环甘露聚糖及其制备方法
1.本发明属于生物医药领域,特别涉及环甘露聚糖及其制备方法。
背景技术:
2.环状聚糖具有独特的主体特性,能够与多种客体分子形成稳定的超分子复合物。此外,环状聚糖具有多个游离羟基,可以对羟基进行共价修饰从而改变其主体特性。天然存在的环状聚糖种类有限,大多是酶促降解多糖的结果或微生物表达的产物。其中,环糊精是最常见的天然环糖,其疏水空腔可以捕获多种客体分子,形成稳定的包合物。这种独特的性质使其广泛应用于医药、分析化学、材料化学和食品等领域。在医药领域,鉴于环糊精中等大小的疏水空腔及优异的生物相容性,环糊精及其衍生物被用于小分子药物的递送,以提高药效,降低药物的毒副作用。
3.环状聚糖能够根据其内部空腔的大小和形状识别不同的分子,因此不同类型和不同大小的环状聚糖可能具有不同于环糊精的特性及功能。环状多糖有可能具有容纳生物大分子药物等多种不同于环状寡糖的性质及潜在应用,但由于缺乏足量纯净的环状多糖,迄今对结构明确的环状多糖的功能了解甚少。因此,获得足量纯净的环状多糖是研究其空间构象及药物高效递送等应用的关键。
4.化学合成是大量制备结构明确的天然或非天然环状聚糖的重要途径,主要包括如下两种策略:(1)长链线性聚糖的环糖苷化,即预先制备所需数目的长链线性环化前体,随后发生环糖苷化反应。(2)寡糖单体的环缩聚反应,即寡糖单体进行低聚化随后发生环化反应,一锅法得到多种大小不同的环状聚糖。其中,寡糖单体的环缩聚方法可以一锅法得到多种大小不同的环状聚糖,效率较高,但环缩聚的程度通常不可控,且生成的环状聚糖极性接近,分离纯化较为困难,目前这种方法适用于中等大小的环状聚糖的合成。stoddart等人利用双官能团化二糖单体的环缩聚反应获得了目前为止人工合成的最大环状聚糖——由d-鼠李糖和l-鼠李糖交替组成的环14糖。长链线性前体的大环化方法主要得到一种预定数目的环状聚糖,但面临环化前体制备步骤冗长、环糖苷化收率低、立体选择性差等难题,尤其是超过10个糖基的超大环状多糖的合成更是具有较大的挑战性。
技术实现要素:
5.针对上述现有技术存在的问题,本发明的目的在于提供了结构明确的环甘露聚糖及其制备方法。
6.为了实现上述目的,本发明采用如下技术方案:
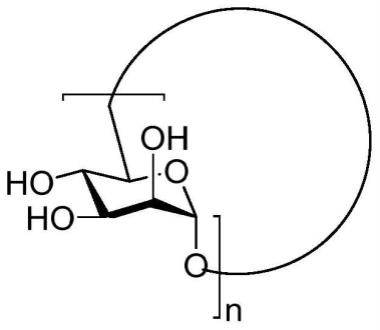
7.本发明的第一方面提供了环甘露聚糖,所述环甘露聚糖的结构通式如下式(ⅰ)所示:
[0008][0009]
其中,n独立地取自1~32的整数。
[0010]
本发明的第二方面提供了上述环甘露聚糖的制备方法,包括以下步骤:
[0011]
s1、通过以下两种方式构建环甘露聚糖骨架:(1)通过将双官能团化的甘露糖(z)-炔烯酸酯前体进行基于金催化的环糖苷化反应,立体选择性地构建环-α-1,6-糖苷键,制备环甘露聚糖骨架;或者(2)通过将双官能团化的甘露糖硫苷线性前体进行基于碘离子活化的环糖苷化反应,立体选择性地构建环-α-1,6-糖苷键,制备环甘露聚糖骨架;
[0012]
s2、将构建的环甘露聚糖骨架进行脱保护基处理,得到环甘露聚糖。
[0013]
优选地,步骤s1中,所述双官能团化的甘露糖(z)-炔烯酸酯前体,通过以甘露糖硫苷为起始原料,在碘离子活化下与(z)-3-碘代丙烯酸发生糖苷化反应,然后与1-己炔发生sonogashira偶联反应,得到甘露糖炔烯酸酯,然后选择性脱除所得甘露糖炔烯酸酯c6位羟基的tbdps保护基而得到。
[0014]
优选地,步骤s1中,所述双官能团化的甘露糖硫苷线性前体,通过以甘露糖硫苷为起始原料,选择性脱除其c6位羟基的tbdps保护基而得到。
