.hrms(esi)m/zcalcdforc55h59o10nna[m+na]+916.4037,found916.4020.
化合物15*:根据反应条件3.2,化合物14*(960mg,1.12mmol)脱去酯基得到化合物15*(908mg,quan.)。rf=0.34,petroleumether/etoac=2:1.[α]25d=+26.8(c1.0,ch3cl).1hnmr(400mhz,chloroform-d)δ7.46–7.08(m,30h,arom.h),5.23–5.11(m,2h,ph-ch2)4.79(d,j=10.8hz,1h,ph-ch2),4.75–4.60(m,3h,ph-ch2),4.66(s,1h,1-h),4.58(d,j=10.8hz,1h,ph-ch2),4.51–4.39(m,5h,ph-ch2),4.02–3.93(m,1h,6-h),3.89(s,1h,2-h),3.88–3.77(m,3h,3-h/4-h/5-h),3.74(dd,j=10.4,4.7hz,1h,7-h),3.68(dd,j=10.4,6.7hz,1h,7-h’),3.74–3.58(m,1h,ch2),3.45–3.11(m,3h,ch2),2.35(d,j=13.5hz,1h,2-oh),1.88–1.62(m,2h,ch2).13cnmr(101mhz,chloroform-d)δ156.4(d,j=47.1hz),138.7,138.4,138.4,137.9,137.8,136.8,128.6,128.5,128.5,128.3,128.3,128.2,127.9,127.9,127.8,127.7,127.6,127.4,127.4,99.0,80.7,78.0,74.7,74.3,73.2,72.5,72.0,71.9,70.6,68.2,67.2,65.0,50.7,44.2(d,j=86.6hz),27.9(d,j=40.6hz).ir(film):ν=3482,3030,2920,1698,1453,1217,1092,1053,1027,734,696cm-1.hrms(esi)m/zcalcdforc53h57o9nna[m+na]+874.3931,found874.3916.
化合物16*:根据反应条件3.1,糖基供体13*(472mg,0.72mmol)和糖基受体15*(908mg,1.06mmol)反应得到16*(793mg,76%)。rf=0.51,petroleumether/etoac=3:1.[α]25d=+11.7(c1.0,ch3cl).1hnmr(400mhz,chloroform-d)δ7.72–6.78(m,50h,arom.h),5.45(t,j=2.3hz,1h,2-h),5.14(d,j=11.4hz,2h,ph-ch2),4.89(s,1h,1’-h),4.88(s,1h,1-h),4.88–4.30(m,14h,ph-ch2),4.25(d,j=11.0hz,1h,ph-ch2),4.15–4.09(m,1h),3.99(dt,j=12.5,5.8hz,2h),3.94–3.80(m,3h),3.77(dd,j=10.3,4.9hz,2h),3.71(dd,j=5.8,2.6hz,2h),3.67(dd,j=10.4,6.5hz,1h,7-h),3.50–3.27(m,1h,ch2),3.27–3.05(m,2h,ch2),3.05–2.83(m,1h,ch2),2.07(s,3h,ch3co),1.72–1.43(m,2h,ch2).13cnmr(101mhz,chloroform-d)δ169.9,156.3,156.3,138.8,138.5,138.4,138.4,138.3,137.8,128.5,128.4,128.3,128.3,128.3,128.2,128.2,128.0,128.0,127.9,127.7,127.7,127.6,127.6,127.6,127.5,127.5,127.4,127.3,127.2,99.9(c-1),98.2(c-1’),79.9,78.7,78.5,77.6,76.3,74.9,74.5,74.4,73.3,73.1,72.5,72.4,72.3,72.2,72.0,71.7,71.2,70.3,68.6,67.1,64.8,60.4,50.6,44.0(d,j=83.2hz),27.9(d,j=38.4hz),21.0.ir(film):ν=3030,2922,1744,1699,1454,1368,1234,1095,1028,736,697cm-1.hrms(esi)m/zcalcdforc90h95o16nna[m+na]+1468.6549,found1468.6521.
化合物17*:根据反应条件3.2,化合物16*(753mg,0.52mmol)脱去乙酰基得到化合物17*(657mg,90%)。rf=0.32,petroleumether/etoac=3:1.[α]25d=+13.5(c1.0,ch3cl).1hnmr(400mhz,chloroform-d)δ7.50–6.97(m,36h,arom.h),5.14(d,j=11.3hz,2h),4.94(s,1h,1’-h),4.93(s,1h,1-h),4.87(d,j=10.7hz,1h),4.77(d,j=11.0hz,1h),4.68(s,1h),4.66–4.53(m,4h),4.50–4.34(m,7h),4.10(d,j=9.6hz,1h),4.06(s,1h),3.99(q,j=6.1hz,2h),3.92(d,j=9.5hz,1h),3.85–3.65(m,7h),3.35(d,j=37.4hz,1h),3.14(d,j=34.3hz,2h),2.93(d,j=42.3hz,1h),2.33(d,j=2.3hz,1h),1.79–1.44(m,2h).13cnmr(101mhz,chloroform-d)δ156.6,156.0,138.9,138.5,138.5,138.4,138.4,138.3,137.8,136.9,128.5,128.4,128.4,128.3,128.2,128.2,128.2,127.9,127.9,127.8,127.8,127.7,127.7,127.5,127.5,127.4,127.4,127.4,127.3,127.2,101.6,98.3,80.5,80.0,78.9,76.3,74.8,74.6,74.4,73.2,73.1,72.3,72.3,72.2,71.9,71.3,70.2,68.3,67.1,64.8,50.6,50.4,44.4,43.6,29.7,28.1,27.7.ir(film):ν=3030,2918,1698,1453,1216,1054,1027,734,696cm-1.hrms(esi)m/zcalcdforc88h93o15nna[m+na]+1426.6443,found1426.6480.
