一种卡瓦胡椒素B类似物的制备方法及其用途
一种卡瓦胡椒素b类似物的制备方法及其用途
技术领域
1.本发明涉及化学医药领域,具体而言,涉及一种卡瓦胡椒素b类似物的制备方法及其用途。
背景技术:
2.癌症是严重威胁人类健康的常见疾病,位居人类三大主要致死疾病之首,是目前我国乃至世界范围内仍未解决的首要难题。目前,通过化学药物治疗在癌症治疗中起着不可替代的作用。硫氧还蛋白还原酶(thioredoxin reductase,trxr)是存在于细胞中的含硒同型二聚体黄素酶,它与硫氧还蛋白(thioredoxin,trx)、还原型烟酰胺腺嘌呤二核苷磷酸(nadph)共同构成硫氧还蛋白系统(thioredoxin system)。该系统控制多种涉及细胞存活与增殖信号通路中的氧化还原过程,能够抑制细胞凋亡、促进细胞增长、调节免疫功能、直接参与抗氧化作用、活化多种转录因子等。研究发现,trxr在多种肿瘤组织中过表达,它影响着肿瘤细胞的存活、增殖与凋亡,通过化学药物抑制trxr的活性杀死肿瘤细胞已经成为一种有效的癌症靶向治疗策略。
技术实现要素:
3.基于上述现有技术研究的现状,本发明提供一种卡瓦胡椒素b类似物的制备方法及其用途。本发明所述卡瓦胡椒素b类似物对硫氧还蛋白还原酶活性具有很好的抑制作用,并发现该类分子通过抑制硫氧还蛋白还原酶碳末端的硒半胱氨酸来抑制硫氧还蛋白还原酶的活性进而杀死肿瘤细胞。
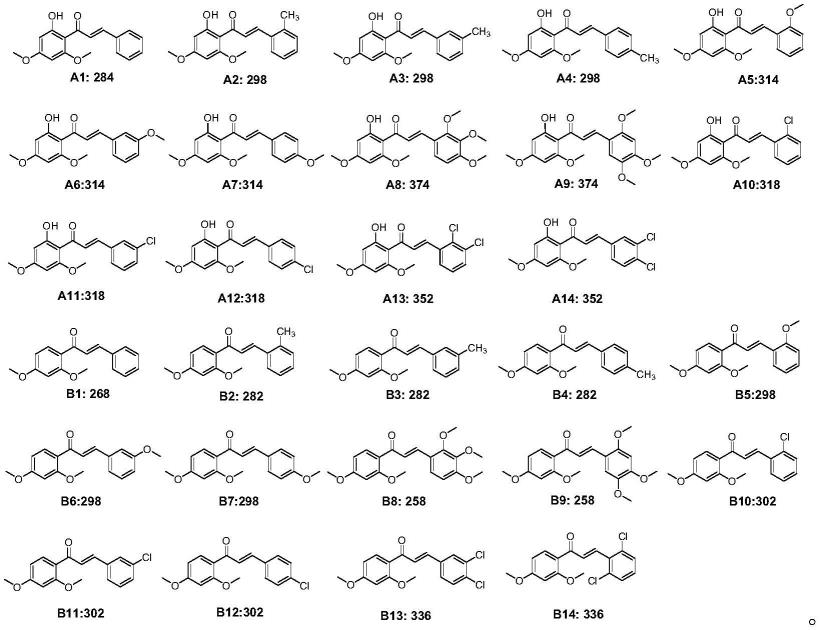
4.具体地,本发明提供卡瓦胡椒素b类似物,其结构如下述式(ⅰ)所示:
[0005][0006]
其中,r1:氢、羟基;r2,r3,r4,r5,r6:氢、甲基、甲氧基、氯取代杂芳基
[0007]
中的任一种。其中,所述化合物为下述中的任一种:
[0008][0009]
本发明还提供一种卡瓦胡椒素b类似物的用途,具体将其用于其抑制硫氧还蛋白还原酶的活性。机制研究表明该类分子是通过抑制硫氧还蛋白还原酶碳末端的硒半胱氨酸来抑制硫氧还蛋白还原酶的活性进而杀死肿瘤细胞。
[0010]
本发明所述卡瓦胡椒素b类似物的具体制备方法如下:将1eq不同取代的苯乙酮溶解到甲醇溶液中,之后加入10%的氢氧化钾溶液,半小时后把1.1eq的拥有不同取代基的醛加入溶液中,室温搅拌至原料完全反应,反应进程用tlc检测,反应结束之后旋蒸除去乙醇溶液,用乙酸乙酯进行萃取干燥。合并有机相,柱层析分离得到最终目标化合物。
附图说明
[0011]
图1是实施例1-28的卡瓦胡椒素b类似物对hela细胞细胞毒活性结果示意图。
[0012]
图2是实施例1-28的卡瓦胡椒素b类似物活性最优化合物(b11)在体外对硫氧还蛋白还原酶及其相关酶活性的抑制结果示意图。
[0013]
图3是实施例1-28的卡瓦胡椒素b类似物活性最优化合物(b11)抑制hela细胞生长进行的划痕实验结果示意图。
[0014]
实施例1
[0015]
(e)-1-(2-hydroxy-4,6-dimethoxyphenyl)-3-phenylprop-2-en-1-one(a1)1h nmr(400mhz,cdcl3)δ14.30(s,1h),7.94(d,j=15.6hz,1h),7.81(d,j=15.6hz,1h),7.63(dd,j=7.6,2.0hz,2h),7.436(m,3h),6.15(d,j=2.4hz,1h),5.98(d,j=2.4hz,1h),3.93(s,3h),3.85(s,3h);