[0015]
优选地,步骤s1中,所述基于金催化的环糖苷化反应的条件:所用促进剂为pph3auotf、pph3auntf2、sphosauotf和sphosauntf2中的一种或者两者以上的组合;所用添加剂为tfoh或者tmsotf,所用溶剂为二氯甲烷或甲苯,并加入分子筛干燥剂除水,反应温度为-40℃~室温,反应时间为1~5h。
[0016]
优选地,在基于金催化的环糖苷化反应中,所述双官能团化的甘露糖(z)-炔烯酸酯前体的浓度为0.0001~0.01m,所述促进剂、添加剂以及双官能团化的甘露糖(z)-炔烯酸酯前体的摩尔比为(0.1~2.0):(0.1~0.3):1。
[0017]
优选地,步骤s1中,所述基于碘离子活化的环糖苷化反应的条件为:所用促进剂为nis/tmsotf、nis/tfoh和ibr/agotf中的任一种,所用溶剂为二氯甲烷,并加入分子筛干燥剂除水,反应温度为0℃~室温,反应时间为1~5h。
[0018]
优选地,在基于碘离子活化的环糖苷化反应中,所述双官能团化的甘露糖硫苷线性前体的浓度为0.0001~0.01m,所述促进剂为nis/tmsotf、nis/tfoh或者ibr/agotf中的任一种;当所述促进剂为nis/tmsotf或者nis/tfoh时,所述促进剂与双官能团化的甘露糖硫苷线性前体的摩尔比为((1~10)/(0.1~0.3)):1;当所述促进剂为ibr/agotf时,所述促进剂与双官能团化的甘露糖硫苷线性前体的摩尔比为((1~10)/(1~24)):1。
[0019]
优选地,步骤s2中,所述脱保护基处理为:将得到的环甘露聚糖骨架依次进行皂化反应和氢化反应,其中,进行皂化反应用于脱除所述环甘露聚糖骨架中的酯基,其反应条件为:以甲醇钠作为皂化试剂,以二氯甲烷与甲醇的混合物作为溶剂,室温下反应12~72h;进
行氢化反应用于脱除所述环甘露聚糖骨架中的芳香基团,其反应条件为:以pd/c作为催化剂,以四氢呋喃、水的混合物作为溶剂,氢气氛围下室温反应24~72h。
[0020]
本发明具备如下有益效果:
[0021]
(1)本发明提供了一种环甘露聚糖的制备方法,具体地,通过两种方式构建环甘露聚糖骨架:将双官能团化的甘露糖(z)-炔烯酸酯前体在高稀释浓度(0.0001~0.01m)下进行基于金催化的环糖苷化反应,立体选择性地构建环-α-1,6-糖苷键,制备环甘露聚糖骨架;或者通过将双官能团化的甘露糖硫苷线性前体在高稀释浓度(0.0001~0.01m)下进行基于碘离子活化的环糖苷化反应,立体选择性地构建环-α-1,6-糖苷键,制备环甘露聚糖骨架;然后将构建的环甘露聚糖骨架进行脱保护基处理,即得到环甘露聚糖。该制备方法操作简单,通用性强,可以实现快速,高效,可靠地合成超大环状多糖。
[0022]
(2)本发明制备的环甘露聚糖结构明确,可以制作成小型环甘露聚糖分子库,其包含一系列由不同数量的重复单元组成的环甘露聚糖,为进一步研究其空间构象及在药物递送等方面的应用提供物质基础。
附图说明
[0023]
为了更清楚地说明本发明实施例中的技术方案,下面将对实施例中所需要使用的附图作简单地介绍,显而易见地,下面描述中的附图仅仅是本发明的一些实施例,对于本领域普通技术人员来讲,在不付出创造性劳动的前提下,还可以根据这些附图获得其他的附图。
[0024]
图1为本发明提供的环甘露聚糖的结构通式示意图;
[0025]
图2为双官能团化的甘露糖(z)-炔烯酸酯线性前体的合成路线图;
[0026]