化合物18*:根据反应条件3.1,糖基供体11*(258mg,0.42mmol)和糖基受体17*(487mg,0.42mmol)反应得到18*(441mg,66%)。rf=0.37,toluene/etoac=9:1.[α]25d=+16.8(c1.0,ch3cl).1hnmr(400mhz,chloroform-d)δ7.48–6.99(m,60h,arom.h),5.61–5.54(m,1h),5.38(d,j=3.3hz,1h),5.36(s,1h),5.13(d,j=9.9hz,2h),5.11(s,1h,1”-h),4.93(s,1h,1’-h),4.82(t,j=10.8hz,3h),4.75(s,1h,1-h),4.72–4.57(m,7h),4.53–4.28(m,12h),4.10(dd,j=10.1,5.5hz,2h),3.98(q,j=6.9,4.7hz,3h),3.93–3.63(m,12h),3.54(dd,j=10.1,6.6hz,1h),3.44–3.21(m,1h),3.20–2.96(m,2h),2.98–2.71(m,1h),2.61(qd,j=10.9,6.6,5.4hz,1h),2.50(t,j=6.4hz,2h),2.37(m,1h),2.06(s,3h),1.98(s,3h),1.94(s,3h),1.64–1.47(m,2h).13cnmr(101mhz,chloroform-d)δ206.2,171.8,169.6,169.4,156.2,156.2,138.9,138.8,138.7,138.5,138.5,138.0,137.9,136.8,128.5,128.3,128.3,128.3,128.2,128.2,128.1,128.0,127.9,127.8,127.7,127.7,127.6,127.6,127.6,127.5,127.4,127.4,127.3,127.3,127.1,101.2,99.5,98.4,79.9,79.6,78.9,74.9,74.8,74.7,74.5,74.4,73.5,73.1,73.1,72.9,72.4,72.2,72.1,71.7,71.3,70.2,70.0,68.1,67.1,65.0,65.0,60.4,50.7,50.4,44.5,43.7,37.7,29.7,28.2,27.8,20.8,20.8,14.2.ir(film):ν=3030,2919,1749,1698,1453,1365,1238,1216,1070,1027,735,696cm-1.hrms(esi)m/zcalcdforc118h131o25n2[m+nh4]+1975.9035,found1975.9043.
化合物19*:将化合物18*(550mg,0.28mmol)溶于ch2cl2/meoh(20/1)(3.3ml)中,加入醋酸肼(40mg,0.42mmol),反应在室温下搅拌3h。tlc检测反应完全后,加入适量dcm稀释,然后反应混合物用饱和nahco3和食盐水洗,无水na2so4干燥,浓缩后硅胶柱层析分离纯化(石油醚/乙酸乙酯:6/1→4/1)得到化合物19*(494mg,95%)。rf=0.35,petroleumether/etoac=3:1.[α]25d=+25.2(c1.0,ch3cl).1hnmr(400mhz,chloroform-d)δ7.49–7.02(m,60h,arom.h),5.39(m,2h),5.15(s,2h,1c-h),5.10(s,1h),4.97(s,1h,1a-h),4.88(s,1h,1b-h),4.78(d,j=11.0hz,2h),4.74–4.26(m,19h),4.12(m,2h),4.05–3.67(m,15h),3.57(dd,j=9.9,4.4hz,1h),3.48(dd,j=9.8,7.4hz,1h),3.42–3.19(m,1h),3.16–3.01(m,2h),3.01–2.77(m,1h),2.66(s,1h),2.07(s,3h,oac),1.93(s,3h,oac),1.69–1.41(m,2h,ch2).13cnmr(101mhz,chloroform-d)δ169.8,169.6,156.8,156.2,139.1,139.0,138.9,138.7,138.7,138.6,138.6,138.0,128.7,128.6,128.6,128.5,128.4,128.4,128.3,128.2,128.1,128.0,127.9,127.8,127.8,127.6,127.5,127.4,127.4,127.3,101.3,99.3,98.4,80.2,79.7,79.2,76.7,75.5,75.1,75.0,74.8,74.6,73.7,73.6,73.4,73.3,73.3,73.1,72.5,72.4,72.3,71.6,70.9,70.6,70.2,67.3,65.1,28.0,21.1.ir(film):ν=3030,2920,2360,1750,1698,1453,1366,1239,1218,1072,1028,913,735,696cm-1.hrms(esi)m/zcalcdforc113h125o23n2[m+nh4]+1877.8668,found1877.8698.