13
c nmr(100mhz,cdcl3)δ192.52,168.33,166.17,162.44,142.18,135.48,129.96,128.79,128.26,127.45,106.23,93.75,91.15,55.74,55.46.
[0016]
实施例2
[0017]
(e)-1-(2-hydroxy-4,6-dimethoxyphenyl)-3-(2-methylphenyl)prop-2-en-1-one(a2)1h nmr(400mhz,cdcl3)δ14.29(s,1h),8.09(d,j=15.6hz,1h),7.84(d,j=15.6hz,1h),7.65(d,j=7.6hz,1h),7.30(t,1h),7.24(dd,j=12.4,8.4hz,2h),6.12(d,j=2hz,1h),5.97(d,j=2.4hz,1h),3.91(s,3h),3.84(s,3h),2.50(s,3h);
13
c nmr(100mhz,cdcl3)δ192.68,168.36,166.20,162.49,139.91,138.11,134.48,130.82,129.74,128.58,126.58,126.23,106.32,93.77,91.20;55.79,55.52,55.47,19.90.
[0018]
实施例3
[0019]
(e)-1-(2-hydroxy-4,6-dimethoxyphenyl)-3-(3-methylphenyl)prop-2-en-1-one(a3)1h nmr(400mhz,cdcl3)δ14.31(s,1h),7.89(d,j=15.2hz,1h),7.77(d,j=15.6hz,1h),7.42(m,2h),7.31(m,1h),7.20(d,j=7.6hz,1h),6.11(d,j=2.4hz,1h),5.96(d,j=2.4hz,1h),3.91(s,3h),3.83(s,3h),2.389(s,3h);
13
c nmr(100mhz,cdcl3)δ192.61,168.32,166.14,162.46,142.47,138.41,135.47,130.85,129.16,128.68,127.28,125.30,106.28,93.75,91.17,55.78,55.48,21.31.
[0020]
实施例4
[0021]
(e)-1-(2-hydroxy-4,6-dimethoxyphenyl)-3-(4-methylphenyl)prop-2-en-1-one(a4)1h nmr(400mhz,cdcl3)δ14.34(s,1h),7.89(d,j=15.6hz,1h),7.80(d,j=15.6hz,1h),7.52(d,j=8.4hz,2h),7.22(d,j=8hz,2h),6.11(d,j=2.4hz,1h),5.97(d,j=2.4hz,1h),3.92(s,3h),3.84(s,3h),2.39(s,3h);
13
c nmr(100mhz,cdcl3)δ192.61,168.31,168.31,162.43,142.43,140.45,132.75,129.55,128.31,126.43,106.27,93.74,91.14,55.74,55.47,21.43.