图3为由双官能团化的甘露糖(z)-炔烯酸酯线性前体制备环甘露聚糖骨架的合成路线图;
[0027]
图4为双官能团化的甘露糖硫苷线性前体的合成路线图;
[0028]
图5为由双官能团化的甘露糖硫苷线性前体制备环甘露聚糖骨架的合成路线图;
[0029]
图6为由环甘露聚糖骨架制备环甘露聚糖的合成路线图。
具体实施方式
[0030]
以下描述中,为了说明而不是为了限定,提出了诸如特定系统结构、技术之类的具体细节,以便透彻理解本发明实施例。然而,本领域的技术人员应当清楚,在没有这些具体细节的其它实施例中也可以实现本发明。在其它情况中,省略对众所周知的系统、装置、电路以及方法的详细说明,以免不必要的细节妨碍本发明的描述。
[0031]
(一)在本发明中,提供了环甘露聚糖,其结构通式如下式(1)所示:
[0032][0033]
其中,n=2,4,8,16或32。
[0034]
上述环甘露聚糖的制备方法,包括以下步骤:
[0035]
s1、通过以下两种方式构建环甘露聚糖骨架:
[0036]
(1)、将双官能团化的甘露糖(z)-炔烯酸酯前体进行基于金催化的环糖苷化反应,立体选择性地构建环-α-1,6-糖苷键,制备环甘露聚糖骨架;其合成路线如图3所示;
[0037]
其中,基于金催化的环糖苷化反应的条件:所用促进剂为pph3auotf、pph3auntf2、sphosauotf和sphosauntf2中的一种或者两者以上的组合;所用添加剂为tfoh或者tmsotf,所用溶剂为二氯甲烷或甲苯,并加入分子筛干燥剂除水,反应温度为-40℃~室温;双官能团化的甘露糖(z)-炔烯酸酯前体的浓度为0.0001m~0.01m,促进剂、添加剂以及双官能团化的甘露糖(z)-炔烯酸酯前体的摩尔比为(0.1~2.0):(0.1~0.3):1;
[0038]
双官能团化的甘露糖(z)-炔烯酸酯线性前体的合成路线如图2所示,具体步骤为:将甘露糖硫苷与(z)-3-碘代丙烯酸在碘离子活化下发生糖苷化反应,然后与1-己炔发生sonogashira偶联反应,得到甘露糖炔烯酸酯,然后选择性脱除所得甘露糖炔烯酸酯c6位羟基的tbdps保护基,即得到双官能团化的甘露糖(z)-炔烯酸酯前体。
[0039]
或者(2)、将双官能团化的甘露糖硫苷线性前体进行基于碘离子活化的环糖苷化反应,立体选择性地构建环-α-1,6-糖苷键,制备环甘露聚糖骨架;具体合成路线如图5所示。
[0040]
其中,基于碘离子活化的环糖苷化反应的条件为:所用促进剂为nis/tmsotf、nis/tfoh和ibr/agotf中的任一种,所用溶剂为二氯甲烷,并加入分子筛干燥剂除水,反应温度为0℃~室温;双官能团化的甘露糖硫苷线性前体的浓度为0.0001m~0.01m,促进剂为nis/tmsotf、nis/tfoh或者ibr/agotf中的任一种;当促进剂为nis/tmsotf或者nis/tfoh时,促进剂与双官能团化的甘露糖硫苷线性前体的摩尔比为((1~10)/(0.1~0.3)):1;当促进剂为ibr/agotf时,促进剂与双官能团化的甘露糖硫苷线性前体的摩尔比为((1~10)/(1~24)):1;
[0041]
双官能团化的甘露糖硫苷线性前体的合成路线如图4所示,具体步骤为:以甘露糖硫苷为起始原料,选择性脱除甘露糖硫苷c6位tbdps保护基,即得到双官能团化的甘露糖硫苷前体。
[0042]
s2、将构建的环甘露聚糖骨架进行脱保护基处理,得到环甘露聚糖,具体合成路线如图6所示,具体步骤为:将得到的环甘露聚糖骨架依次进行皂化反应和氢化反应,然后经纯化,得到环甘露聚糖;其中,进行皂化反应用于脱除环甘露聚糖骨架中的酯基,其反应条件为:以甲醇钠作为皂化试剂,以二氯甲烷与甲醇的混合物作为溶剂,室温下反应12~72h;