实施例4重复二糖和三糖的合成:
合成路线如图6所示。
具体试验操作和步骤:
4.1此发明中如未说明,脱去lev基条件为:将起始原料溶解于ch2cl2/meoh(20/1,0.1m)中,加入醋酸肼(2eq),反应在室温下搅拌3h。tlc检测反应完全后,加入适量dcm稀释,然后反应混合物用饱和nahco3和食盐水洗,无水na2so4干燥,浓缩后硅胶柱层析分离纯化得到脱lev受体。
化合物20*:根据反应条件3.1,活化试剂只加tmsotf(0.12eq),糖基供体8*(674mg,0.817mmol)和糖基受体6*(428mg,0.68mmol)反应得到20*(670mg,75%)。rf=0.36,petroleumether/etoac=3:1.[α]25d=+18.3(c1.0,ch3cl).1hnmr(400mhz,chloroform-d)δ8.17–6.93(m,40h,arom.h),5.58–5.53(m,2h),5.40(d,j=1.7hz,2h,1-h/2-h),5.21(d,j=2.0hz,1h,1-h),4.94(d,j=12.1hz,1h),4.88(d,j=10.6hz,1h),4.80(d,j=12.1hz,1h),4.73(d,j=10.7hz,1h),4.69(d,j=12.3hz,1h),4.58(d,j=11.0hz,1h),4.55–4.45(m,3h),4.39–4.35(m,2h),4.30(dd,j=9.5,3.3hz,1h),4.27(d,j=7.2hz,1h),4.20(t,j=9.6hz,1h),4.06–3.94(m,3h),3.78(dd,j=10.2,5.0hz,1h),3.70(dd,j=10.2,6.7hz,1h),3.52(dd,j=10.5,7.8hz,1h),3.29(dd,j=10.5,3.6hz,1h),2.71–2.46(m,5h),2.43–2.27(m,2h,ch2),2.03(s,3h,ch3co),1.25(t,j=7.4hz,3h,ch3).13cnmr(101mhz,chloroform-d)δ206.1,171.6,165.7,165.2,139.3,138.6(d,j=2.7hz),138.3,138.0,137.8,133.3,129.9,129.7,129.7,129.4,128.6,128.5,128.3,128.3,128.2,128.2,127.5,127.4,127.4,127.4,127.3,127.3,127.1,99.7,82.0,79.7,79.4,78.5,75.3,75.0,74.2,74.0,73.6,73.5,73.3,72.8,72.6,72.5,72.5,71.8,70.9,70.3,37.8,29.7,28.0,25.5,14.9.ir(film):ν=3030,2870,1720,1452,1264,1148,1093,1026,736,712,697cm-1.hrms(esi)m/zcalcdforc77h84o16sn[m+nh4]+1310.5505,found1310.5540.
化合物21*:根据反应条件4.1,化合物20*(440mg,0.34mmol)脱去lev基得到化合物21*(405mg,quan.)。rf=0.45,petroleumether/etoac=3:1.[α]25d=+18.3(c1.0,ch3cl).1hnmr(400mhz,chloroform-d)δ8.20–6.99(m,40h,arom.h),5.56(dd,j=3.1,1.7hz,1h),5.36(d,j=1.6hz,1h),5.34(dd,j=3.2,1.8hz,1h),5.25(d,j=1.7hz,1h),4.92(d,j=12.1hz,1h),4.85–4.75(m,3h),4.70(dd,j=11.3,7.8hz,3h),4.60(d,j=11.4hz,1h),4.49(d,j=1.2hz,2h),4.46(d,j=12.2hz,1h),4.41–4.36(m,2h),4.32(dd,j=9.3,3.1hz,1h),4.22(t,j=9.6hz,1h),4.09(pd,j=6.6,6.0,3.5hz,1h),4.01(dq,j=5.5,2.6,1.9hz,4h),3.79(dd,j=10.2,5.1hz,1h),3.70(dd,j=10.2,6.7hz,1h),3.63(dd,j=10.4,7.5hz,1h),3.52(dd,j=10.3,4.3hz,1h),2.72–2.46(m,2h,ch2),1.91(d,j=5.0hz,1h,oh),1.23(t,j=7.4hz,3h,ch3).13cnmr(101mhz,chloroform-d)δ165.8,165.4,139.2,138.7,138.6,138.3,138.2,137.7,133.3,133.2,129.8,129.8,129.5,128.6,128.4,128.3,128.3,128.2,128.2,128.0,127.8,127.6,127.5,127.5,127.4,127.4,127.3,127.2,99.4,82.1,79.2,78.6,77.7,75.6,75.4,75.1,74.2,73.8,73.3,73.2,73.0,72.8,72.5,72.5,71.4,71.0,70.4,25.4,14.8.ir(film):ν=3050,2926,1720,1452,1265,1093,1070,1026,825,736,711,698cm-1.hrms(esi)m/zcalcdforc72h78o14sn[m+nh4]+1212.5138,found1212.5189.
化合物22*:根据反应条件3.1,活化试剂只加tmsotf(0.12eq),糖基供体8*(239mg,0.29mmol)和糖基受体21*(288mg,0.24mmol)反应得到22*(285mg,64%)。rf=0.23,petroleumether/etoac=3:1.[α]25d=+10.2(c1.0,ch3cl).1hnmr(400mhz,chloroform-d)δ8.24–6.85(m,60h,arom.h),5.57(s,1h),5.56–5.52(m,1h),5.49–5.42(m,1h),5.39–5.36(m,1h),5.37(d,j=1.8hz,1h,1c-h),5.33(d,j=1.9hz,1h,1b-h),4.97(d,j=1.9hz,1h,1a-h),4.93(d,j=10.6hz,1h),4.87(dd,j=11.6,5.7hz,2h),4.78(d,j=12.1hz,1h),4.75–4.62(m,4h),4.58–4.50(m,2h),4.49–4.41(m,2h),4.41–4.31(m,3h),4.28(d,j=12.3hz,1h),4.17(t,j=9.8hz,1h),4.10(d,j=12.4hz,1h),3.97(ddd,j=11.9,7.3,4.2hz,3h),3.87(d,j=9.7hz,1h),3.82(dd,j=8.5,2.9hz,1h),3.72(dd,j=10.3,4.8hz,1h),3.65(dd,j=10.3,6.9hz,1h),3.57(dd,j=10.4,7.6hz,1h),3.40–3.30(m,2h),3.03(dd,j=10.5,3.0hz,1h),2.71–2.42(m,4h,ch2),2.40–2.23(m,2h,ch2),2.01(s,3h,ch3co),1.22(t,j=7.4hz,3h,ch3).13cnmr(101mhz,chloroform-d)δ206.1,171.6,165.5,165.5,165.1,139.4,139.2,138.7,138.7,138.3,137.9,137.9,137.7,133.2,133.2,129.9,129.8,129.8,129.7,129.4,129.4,128.5,128.4,128.3,128.2,128.2,128.1,128.1,128.1,127.5,127.5,127.4,127.4,127.4,127.3,127.2,127.2,127.2,127.1,127.0,127.0,99.8,98.7,82.0,80.3,79.2,78.6,78.2,75.4,75.3,74.9,74.2,74.0,73.7,73.6,73.2,73.2,72.9,72.6,72.5,72.4,72.2,72.1,71.6,71.0,70.2,37.8,29.7,27.9,25.4,14.9.ir(film):ν=3030,2868,1721,1452,1263,1149,1092,1026,735,711,696cm-1.hrms(esi)m/zcalcdforc112h118o23sn[m+nh4]+1876.7810,found1876.7877.