[0022]
实施例5
[0023]
(e)-1-(2-hydroxy-4,6-dimethoxyphenyl)-3-(2-methoxyphenyl)prop-2-en-1-one(a5)1h nmr(400mhz,cdcl3)δ14.43(s,1h),8.19(d,j=16hz,1h),8.01(d,j=15.6hz,1h),7.65(dd,j=8,1.6hz,1h),7.40(m,1h),7.03(t,1h),6.97(d,j=8.4hz,1h),6.14(d,j=2.4hz,1h),5.99(d,j=2.4hz,1h),3.94(s,3h),3.94(s,3h),3.86(s,3h);
13
c nmr(100mhz,cdcl3)δ193.00,168.30,166.00,162.47,158.59,37.77,131.29,128.71,127.82,124.50,120.63,111.14,106.38,93.74,91.11,55.68,55.47,55.42.
[0024]
实施例6
[0025]
(e)-1-(2-hydroxy-4,6-dimethoxyphenyl)-3-(3-methoxyphenyl)prop-2-en-1-one(a6)1h nmr(400mhz,cdcl3)δ14.27(s,1h),7.90(d,j=15.6hz,1h),7.76(d,j=15.6hz,1h),7.35(t,1h),7.22(d,j=7.6hz,1h),7.13(d,j=2hz,1h),6.95(dd,j=5.6,2hz,1h),6.12(d,j=2.4hz,1h),5.97(d,j=2hz,1h),3.92(s,3h),3.86(s,3h),3.84(s,3h);
13
c nmr(100mhz,cdcl3)δ192.49,168.33,,166.20,162.44,159.80,142.04,136.90,129.76,127.80,120.81,115.51,113.56,106.24,93.75,91.16,55.74,55.47,55.18.
[0026]
实施例7
[0027]
(e)-1-(2-hydroxy-4,6-dimethoxyphenyl)-3-(4-methoxyphenyl)prop-2-en-1-one(a7)1h nmr(400mhz,cdcl3)δ14.41(s,1h),7.84(d,j=16hz,1h),7.80(d,j=16hz,1h),7.58(dd,j=6.8,1.6hz,2h),6.94(d,j=8.8hz,2h),6.13(d,j=1.2hz,1h),5.97(d,j=2hz,1h),,3.92(s,3h),3.85(s,3h),3.84(s,3h);
13
c nmr(100mhz,cdcl3)δ192.51,168.31,
165.96,162.40,161.30,142.38,130.04,128.23,125.05,114.29,106.27,93.76,91.13,55.75,55.48,55.31.
[0028]
实施例8
[0029]
(e)-1-(2-hydroxy-4,6-dimethoxyphenyl)-3-(2,3,4-trimethoxyphenyl)prop-2-en-1-one(a8)1h nmr(400mhz,cdcl3)δ14.44(s,1h),8.03(d,j=16hz,1h),7.94(d,j=16hz,1h),7.36(d,j=8.8hz,1h),6.73(d,j=8.8hz,1h),6.12(d,j=2.4hz,1h),5.97(d,j=2.4hz,1h),3.96(s,3h),3.91(s,3h),3.89(s,3h);3.84(s,3h);
13
c nmr(100mhz,cdcl3)δ192.81,168.31,165.92,162.42,155.44,153.66,142.43,137.80,126.45,123.67,122.60,107.59,106.38,93.76,91.14,61.37,60.86,56.01,55.74,55.49.
[0030]
实施例9
[0031]
(e)-1-(2-hydroxy-4,6-dimethoxyphenyl)-3-(2,4,5-trimethoxyphenyl)prop-2-en-1-one(a9)1h nmr(400mhz,cdcl3)δ14.54(s,1h),8.15(d,j=15.6hz,1h),7.89(d,j=15.6hz,1h),7.12(s,1h),6.52(s,1h),6.11(d,j=2hz,1h),5.97(d,j=2hz,1h),3.95(s,3h),3.91(s,3h),3.89(s,3h);3.84(s,3h);
13
c nmr(100mhz,cdcl3)δ192.67,168.26,165.73,162.29,154.41,152.16,143.09,137.85,125.12,116.06,111.36,106.30,6.8,93.74,91.03,56.35,56.22,55.95,55.58,55.41.