进行氢化反应用于脱除环甘露聚糖骨架中的芳香基团,其反应条件为:以pd/c作为催化剂,以四氢呋喃、水的混合物作为溶剂,氢气氛围下室温反应24~72h,得到环甘露聚糖。
[0043]
实施例1
[0044]
环甘露二糖26的制备方法,包括以下步骤:
[0045]
(1)制备甘露二糖炔烯酸酯线性前体11:
[0046]
(a)化合物6的制备:将化合物1(52.0mg,0.040mmol)和(z)-3-碘代丙烯酸(10.0mg,0.048mmol)溶于无水ch2cl2(2ml)中,加入ms(90mg),室温搅拌30min。加入nis(11.0mg,0.048mmol)和tfoh的ch2cl2溶液(0.1ml,0.06m),室温搅拌30min,tlc显示反应完全后,将反应液用et3n淬灭,硅藻土过滤,滤液用饱和na2s2o3溶液洗涤,ch2cl2萃取3次,合并有机相,饱和nacl溶液洗涤,无水na2so4干燥,滤除干燥剂,浓缩,粗品经硅胶柱层析(petroleum ether/etoac=10/1)纯化,得到白色固体(48.0mg,90%);将得到的白色固体(46.5mg,0.035mmol)溶于无水ch3cn/thf(3ml,v/v,2/1),加入pd(pph3)2cl2(3.0mg,0.004mmol)和cui(1.0mg,0.006mmol)。反应液用氩气置换空气5次,室温下依次加入et3n(10μl,0.070mmol)和1-己炔(5.0μl,0.042mmol),室温搅拌1h,tlc显示反应完全后,反应液用饱和nh4cl溶液淬灭,ch2cl2萃取3次,合并有机相,饱和nacl溶液洗涤,无水na2so4干燥,滤除干燥剂,浓缩,粗品经硅胶柱层析(petroleum ether/etoac=15/1)纯化,得到白色固体6(40.2mg,90%)。1h nmr(400mhz,cdcl3)δ8.18-8.15(m,4h),7.76(dd,j=1.2,8.0hz,2h),7.56-7.49(m,4h),7.41-7.20(m,20h),7.13-7.06(m,8h),6.39(d,j=2.0hz,1h),6.29(dt,j=2.4,11.6hz,1h),6.02(d,j=11.6hz,1h),5.84(dd,j=2.0,2.8hz,1h),5.73(dd,j=2.4,3.2hz,1h),5.14(d,j=1.6hz,1h),4.94(d,j=11.2hz,1h),4.88(d,j=10.8hz,1h),4.83(d,j=10.8hz,1h),4.79(d,j=11.2hz,1h),4.63(d,j=10.8hz,1h),4.53(t,j=11.6hz,2h),4.39(d,j=11.2hz,1h),4.23(t,j=9.6hz,1h),4.17(dd,j=3.2,9.2hz,1h),4.08(dd,j=9.6,3.2hz,1h),4.03(d,j=9.6hz,1h),3.99-3.97(m,2h),3.90(dd,j=2.8,11.2hz,1h),3.78(d,j=11.2hz,1h),3.74(d,j=10.4hz,1h),3.65(d,j=9.6hz,1h),2.41-2.35(m,2h),1.53-1.44(m,2h),1.34-1.29(m,2h),1.10(s,9h),0.84(t,j=7.2hz,3h);