还原端五糖和八糖的合成路线如图7所示。
化合物24*:根据反应条件3.1,糖基供体20*(417mg,0.30mmol,1.2equiv)和糖基受体23*(452mg,0.243mmol,1equiv)反应得到24*(488mg,65%)。rf=0.18,petroleumether/etoac=2:1.[α]25d=+14.8(c1.0,ch3cl).1hnmr(400mhz,chloroform-d)δ8.04(d,j=7.7hz,2h,arom.h),7.93(d,j=7.6hz,2h,arom.h),7.69–6.82(m,96h,arom.h),5.73(t,j=2.4hz,1h),5.61(t,j=2.5hz,1h),5.49(d,j=1.8hz,1h,1-h),5.45(dd,j=9.8,3.2hz,1h),5.41(dd,j=9.0,2.9hz,1h),5.37(d,j=2.3hz,1h),5.22(s,1h,1-h),5.10(d,j=4.5hz,2h),4.94(s,1h,1-h),4.91(d,j=2.5hz,1h,1-h),4.90–4.81(m,3h),4.80(d,j=1.4hz,1h,1-h),4.79–4.70(m,4h),4.70–4.49(m,7h),4.49–4.08(m,23h),4.04(d,j=9.8hz,1h),4.00–3.64(m,16h),3.62–3.50(m,2h),3.45(tq,j=7.5,4.2,3.2hz,3h),3.39–3.15(m,2h),3.16–2.93(m,2h),2.94–2.70(m,1h),2.61(dt,j=18.2,7.3hz,1h),2.50(dt,j=18.2,6.4hz,1h),2.35(q,j=6.8hz,2h),2.08(s,3h,oac),2.02(s,3h,oac),1.77(s,3h,ch3co),1.61–1.41(m,2h,ch2).13cnmr(101mhz,chloroform-d)δ206.0,171.6,170.2,169.5,165.5,165.2,139.1,139.1,139.0,138.9,138.8,138.7,138.6,138.6,138.2,138.2,138.1,138.0,137.9,137.8,136.9,133.3,129.9,129.7,129.7,129.5,128.6,128.5,128.4,128.3,128.3,128.3,128.3,128.2,128.2,128.1,128.1,128.1,128.0,127.9,127.8,127.7,127.7,127.7,127.5,127.5,127.4,127.3,127.3,127.3,127.2,127.2,127.1,127.1,101.4,100.3,99.1,98.3,96.0,80.1,79.6,79.3,79.1,78.9,77.5,76.0,75.9,75.3,75.2,75.0,74.8,74.7,74.6,74.2,73.4,73.4,73.3,73.1,73.1,73.0,73.0,72.9,72.9,72.7,72.7,72.4,72.3,72.3,72.3,72.2,72.1,72.1,71.6,71.5,71.4,70.5,70.4,69.8,67.1,65.0,50.6,43.7,37.8,29.6,28.5,28.0,20.6,20.6,7.9.ir(film):ν=3030,2917,1748,1722,1453,1365,1265,1240,1071,1026,988,734,696cm-1.hrms(esi)m/zcalcdforc188h195no39na2[m+2na]2+1568.1545,found1568.1525.
化合物25*:根据反应条件4.1,化合物24*(393mg,0.127mmol)脱去lev基得到化合物25*(353mg,93%)。rf=0.48,petroleumether/etoac=2:1.[α]25d=+19.2(c1.0,ch3cl).1hnmr(600mhz,chloroform-d)δ8.03(d,j=7.7hz,2h,arom.h),7.92(d,j=7.7hz,2h,arom.h),7.69–6.87(m,94h,arom.h),5.77(s,1h),5.53(s,1h),5.47(dd,j=9.9,3.1hz,1h),5.44(d,j=3.2hz,1h),5.38(d,j=3.1hz,1h),5.31(s,1h),5.13(d,j=9.9hz,2h),4.98(d,j=22.1hz,1h),4.94–4.85(m,5h),4.84–4.59(m,14h),4.59–4.47(m,5h),4.47–4.10(m,24h),4.06(d,j=9.8hz,1h),4.02–3.93(m,7h),3.90(t,j=9.7hz,1h),3.87–3.68(m,8h),3.62(t,j=9.3hz,1h),3.58–3.47(m,4h),3.44(dd,j=10.4,4.2hz,1h),3.37–3.21(m,1h),3.18–2.96(m,3h),2.98–2.72(m,1h),2.08(s,3h,ch3co),1.97(d,j=5.0hz,1h,3-oh),1.76(s,3h,ch3co),1.64–1.46(m,2h,ch2).13cnmr(101mhz,chloroform-d)δ170.1,169.5,165.9,165.4,139.2,139.1,139.0,138.9,138.8,138.7,138.6,138.2,138.2,138.1,138.0,137.9,136.9,133.3,133.2,129.8,129.8,129.6,128.6,128.5,128.4,128.4,128.3,128.3,128.3,128.1,128.0,127.9,127.9,127.8,127.8,127.7,127.6,127.6,127.5,127.4,127.4,127.3,127.2,127.2,127.2,127.1,101.4,100.0,99.1,98.3,96.0,80.1,79.5,79.3,78.9,78.6,78.4,77.5,76.0,75.7,75.5,75.2,75.0,74.8,74.6,74.1,73.4,73.3,73.2,73.2,73.1,73.0,73.0,72.8,72.4,72.4,72.3,72.2,72.1,72.1,71.6,71.6,71.3,70.8,70.5,69.8,67.1,65.0,60.4,50.7,31.6,29.1,22.6,20.6,20.6,14.1,14.1.ir(film):ν=3030,2918,1749,1722,1453,1266,1071,1027,734,696cm-1.hrms(esi)m/zcalcdforc183h189no37na2[m+2na]2+1519.1361,found1519.1348.