[0032]
实施例10
[0033]
(e)-3-(2-chlorophenyl)-1-(2-hydroxy-4,6-dimethoxyphenyl)prop-2-en-1-one(a10)1h nmr(400mhz,cdcl3)δ14.20(s,1h),8.17(d,j=15.6hz,1h),7.90(d,j=15.6hz,1h),7.71(t,1h),7.45(m,1h),7.32(dd,j=4.4,2.4hz,2h),6.12(d,j=2.4hz,1h),5.97(d,j=2.4hz,1h),3.91(s,3h),3.85(s,3h);.
[0034]
实施例11
[0035]
(e)-3-(3-chlorophenyl)-1-(2-hydroxy-4,6-dimethoxyphenyl)prop-2-en-1-one(a11)1h nmr(400mhz,cdcl3)δ14.18(s,1h),7.89(d,j=15.6hz,1h),7.70(d,j=15.6hz,1h),7.57(s,1h),7.31(m,1h),7.20(dd,j=4,0.8hz,2h),6.12(d,j=2.4hz,1h),5.97(d,j=2.4hz,1h),3.93(s,3h),3.85(s,3h);
13
c nmr(100mhz,cdcl3)δ192.21,168.42,166.42,162.47,140.37,137.44,134.80,130.06,129.77,128.85,127.83,126.55,106.25,93.79,91.29,55.89,55.58;.
[0036]
实施例12
[0037]
(e)-3-(4-chlorophenyl)-1-(2-hydroxy-4,6-dimethoxyphenyl)prop-2-en-1-one(a12)1h nmr(400mhz,cdcl3)δ14.22(s,1h),7.88(d,j=15.6hz,1h),7.36(d,j=15.6hz,1h),7.54(d,j=8.4hz,2h),7.39(d,j=8hz,2h),6.12(d,j=2.4hz,1h),5.97(d,j=2.4hz,1h),3.92(s,3h),3.84(s,3h);
13
c nmr(100mhz,cdcl3)δ192.28,168.42,166.34,162.44,140.71,135.81,134.06,129.42,129.10,128.01,106.25,93.80,91.28,55.84,55.57.
[0038]
实施例13
[0039]
(e)-3-(2,3-dichlorophenyl)-1-(2-hydroxy-4,6-dimethoxyphenyl)prop-2-en-1-one(a13)1h nmr(400mhz,cdcl3)δ14.13s,1h),8.14(d,j=15.6hz,1h),7.85(d,j=15.6hz,1h),7.59(dd,j=8,1.2hz,1h),7.49(dd,j=8,1.2hz,1h),7.24(d,j=8hz,1h),
6.12(d,j=2.4hz,1h),5.96(d,j=2hz,1h),3.90(s,3h),3.85(s,3h);
13
c nmr(100mhz,cdcl3)δ192.02,168.49,166.55,162.45,137.61,136.26,133.98,133.29,131.23,131.10,127.26,125.89,106.24,93.83,91.33,55.88,55.61.
[0040]
实施例14
[0041]
(e)-3-(2,6-dichlorophenyl)-1-(2-hydroxy-4,6-dimethoxyphenyl)prop-2-en-1-one(a14)
[0042]1h nmr(400mhz,cdcl3)δ14.14(s,1h),7.86(d,j=15.6hz,1h),7.66(d,j=2hz,1h),7.65(d,j=15.6hz,1h),7.48(d,j=8hz,1h),7.42(dd,j=8,2hz,1h),6.12(d,j=2.4hz,1h),5.97(d,j=2.4hz,1h),3.92(s,3h),3.85(s,3h);
13
c nmr(100mhz,cdcl3)δ191.91,168.46,166.5,162.42,139.17,135.68,133.70,133.08,130.79,129.65,129.19,127.25,106.19,93.81,91.31,55.90,55.59;
[0043]
实施例15
[0044]
(e)-1-(2,4-dimethoxyphenyl)-3-phenylprop-2-en-1-one(b1)1h nmr(400mhz,cdcl3)δ7.77(d,j=8.4hz,1h),7.70(d,j=15.6hz,1h),7.61(m,2h),7.54(d,j=15.6hz,1h),6.59(dd,j=8.4,2hz,1h),6.51(d,j=2hz,1h),5.56(m,1h),3.91(s,3h),3.88(s,3h);
13
c nmr(100mhz,cdcl3)δ190.28,164.11,160.32,141.80,135.35,132.74,129.82,128.70,128.15,127.09,122.04,105.16,98.5,55.61,55.40.