13
c nmr(100mhz,cdcl3)δ165.8,165.6,162.3,138.8,138.3,137.9,137.8,136.0,135.8,133.7,133.6,133.3,133.2,130.2,130.1,129.8,129.7,128.8,128.5,128.4,128.3,127.9,127.8,127.7,127.6,127.5,126.1,126.0,106.4,98.7,91.7,78.3,78.2,77.4,75.5,75.3,74.0,73.7,73.5,72.9,71.9,71.5,68.9,67.9,65.7,62.7,30.5,27.0,22.1,20.0,19.5,13.7;hrms(esi)m/z calcd for c
79h82o14
sina[m+na]
+
1305.5372;found 1305.5371.
[0047]
(b)化合物11的制备:将得到的化合物6(256.5mg,0.2mmol)溶于thf(4ml),滴加hf
·
py(0.4ml),室温条件下搅拌过夜,tlc显示反应完全后,反应液用饱和nahco3溶液淬灭,乙酸乙酯萃取3次,合并有机层,饱和nacl溶液洗涤,无水na2so4干燥,滤除干燥剂,浓缩,粗品经硅胶柱层析(petroleum ether/etoac=4/1)纯化,得到白色固体,即化合物11(185.0mg,89%)。(185.0mg,89%)。1h nmr(400mhz,cdcl3)δ8.17-8.15(m,2h),8.09(d-like,j=7.2hz,2h),7.60(t,j=7.2hz,1h),7.53-7.51(m,3h),7.48(t,j=7.6hz,2h),7.36-7.08(m,20h),6.38(d,j=2.0hz,1h),6.28(dt,j=2.4,11.2hz,1h),6.02(d,j=
11.2hz,1h),5.77(t,j=2.4hz,1h),5.73(t,j=2.4hz,1h),5.10(d,j=1.2hz,1h),4.92-4.85(m,3h),4.75(d,j=11.6hz,1h),4.62(d,j=10.8hz,1h),4.56(d,j=11.2hz,1h),4.49(d,j=11.2hz,1h),4.44(d,j=11.2hz,1h),4.18(dd,j=3.2,8.4hz,1h),4.08(dd,j=3.2,9.6hz,1h),4.03-3.93(m,4h),3.78-3.72(m,3h),3.67-3.63(m,1h),2.41-2.35(m,2h),1.87(s,1h),1.52-1.44(m,2h),1.37-1.31(m,2h),0.85(t,j=7.2hz,3h);
13
c nmr(100mhz,cdcl3)δ165.7,165.6,162.3,138.5,138.4,137.8,133.6,133.4,130.1,130.0,129.8,128.9,128.7,128.6,128.5,128.3,128.2,128.0,127.8,126.2,126.0,106.5,98.5,91.7,78.3,78.2,77.8,75.6,75.3,74.0,73.7,73.4,72.4,72.0,71.4,68.7,67.9,65.9,62.1,30.5,22.1,20.0,13.8;hrms(esi)m/z[m+na]
+
calcd for c
63h64o14
na 1067.4194;found 1067.4191.