化合物26*:根据反应条件3.1,糖基供体22*(228mg,0.126mmol)和糖基受体25*(314mg,0.105mmol)反应得到26*(306mg,61%)。rf=0.29,petroleumether/etoac=2:1.[α]25d=+4.6(c0.5,ch3cl).1hnmr(600mhz,chloroform-d)δ8.02(d,j=7.7hz,2h,arom.h),7.98(d,j=7.7hz,2h,arom.h),7.88(dd,j=12.9,7.8hz,4h,arom.h),7.82(d,j=7.7hz,2h,arom.h),7.62–6.72(m,150h),5.67(s,1h),5.61(s,1h),5.46(s,3h),5.41(d,j=9.8hz,1h),5.37(s,1h),5.33(s,2h),5.09(d,j=8.0hz,3h),5.03(s,1h),4.99(d,j=10.7hz,1h),4.93(d,j=21.1hz,1h),4.85(s,3h),4.77(s,1h),4.68(ddd,j=16.6,11.0,6.0hz,4h),4.63–4.55(m,5h),4.55–4.42(m,8h),4.34(tdd,j=28.2,17.9,10.5hz,11h),4.25–4.10(m,10h),4.10–3.92(m,9h),3.92–3.82(m,4h),3.82–3.64(m,15h),3.60–3.22(m,9h),3.17(d,j=10.1hz,1h),3.12–3.02(m,1h),2.97(s,1h),2.92–2.79(m,3h),2.75(d,j=8.8hz,1h),2.55(dt,j=18.2,7.3hz,1h),2.45(dt,j=18.2,6.5hz,1h),2.30(tt,j=18.1,8.8hz,3h,ch3co),2.05(s,3h,ch3co),1.98(s,3h,ch3co),1.70(s,3h,ch3co),1.54(tt,j=14.6,6.7hz,1h,ch2),1.48–1.38(m,1h,ch2).13cnmr(101mhz,chloroform-d)δ206.0,171.6,170.3,169.6,165.4,165.4,165.2,165.1,139.5,139.5,139.2,139.2,138.9,138.9,138.8,138.8,138.7,138.6,138.6,138.2,138.1,137.9,137.8,137.6,137.6,136.9,133.2,129.9,129.9,129.7,129.5,129.5,129.3,128.6,128.6,128.5,128.4,128.3,128.3,128.2,128.1,128.0,127.9,127.8,127.7,127.6,127.5,127.4,127.3,127.3,127.2,127.2,127.1,127.1,126.9,126.8,101.4,99.9,99.6,99.1,99.0,98.3,96.0,80.6,80.5,80.1,79.6,79.2,78.9,75.9,75.5,75.1,75.0,74.6,74.1,73.9,73.8,73.6,73.6,73.5,73.3,73.1,72.9,72.9,72.8,72.7,72.6,72.6,72.5,72.3,72.2,72.1,71.8,71.7,71.5,71.4,70.5,70.3,69.8,67.1,65.0,60.4,50.6,37.7,29.6,27.9,21.0,20.6.ir(film):ν=3030,2922,2360,1722,1452,1365,1264,1092,1026,733,696cm-1.hrms(esi)m/zcalcdforc293h305n3o60[m+2nh4]2+2412.5448,found2412.5464.
化合物27*:根据反应条件4.1,化合物26*(300mg,0.063mmol)脱去lev基得到化合物27*(264mg,90%)。rf=0.46,petroleumether/etoac=2:1.[α]25d=+25.6(c1.0,ch3cl).1hnmr(600mhz,chloroform-d)δ8.02(d,j=7.7hz,2h,arom.h),7.98(d,j=7.7hz,2h,arom.h),7.91(d,j=7.6hz,2h,arom.h),7.86(d,j=7.7hz,2h,arom.h),7.81(d,j=7.7hz,2h,arom.h),7.51(t,j=7.5hz,2h,arom.h),7.43(q,j=7.9hz,3h,arom.h),7.37–6.79(m,145h,arom.h),5.68(s,1h),5.61(s,1h),5.48–5.45(m,2h),5.44(s,1h),5.41(dd,j=10.0,2.9hz,1h),5.33(s,2h),5.13–5.07(m,5h),5.03(s,1h),4.99(d,j=10.6hz,1h),4.95(s,0h),4.90(s,1h),4.88–4.80(m,5h),4.80–4.73(m,3h),4.72–4.64(m,5h),4.63–4.55(m,5h),4.55–4.48(m,6h),4.48–4.25(m,16h),4.24–4.11(m,10h),4.11–3.82(m,15h),3.81–3.63(m,18h),3.53(t,j=9.1hz,1h),3.47(t,j=9.0hz,1h),3.44–3.37(m,1h),3.35(dd,j=10.5,3.6hz,1h),3.28(dq,j=20.0,9.8hz,3h),3.17(d,j=10.2hz,1h),3.07(s,1h),3.01–2.93(m,2h),2.87(d,j=10.1hz,3h),2.75(s,1h),2.05(s,3h,ch3co),1.76(d,j=5.3hz,1h,3-oh),1.69(s,3h,ch3co),1.47–1.40(m,1h,ch2).13cnmr(101mhz,chloroform-d)δ170.4,169.7,165.7,165.4,165.3,165.3,165.2,156.5,139.5,139.4,139.2,139.1,138.9,138.8,138.7,138.7,138.6,138.5,138.2,138.1,138.0,137.8,137.7,137.6,137.5,136.8,133.3,129.9,129.9,129.8,129.6,129.4,129.3,128.7,128.6,128.5,128.4,128.3,128.3,128.3,128.2,128.2,128.2,128.1,128.1,128.0,127.9,127.9,127.8,127.8,127.7,127.7,127.6,127.4,127.4,127.4,127.3,127.2,127.2,127.1,127.1,127.0,126.9,126.9,101.4,99.5,99.0,98.2,95.9,80.6,80.4,80.2,80.0,79.7,79.6,79.2,78.9,75.7,75.5,75.1,75.0,74.7,74.5,74.1,73.8,73.6,73.6,73.5,73.2,73.1,72.9,72.8,72.8,72.6,72.6,72.5,72.4,72.3,72.2,72.1,71.8,71.7,71.4,70.5,69.7,67.1,50.6,50.4,44.4,43.6,28.2,27.7,20.7,20.7.ir(film):ν=3033,2932,1726,1498,1455,1267,1096,737,698cm-1.hrms(esi)m/zcalcdforc288h299n3o60[m+2nh4]2+2363.5264,found2363.5398.