[0045]
实施例16
[0046]
(e)-1-(2,4-dimethoxyphenyl)-3-(2-methylphenyl)prop-2-en-1-one(b2)h nmr(400mhz,cdcl3)δ7.99(d,j=16hz,1h),7.79(d,j=8.4hz,1h),7.65(d,j=7.6hz,1h),7.46(d,j=15.6hz,1h),7.29(d,j=6.4hz,1h),7.24(t,2h),6.59(dd,j=8.8,2.4hz,1h),6.50(d,j=2hz,1h),3.91(s,3h),3.88(s,3h);
13
c nmr(100mhz,cdcl3)δ190.30,164.14,160.37,139.44,137.98,134.35,132.83,130.68,129.56,128.18,126.40,26.12,122.15,105.17,98.50,55.62,55.42,19.80;
[0047]
实施例17
[0048]
(e)-1-(2,4-dimethoxyphenyl)-3-(3-methylphenyl)prop-2-en-1-one(b3)1h nmr(400mhz,cdcl3)δ7.76(d,j=8.8hz,1h),7.66(d,j=16hz,1h),7.50(d,j=15.6hz,1h),7.42(d,j=8.4hz,2h),7.30(d,j=7.6hz,1h),7.20(d,j=7.2hz,1h),6.59(dd,j=8.8,2.4hz,1h),6.51(d,j=2.4hz,1h),3.91(s,3h),3.87(s,3h),2.382(s,3h);
13
c nmr(100mhz,cdcl3)δ190.32,164,160.24,142.01,138.24,135.24,132.61,130.64,128.87,128.54,126.86,125.19,122.05,105.10,98.44,55.56,55.32,21.16.
[0049]
实施例18
[0050]
(e)-1-(2,4-dimethoxyphenyl)-3-(4-methylphenyl)prop-2-en-1-one(b4)1h nmr(400mhz,cdcl3)δ7.76(d,j=8.4hz,1h),7.67(d,j=16hz,1h),7.50(dd,j=9.6,8.4hz,3h),7.20(d,j=8hz,2h),6.58(dd,j=8.8,2.4hz,1h),6.50(d,j=2hz,1h),3.90(s,3h),3.87(s,3h),2.38(s,3h);
13
c nmr(100mhz,cdcl3)δ190.61,164.03,160.29,142.13,140.31,132.73,132.67,129.52,128.25,126.22,122.31,105.12,98.62,55.70,55.48,21.42.
[0051]
实施例19
[0052]
(e)-1-(2,4-dimethoxyphenyl)-3-(2-methoxyphenyl)prop-2-en-1-one(b5)1h nmr(400mhz,cdcl3)δ8.03(d,j=16hz,1h),7.76(d,j=8.8hz,1h),7.61(dd,j=7.6,1.2hz,1h),7.57(d,j=16hz,1h),7.37(m,1h),6.99(d,j=7.2hz,1h),6.95(t,1h),6.57(dd,j=8.8,2.4hz,1h),6.50(d,j=2.4hz,1h),3.90(s,3h),3.89(s,3h),3.87(s,3h);
13
c nmr(100mhz,cdcl3)δ190.93,163.88,160.23,158.5,137.41,132.68,131.09,128.55,127.63,124.38,122.42,120.55,111.07,105.06,98.55,55.60,55.41,55.37.
[0053]
实施例20
[0054]
(e)-1-(2,4-dimethoxyphenyl)-3-(3-methoxyphenyl)prop-2-en-1-one(b6)1h nmr(400mhz,cdcl3)δ7.70(d,j=8.8hz,1h),7.66(d,j=15.6hz,1h),7.51(d,j=15.6,1h),7.33(t,1h),7.21(d,j=7.6hz,1h),7.12(t,1h),6.94(m,1h),6.58(dd,j=8.8,2.4hz,1h),6.50(d,j=2.4hz,1h),3.90(s,3h),3.87(s,3h),3.84(s,3h);
13
c nmr(100mhz,cdcl3)δ190.12,164.07,160.26,159.65,141.57,136.66,132.63,129.60,127.32,121.86,120.66,115.35,113.26,105.13,98.37,55.50,55.30,55.03.