[0048]
(2)环甘露二糖骨架21的制备:
[0049]
将化合物11(62.7mg,0.06mmol)溶于无水ch2cl2(60ml),加入新活化的ms(100mg),室温搅拌30min,然后加入新鲜制备的ph3pauotf的ch2cl2溶液(60μl,0.1m)和tfoh的ch2cl2溶液(60μl,0.1m),室温搅拌3h,tlc显示反应完全后,将反应液用et3n淬灭,硅藻土滤除分子筛,浓缩,粗品经硅胶柱层析(petroleum ether/etoac=12/1
→
10/1)纯化,得到白色固体,即环甘露二糖骨架21(36.0mg,67%)和化合物22(12.4mg,23%)。
[0050]
(3)环甘露二糖26的制备:
[0051]
将得到的化合物21(50mg,0.056mmol)溶于ch2cl2/meoh的混合溶剂(4ml,v/v,1/1)中,加入甲醇钠(108mg,2mmol),室温搅拌24h,反应液用氢离子树脂调节ph至中性,过滤,浓缩,硅胶柱层析(petroleum ether/etoac=2/1
→
ch2cl2/meoh=60/1)纯化,得到白色皂化产物(33.4mg,87%)。将上述得到的白色皂化产物(15mg,0.02mmol)溶于thf/h2o的混合溶剂中(3.3ml,v/v,10/1),加入10% pd/c(15mg),氢气置换5次,室温反应48h后,tlc监测显示原料反应完全,过滤,浓缩,依次用氯离子树脂(h2o)及反相c18硅胶柱层析(meoh/h2o=10/90)纯化,得到白色固体,即环甘露二糖26(5.4mg,83%)。1h nmr(400mhz,d2o)δ4.87(s,2h),4.27(br s,2h),3.95(m,4h),3.74(s,2h),3.60-3.58(m,4h);
13
c nmr(150mhz,d2o)δ101.9,73.8,73.4,70.7,70.2,68.0;hrms(esi)m/z[m+na]
+
calcd for c
12h20o10
na347.0954;found 347.0958.
[0052]
实施例2
[0053]
环甘露二糖26的制备方法,包括以下步骤:
[0054]
(1)甘露二糖硫苷线性前体16的制备:
[0055]
将化合物1(9.83g,7.5mmol)溶于无水thf(15ml),加入hf
·
py(22.5ml),室温搅拌24h,tlc显示反应完全后,将反应液用饱和nahco3溶液淬灭,etoac萃取3次,合并有机相,用饱和nacl溶液洗涤,无水na2so4干燥,滤除干燥剂,浓缩,粗品经硅胶柱层析(petroleum ether/etoac=10/1)纯化,得到白色泡沫状固体,即甘露二糖硫苷线性前体16(6.74g,84%)。84%)。8.07-8.05(m,2h),7.58(t,j=7.6hz,1h),7.53(d,j=2.0hz,1h),7.49-7.44(m,5h),7.37-7.35(m,2h),7.31-7.18(m,16h),7.15-7.06(m,4h),5.95(dd,j=2.0,2.8hz,1h),5.75(m,1h),5.53(d,j=1.2hz,1h),5.06(d,j=1.6hz,1h),4.92-4.86(m,3h),4.73(d,j=11.2hz,1h),
7.26(m,14h),7.23-6.99(m,34h),6.38(d,j=2.0hz,1h),6.26(dt,j=2.4,11.6hz,1h),6.00(d,j=11.2hz,1h),5.83(br s,2h),5.79(m,1h),5.74(t-like,j=2.4hz,1h),5.10-5.03(m,3h),4.93-4.87(m,4h),4.83-4.73(m,4h),4.60(d,j=10.8hz,1h),4.55(d,j=10.8hz,1h),4.48-4.36(m,5h),4.29-4.17(m,3h),4.08-3.90(m,8h),3.82-3.66(m,6h),3.61-3.50(m,3h),3.44(d,j=10.4hz,1h),2.43-2.31(m,2h),1.50-1.43(m,2h),1.34-1.26(m,2h),1.08(s,9h),0.83(t,j=7.2hz,3h);
13
c nmr(100mhz,cdcl3)δ165.8,165.7,165.6,162.2,138.8,138.6,138.5,138.4,137.8,137.7,136.0,135.8,133.6,133.4,133.3,133.2,130.2,130.1,130.0,129.8,129.7,128.8,128.7,128.5,128.4,128.3,128.1,128.0,127.8,127.7,127.5,127.4,127.3,126.1,125.9,106.4,98.6,98.5,91.7,78.4,78.3,78.2,77.4,75.5,75.3,75.1,74.0,73.9,73.8,73.7,73.3,72.6,71.9,71.5,71.4,71.2,71.0,68.9,68.5,67.9,66.0,65.9,65.6,62.6,30.5,27.0,22.1,20.0,19.5,13.7;hrms(esi)m/z calcd for c
133h134o26
sina[m+na]
+
2197.8830;found 2197.8833.