实施例5幽门螺旋杆菌o:6血清型o-抗原十三糖的合成:
合成路线如图8所示。
化合物30*:根据反应条件3.1,活化试剂为tfoh(0.2eq)和nis(1.2eq),糖基供体29*(39mg,0.036mmol)和糖基受体28*(22mg,0.043mmol)反应得到30*(41mg,70%)。rf=0.35,hexane/etoac=3:2.[α]25d=-12.0(c1.0,ch3cl).1hnmr(400mhz,chloroform-d)δ8.00–7.94(m,2h,arom.h),7.65–7.10(m,33h,arom.h),6.36(d,j=9.3hz,1h,nh),5.49(dd,j=10.0,7.6hz,1h),5.11(dd,j=10.0,7.9hz,1h),5.00(d,j=11.6hz,1h,ph-ch2),4.84(d,j=11.4hz,1h,ph-ch2),4.67(d,j=7.8hz,1h,1-h),4.64–4.56(m,3h),4.48–4.39(m,6h),4.37(d,j=11.7hz,1h,ph-ch2),4.31(d,j=7.9hz,1h,1-h),4.03(dd,j=10.2,3.1hz,1h),4.01–3.88(m,5h),3.81(dd,j=11.1,3.5hz,1h),3.73(dd,j=11.1,1.8hz,1h),3.65(t,j=6.1hz,1h),3.61–3.48(m,5h),3.38(dd,j=8.3,5.1hz,1h),3.33(dd,j=10.1,2.8hz,1h),2.77(ddd,j=18.3,8.0,5.7hz,1h,ch2),2.57(dt,j=18.3,6.0hz,1h,ch2),2.52–2.41(m,2h,ch2),2.40–2.26(m,4h,ch2),2.15(s,3h,ch3co),1.88(s,3h,ch3co),0.69(s,9h,ch3),0.01(s,3h,sich3),-0.07(s,3h,sich3).13cnmr(101mhz,chloroform-d)δ206.5,206.4,172.5,171.1,164.7,162.0,138.6,138.5,138.1,138.0,137.9,137.7,133.2,130.0,129.7,128.9,128.6,128.5,128.5,128.4,128.4,128.2,128.1,128.1,128.0,128.0,127.9,127.8,127.7,127.5,127.5,127.4,101.2,100.5,96.7,91.9,80.2,78.9,75.9,75.0,74.8,74.6,74.2,74.1,74.0,73.5,73.1,72.3,72.2,71.9,71.7,69.2,67.8,67.7,55.9,37.8,37.7,30.0,29.6,27.9,27.8,25.4,17.7,-4.1,-5.4.ir(film):ν=1720,1071,838,700cm-1.
化合物31*:将化合物30*(40mg,0.025mmol)溶于thf(1ml)中,然后加入醋酸(14μl,0.25mmol)搅拌,在0℃条件下加入tbaf/thf(1m,0.25ml),然后在反应在室温下搅拌4h,tlc检测反应完全后,加入适量dcm稀释,后用饱和nahco3和饱和食盐水洗涤,无水na2so4干燥,过滤浓缩后柱层析分离纯化得到相应的半缩醛。
将上面得到的半缩醛溶于ch2cl2(2ml)中,在0℃下加入ccl3cn(18μl,0.125mmol)和dbu(11μl,0.075mmol),反应在室温下搅拌1.5min。tlc检测反应完全之后,在30℃下浓缩反应液,后经硅胶柱层析分离纯化(正己烷/乙酸乙酯:2/1→1/1)得到化合物31*(37mg,87%)。rf=0.44,hexane/etoac=1:1.[α]25d=7.3(c1.0,ch3cl).1hnmr(400mhz,chloroform-d)δ8.03(d,j=7.8hz,2h,arom.h),7.72–6.96(m,38h,arom.h),6.57(d,j=7.7hz,2h),6.41(d,j=9.3hz,1h),5.91(s,1h),5.77(t,j=9.1hz,1h),5.21–5.01(m,2h),5.00–4.77(m,2h),4.77–4.56(m,4h),4.55–4.26(m,9h),4.26–3.88(m,5h),3.90–3.69(m,2h),3.70–3.49(m,6h),3.37(ddd,j=22.6,9.1,4.0hz,2h),2.79(ddd,j=18.3,8.3,5.4hz,1h,ch2),2.67–2.26(m,7h,ch2),2.17(s,3h,ch3co),1.90(s,3h,ch3co).13cnmr(101mhz,chloroform-d)δ206.5,206.3,172.6,171.1,164.6,162.0,143.2,138.5,138.3,137.9,137.8,137.7,137.6,133.7,129.8,129.2,128.9,128.8,128.6,128.5,128.4,128.2,128.0,128.0,127.9,127.8,127.8,127.6,127.6,127.4,124.2,119.2,101.4,100.5,95.4,91.9,80.2,78.6,75.4,75.1,75.0,74.6,74.0,73.5,73.1,72.3,72.0,71.9,71.8,71.6,67.7,55.9,37.7,37.7,30.0,29.6,27.9,27.8.ir(film):ν=2873,1719,1456,12661210,1164,1096,820,737,698cm-1.