[0055]
实施例21
[0056]
(e)-1-(2,4-dimethoxyphenyl)-3-(4-methoxyphenyl)prop-2-en-1-one(b7)1h nmr(400mhz,cdcl3)δ7.76(d,j=8.4hz,1h),7.68(d,j=15.6hz,1h),7.57(d,j=8.4,2h),7.41(d,j=15.6hz,1h),6.39(d,j=8.8hz,2h),6.59(dd,j=8.8,2.4hz,1h),6.51(d,j=2hz,1h),3.91(s,3h),3.88(s,3h),3.85(s,3h);
13
c nmr(100mhz,cdcl3)δ190.56,163.90,161.17,160.19,141.97,132.63,129.90,128.09,124.95,122.39,114.22,105.06,98.6,55.67,55.45,55.29.
[0057]
实施例22
[0058]
(e)-1-(2,4-dimethoxyphenyl)-3-(2,3,4-trimethoxyphenyl)prop-2-en-1-one(b8)1h nmr(400mhz,cdcl3)δ7.89(d,j=16hz,1h),7.76(d,j=8.4hz,1h),7.51(d,j=16,1h),7.37(d,j=8.4hz,1h),6.73(d,j=8.8hz,1h),6.58(dd,j=8.8,2.4hz,1h),6.51(d,j=2.4hz,1h),3.93(s,3h),3.89(s,3h),3.89(s,3h),3.88(s,3h);
13
c nmr(100mhz,cdcl3)δ190.74,163.78,160.09,155.19,153.44,142.29,137.22,132.55,126.25,123.32,122.39,122.33,107.51,104.99,98.48,61.24,60.73,55.89,55.56,55.35.
[0059]
实施例23
[0060]
(e)-1-(2,4-dimethoxyphenyl)-3-(2,4,5-tri methoxyphenyl)prop-2-en-1-one(b9)1h nmr(400mhz,cdcl3)δ7.98(d,j=15.6hz,1h),7.72(d,j=8.4hz,1h),7.40(d,j=16,1h),7.11(s,1h),6.57(dd,j=8.4,2hz,1h),6.51(d,j=2.4hz,2h),3.93(s,3h),3.89(s,3h),3.88(s,3h),3.87(s,3h);
13
c nmr(100mhz,cdcl3)δ190.99,163.56,159.92,154.16,151.93,143.05,137.45,132.37,125.14,122.56,115.82,111.05,104.90,98.53,96.86,56.33,56.31,55.88,55.52,55.33.
[0061]
实施例24
[0062]
(e)-3-(2-chlorophenyl)-1-(2,4-dimethoxyphenyl)prop-2-en-1-one(b10)1h nmr(400mhz,cdcl3)δ8.06(d,j=15.6hz,1h),7.79(d,j=8.4hz,1h),7.71(m,1h),7.51(d,j=16hz,1h),7.43(m,1h),7.30(m,2h),6.59(dd,j=8.8,2.4hz,1h),6.50(d,j=2.4hz,1h),3.91(s,3h),3.88(s,3h);
13
c nmr(100mhz,cdcl3)δ190.09,164.31,160.43,137.60,
135.21,133.74,132.97,130.53,130.09,129.61,127.67,126.90,121.85,105.27,98.54,55.67,55.49.
[0063]
实施例25
[0064]
(e)-3-(3-chlorophenyl)-1-(2,4-dimethoxyphenyl)prop-2-en-1-one(b11)1h nmr(400mhz,cdcl3)δ7.79(d,j=8.8hz,1h),7.62(t,2h),7.53(d,j=16hz,1h),7.47(dd,j=8,1.6hz,1h),7.34(t,2h),6.59(dd,j=8.8,2.4hz,1h),6.51(d,j=2.4hz,1h),3.92(s,3h),3.88(s,3h);
13
c nmr(100mhz,cdcl3)δ189.77,164.35,160.45,139.89,137.28,134.63,132.87,129.94,129.58,128.30,127.69,126.41,121.72,105.29,98.45,55.65,55.43.