[0063]
(b)甘露四糖炔烯酸酯线性前体12的制备:将化合物7(232.4mg,0.107mmol)溶于thf(3ml),滴加hf
·
py(0.3ml),室温条件下搅拌过夜。tlc显示反应完全后,反应液用饱和nahco3溶液淬灭,乙酸乙酯萃取3次,合并有机层,饱和nacl溶液洗涤,无水na2so4干燥,滤除干燥剂,浓缩,粗品经硅胶柱层析(petroleum ether/etoac=4/1)纯化,得到白色固体,即甘露四糖炔烯酸酯线性前体12(157.8mg,76%)。1h nmr(400mhz,cdcl3)δ8.17-8.16(m,6h),8.09-8.07(m,2h),7.60(t,j=7.6hz,1h),7.51-7.45(m,10h),7.35-7.27(m,8h),7.22-7.05(m,33h),6.39(d,j=2.0hz,1h),6.27(dt,j=2.4,11.6hz,1h),6.01(d,j=11.6hz,1h),5.83(t-like,j=2.4hz,1h),5.80(t-like,j=2.8hz,1h),5.76(t-like,j=2.4hz,1h),5.74(t-like,j=2.8hz,1h),5.10(d,j=1.2hz,1h),5.04(br s,2h),4.91-4.87(m,5h),4.84-4.80(m,2h),4.72(d,j=11.2hz,1h),4.60(d,j=11.2hz,1h),4.55(d,j=10.8hz,1h),4.49-4.37(m,6h),4.20-4.18(m,1h),4.09-3.91(m,10h),3.82(dd,j=3.2,11.2hz,1h),3.77-3.73(m,3h),3.67(m,3h),3.59-3.54(m,2h),3.50(d,j=11.2hz,1h),2.43-2.33(m,2h),1.51-1.44(m,2h),1.36-1.29(m,2h),0.84(t,j=7.2hz,3h);
13
c nmr(100mhz,cdcl3)δ165.7,165.6,162.3,138.6,138.5,138.4,137.8,137.7,133.6,133.4,130.2,130.1,129.8,128.9,128.8,128.7,128.5,128.3,128.2,128.0,127.9,127.8,127.7,127.6,127.5,127.4,126.2,126.0,106.5,98.7,98.5,98.3,91.7,78.4,78.2,77.9,77.4,75.5,75.3,75.2,75.1,74.0,73.9,73.7,73.4,72.2,72.0,71.5,71.4,71.2,71.0,68.7,68.6,68.5,68.0,66.1,66.0,65.7,62.0,30.5,22.1,20.0,13.8;hrms(esi)m/z[m+na]
+
calcd for c
117h116o26
na 1959.7653;found 1959.7292.