化合物32*:根据反应条件3.1,活化试剂只加tmsotf(0.15eq),糖基供体31*(37mg,0.0223mmol,1.5eq)和糖基受体27*(70mg,0.0149mmol,1eq)反应得到32*(59mg,65%)。rf=0.26,hexane/etoac=3:2.[α]25d=+11.8(c1.0,ch3cl).1hnmr(700mhz,chloroform-d)δ8.04(d,j=7.6hz,2h,arom.h),7.98(d,j=7.6hz,2h,arom.h),7.93(d,j=7.7hz,2h,arom.h),7.85(d,j=7.6hz,2h,arom.h),7.62–6.56(m,187h,arom.h),6.06(d,j=9.2hz,1h,nh),5.69(s,1h),5.62(d,j=3.1hz,1h),5.48(s,1h),5.47(s,1h),5.43(d,j=12.4hz,2h),5.39–5.32(m,3h),5.18–5.09(m,4h),5.09–5.04(m,2h),5.00(d,j=10.6hz,1h),4.97–4.92(m,2h),4.91–4.57(m,27h),4.56–4.29(m,33h),4.27–4.08(m,17h),4.05–3.85(m,18h),3.84–3.65(m,15h),3.63(d,j=8.8hz,1h),3.60–3.47(m,7h),3.46–3.33(m,7h),3.30(t,j=9.5hz,1h),3.25(t,j=9.6hz,1h),3.18(d,j=9.8hz,1h),3.10(dd,j=8.9,5.0hz,2h),2.98(q,j=9.3,6.7hz,2h),2.87(d,j=9.7hz,1h),2.84–2.75(m,1h),2.73(d,j=9.9hz,1h),2.65–2.57(m,2h),2.50(ddd,j=14.2,8.2,5.4hz,1h),2.44(dd,j=16.4,7.5hz,1h),2.41–2.26(m,4h,ch2),2.18(s,3h,ch3co),2.07(s,3h,ch3co),1.87(s,3h,ch3co),1.72(s,3h,ch3co),1.50–1.41(m,1h).13cnmr(176mhz,chloroform-d)δ206.2,206.1,172.4,171.1,170.3,169.6,165.3,165.2,165.0,164.0,161.8,139.6,139.5,139.5,139.2,139.1,139.0,138.9,138.9,138.8,138.8,138.7,138.6,138.6,138.5,138.5,138.1,138.1,138.0,138.0,137.9,137.9,137.8,137.7,137.4,132.5,129.9,129.9,129.6,129.4,129.3,129.2,129.1,129.0,128.8,128.6,128.6,128.5,128.5,128.4,128.3,128.3,128.2,128.2,128.1,128.1,128.1,128.0,128.0,128.0,127.9,127.9,127.8,127.8,127.8,127.7,127.7,127.6,127.6,127.5,127.5,127.4,127.4,127.3,127.3,127.3,127.2,127.1,127.1,127.1,127.0,127.0,127.0,126.9,126.9,126.8,126.7,101.3,101.1,100.5,99.5,99.0,98.8,91.7,80.8,80.3,80.0,79.2,78.5,75.9,75.8,75.1,75.0,75.0,74.7,74.5,74.5,74.3,74.0,73.9,73.7,73.5,73.5,73.2,73.1,73.1,72.9,72.8,72.7,72.6,72.4,72.4,72.3,72.2,72.2,72.1,72.0,71.8,71.7,71.3,70.5,69.7,69.0,67.7,67.5,67.0,55.7,37.7,29.9,29.5,27.8,20.6,20.6.ir(film):ν=2928,1724,1455,1267,1097,737,698cm-1.