[0065]
实施例26
[0066]
(e)-3-(4-chlorophenyl)-1-(2,4-dimethoxyphenyl)prop-2-en-1-one(b12)1h nmr(400mhz,cdcl3)δ7.78(d,j=8.8hz,1h),7.64(d,j=15.6hz,1h),7.54(t,3h),7.37(d,j=8.8hz,2h),6.59(dd,j=8.4,2hz,1h),6.50(d,j=2.4hz,1h),3.91(s,3h),3.88(s,3h);
13
c nmr(100mhz,cdcl3)δ189.92,164.29,160.41,140.22,135.6,133.95,132.88,129.33,128.98,127.58,121.90,105.26,98.53,55.67,55.46.
[0067]
实施例27
[0068]
(e)-3-(2,3-dichlorophenyl)-1-(2,4-dimethoxyphenyl)prop-2-en-1-one(b13)1h nmr(400mhz,cdcl3)δ7.79(d,j=8.8hz,1h),7.67(d,j=2hz,1h),7.54(d,j=9.6hz,2h),7.47(d,j=8.4hz,1h),7.42(dd,j=8.4,2hz,1h),6.59(dd,j=8.8,2.4hz,1h),6.50(d,j=2hz,1h),3.92(s,3h),3.88(s,3h);
13
c nmr(100mhz,cdcl3)δ189.46,164.47,160.50,138.68,135.56,133.46,132.95,132.91,130.66,129.53,128.63,127.17,121.56,105.35,98.45,55.67,55.45;
[0069]
实施例28
[0070]
(e)-3-(2,6-dichlorophenyl)-1-(2,4-dimethoxyphenyl)prop-2-en-1-one(b14)1h nmr(400mhz,cdcl3)δ7.83(d,j=8.8hz,1h),7.79(d,j=16hz,1h),7.70(d,j=16.4hz,1h),7.37(d,j=8hz,2h),7.19(t,1h),6.59(dd,j=8.8,2.4hz,1h),6.48(d,j=2.4hz,1h),3.89(s,3h),3.87(s,3h);
13
c nmr(100mhz,cdcl3)δ189.57,164.53,160.73,135.07,134.84,134.82,133.15,133.13,133.02,129.30,128.68,127.54,121.53,105.34,98.39,55.63,55.46;
[0071]
试验例1:卡瓦胡椒素b类似物对hela细胞细胞毒活性试验
[0072]
实验方法:于96孔板中,每孔加入5000个hela细胞,分别以实施例1-28中的化合物作用72h,最后用mtt法测定细胞毒活。
[0073]
实验结果:结果如图1所示,结果表明实施例1-28中的施例1、实施例3、实施例6、实施例10、施例11对hela细胞有很好的细胞毒活性。
[0074]
试验例2:实施例25(b11)所示化合物在体外对硫氧还蛋白还原酶相关酶活性抑制数据
[0075]
实验方法:按照文献“duan,d.;zhang,b.;yao,j.;liu,y.;sun,j.;ge,c.;peng,s.;fang,j.;free radical biol.med.2014,69,15-25”的方法测定实施例19在体外对各种酶活性的抑制情况(u498c trxr:该酶是将trxr的498位的sec突变为cys。gr:谷胱甘肽还原
酶,该酶是一种与trxr拥有相似结构的酶,同时也是谷胱甘肽系统的重要组成部分。gpx:谷胱甘肽过氧化物酶,是一种含硒半胱氨酸的酶)。
[0076]
实验结果:实验结果如图2所示,实施例25所示化合物(b11)通过选择性作用硒半胱氨酸来抑制硫氧还蛋白还原酶的活性。
[0077]
试验例3:实施例25(b11)所示化合物抑制hela细胞生长进行的划痕实验结果数据
[0078]
实验方法:在细胞培养皿均匀种植一定数量的hela细胞,用微量枪头在中央区域进行划线标记,之后去除中央区域的细胞,然后用不用浓度的化合物培养细胞至实验设定的时间,最后取出细胞培养皿,显微镜下面观察细胞并拍照。
[0079]
实验结果:实验结果如图3所示,实施例25所示化合物(b11)可以很好的抑制肿瘤细胞(hela细胞)的生长。
相关技术
网友询问留言
已有0条留言
- 还没有人留言评论。精彩留言会获得点赞!
1