化合物33*:将化合物32*(20mg,0.0032mmol)溶于吡啶(0.5ml)中,加入醋酸肼(2mg,0.016mmol),反应在室温下搅拌3h。tlc检测反应完全后,浓缩除去吡啶,加入适量dcm稀释,然后反应混合物分别用1mhcl、饱和nahco3和食盐水洗,无水na2so4干燥,浓缩后硅胶柱层析分离纯化(石油醚/乙酸乙酯:2/1→3/2)得到化合物33*(17mg,89%)。rf=0.34,hexane/etoac=3:2.[α]25d=+13.4(c1.0,ch3cl).1hnmr(700mhz,chloroform-d)δ8.04(d,j=7.6hz,2h,arom.h),7.98(d,j=7.7hz,2h,arom.h),7.93(d,j=7.7hz,2h,arom.h),7.86(d,j=7.5hz,2h,arom.h),7.53–6.55(m,187h,arom.h),6.04(d,j=8.6hz,1h,nh),5.69(s,1h),5.63(s,1h),5.48(s,1h),5.47(s,2h),5.42(q,j=10.5hz,3h),5.34(d,j=7.8hz,2h),5.12(d,j=10.4hz,3h),5.08(s,2h),5.01(d,j=10.7hz,1h),4.98–4.90(m,3h),4.90–4.45(m,40h),4.37(ddt,j=45.4,24.8,10.6hz,13h),4.28–4.15(m,11h),4.15–4.07(m,6h),4.01(dt,j=20.8,9.3hz,10h),3.94–3.84(m,6h),3.83–3.65(m,16h),3.62(d,j=9.1hz,2h),3.52(tq,j=28.2,9.6,8.4hz,7h),3.37(dtt,j=50.0,18.4,10.2hz,7h),3.25(t,j=9.7hz,1h),3.19(t,j=13.6hz,1h),3.10(d,j=6.6hz,2h),2.99(q,j=8.3,6.7hz,2h),2.88(t,j=15.7hz,1h),2.81–2.75(m,1h),2.72(d,j=10.0hz,1h),2.62(d,j=10.3hz,1h),2.07(s,3h,ch3co),1.72(s,3h,ch3co),1.50–1.41(m,1h).13cnmr(176mhz,chloroform-d)δ170.3,169.6,165.3,165.3,165.2,165.2,165.1,164.1,161.8,139.6,139.5,139.5,139.2,139.1,139.0,139.0,138.8,138.8,138.7,138.6,138.6,138.5,138.4,138.1,138.1,138.1,137.9,137.9,137.8,137.8,137.8,137.7,137.4,137.4,137.3,133.2,133.1,132.8,132.6,129.9,129.9,129.6,129.4,129.3,129.2,129.2,129.1,129.0,128.7,128.6,128.6,128.5,128.5,128.3,128.3,128.3,128.2,128.2,128.2,128.2,128.1,128.1,128.0,128.0,128.0,127.9,127.9,127.9,127.8,127.8,127.7,127.7,127.6,127.6,127.4,127.4,127.3,127.3,127.2,127.1,127.1,127.1,127.0,127.0,127.0,126.9,126.9,126.8,126.7,104.2,101.3,100.3,99.5,99.0,98.8,97.7,95.9,92.1,82.7,81.9,81.2,81.1,80.8,80.5,80.0,79.6,79.2,78.9,77.9,75.8,75.5,75.1,75.0,74.7,74.5,74.5,74.4,74.0,74.0,73.9,73.8,73.7,73.6,73.4,73.2,73.2,73.1,72.9,72.8,72.8,72.6,72.5,72.4,72.3,72.3,72.2,72.1,71.9,71.8,71.7,71.4,71.3,71.0,70.5,69.7,69.0,68.9,68.4,67.5,67.1,57.3,50.6,45.3,29.7,20.6,20.6.ir(film):ν=3432,3066,3033,2926,2869,1725,1603,1498,1455,1367,1267,1218,1096,1028,912,820,736,698cm-1.
化合物35*:将糖基供体34*(7mg,0.0107mmol)和糖基受体33*(15mg,0.00268mmol,1eq)混合,溶于甲苯中共蒸两次。加入干燥的dcm/et2o(v/v,1:1)(0.2ml)中,加入活化的
化合物36*:将化合物35*(9mg,0.0014mmol,52%)溶于acoh(1ml)中,加入新活化的锌粉(100mg),反应在室温下搅拌12h,tlc检测原料消失后,溶液过滤并减压浓缩,然后加入适量的dcm稀释,饱和nahco3洗涤,无水na2so4干燥,溶液过滤后减压浓缩,真空干燥得到nac中间产物。将上述中间产物溶于thf/meoh(1:1,1ml)中,加入meona(30mg),室温下搅拌15min,然后加入naoh(aq,1m,100μl),反应在室温下搅拌12h,tlc检测反应完全后,加入amerliteir120(h+)树脂中和反应液ph达到7,溶液过滤并减压浓缩,硅胶柱层析(二氯甲烷/甲醇:50/1)分离纯化得到中间产物。将上述脱酰基化合物溶于meoh/thf/h2o/acoh(10:5:4:1,12ml)中,加入10%pd/c(50mg),反应混合物在氢气(1bar)条件下搅拌24h,经飞行时间质谱检测反应完成后,溶液过滤并浓缩,真空干燥,后经sephadexlh20凝胶柱分离纯化后得到化合物36*。1hnmr(700mhz,deuteriumoxide)δ5.20(d,j=2.8hz,1h,1-h),5.13(s,1h,1-h),5.10(s,1h,1-h),5.09(s,1h,1-h),5.06–5.01(m,5h,1-h),4.95(s,1h,1-h),4.82–4.78(m,1h),4.65(d,j=8.6hz,1h,1-h),4.44(dd,j=7.8,4.1hz,2h),4.21–4.14(m,6h),4.12(s,1h),4.06(s,1h),3.99(t,j=9.7hz,12h),3.95–3.86(m,10h),3.86–3.68(m,44h),3.64(dt,j=21.4,9.9hz,15h),3.60–3.50(m,6h),3.41–3.36(m,1h),3.11–3.01(m,2h,ch2),1.95(s,3h,ch3co),1.94–1.89(m,2h,ch2),1.19(d,j=6.5hz,3h,fucose-ch3),1.18–1.13(d,j=6.5hz,3h,fucose-ch3).13cnmr(176mhz,deuteriumoxide)δ158.8,102.1,102.0,101.9,101.7,101.2,100.5,100.2,99.4,98.5,98.1,82.1,79.7,79.0,78.6,78.6,78.2,77.9,77.8,76.3,75.4,74.8,74.8,74.0,73.9,73.8,73.7,73.5,73.2,73.1,72.1,71.9,71.7,71.5,71.4,71.4,71.3,70.6,70.4,70.1,69.9,69.7,69.5,69.4,69.1,68.7,68.4,68.3,67.7,67.7,67.5,67.5,66.9,66.8,66.8,66.7,66.0,65.7,64.9,62.1,62.0,61.7,61.6,61.5,61.5,,61.0,59.8,37.5,26.6,22.3,15.4(d,j=3.2hz).
上面结合具体实施例对本发明进行了示例性的描述,显然本发明的实现并不受上述方式的限制,只要采用了本发明的方法构思和技术方案进行的各种改进,或未经改进将本发明的构思和技术方案直接应用于其它场合的,均在本发明的保护范围内。
- 还没有人留言评论。精彩留言会获得点赞!