[0064]
(2)环甘露四糖骨架22的制备:
[0065]
将化合物12(58.1mg,0.03mmol)溶于无水ch2cl2(30ml),加入新活化的ms(50mg),室温搅拌30min。加入新鲜制备的ph3pauotf的ch2cl2溶液(30μl,0.1m)和tfoh的ch2cl2溶液(30μl,0.1m),室温搅拌3h。反应液用et3n淬灭,硅藻土滤除分子筛,浓缩,粗品经硅胶柱层析(petroleum ether/etoac=5/1)纯化,得到白色固体,即环甘露四糖骨架22(49.2mg,92%)。1h nmr(600mhz,cdcl3)δ8.08(d,j=7.2hz,8h),7.57(t,j=7.2hz,4h),7.50(t,j=7.2hz,8h),7.31-7.28(m,20h),7.23-7.16(m,
20h),5.72(br s,4h),5.19(s,4h),4.88(d,j=10.8hz,4h),4.82(d,j=10.8hz,4h),4.56(d,j=10.8hz,4h),4.50(d,j=11.4hz,4h),4.07(dd,j=3.0,9.0hz,4h),4.04(d,j=12.6hz,4h),4.01-3.99(m,4h),3.95(dd,j=4.8,12.0hz,4h),3.75(t,j=9.6hz,4h);
13
c nmr(150mhz,cdcl3)δ165.8,138.5,137.9,133.3,130.1,130.0,128.7,128.5,128.4,127.8,127.7,98.9(1j
c1,h1
=171.6hz),78.2,75.1,74.4,72.8,71.5,68.9,67.6;hrms(maldi-tof)m/z[m+na]
+
calcd for c
108h104o24
na1807.6815;found 1807.4950.
[0066]
(3)环甘露四糖27的制备:
[0067]
将环甘露四糖骨架22(51.5mg,0.029mmol)溶于ch2cl2/meoh的混合溶剂(2ml,v/v,1/1)中,加入甲醇钠(54mg,1.0mmol),室温搅拌72h,将反应液用氢离子树脂调节ph至中性,过滤,浓缩,硅胶柱层析(ch2cl2/meoh=40/1)纯化,得到白色皂化产物(33.4mg,84%)。将上述得到的白色皂化产物(14mg,0.01mmol)溶于thf/h2o的混合溶剂中(3.3ml,v/v,10/1),加入10%pd/c(14mg),氢气置换5次,室温反应48h后,tlc监测显示原料反应完全,过滤,浓缩,依次用氯离子树脂(h2o),lh-20凝胶柱层析(h2o)及反相c18硅胶柱层析(meoh/h2o=10/90)纯化,得到白色固体,即环甘露四糖27(5.6mg,86%)。1h nmr(600mhz,d2o)δ4.25(s,4h),4.18-4.16(m,8h),3.95(dd,j=1.8,3.6hz,4h),3.81(dd,j=3.6,9.6hz,4h),3.75(t,j=10.8hz,4h),3.48(t,j=9.6hz,4h);
13
c nmr(150mhz,d2o)δ97.2,69.9,69.4,68.5,67.1;hrms(esi)m/z[m+na]
+
calcd for c
24h40o20
na 671.2011;found671.2013.
[0068]
实施例4
[0069]
环甘露四糖27的制备方法,包括以下步骤:
[0070]
(1)甘露四糖硫苷线性前体17的制备:
[0071]
将化合物2(4.19g,1.9mmol)溶于无水thf(4ml),加入hf
·
py(5ml),室温搅拌1h。tlc显示反应完全后,反应液用饱和nahco3溶液淬灭,etoac萃取4次,合并有机相,用饱和nacl溶液洗涤,无水na2so4干燥,滤除干燥剂,浓缩,粗品经硅胶柱层析(petroleum ether/etoac=4/1)纯化得白色泡沫状固体,即甘露四糖硫苷线性前体17(3.29g,88%)。1h nmr(400mhz,cdcl3)δ8.16-8.07(m,8h),7.59(t,j=7.6,1h),7.54-7.45(m,11h),7.36-7.06(m,43h),5.97(d,j=1.2hz,1h),5.83(s,1h),5.80(s,1h),5.76(d,j=1.6hz,1h),5.53(s,1h),5.07-5.02(m,3h),4.95-4.78(m,7h),4.72(d,j=11.6hz,1h),4.60(dd,j=3.2,10.8hz,2h),4.48-4.33(m,7h),4.15-4.01(m,6h),3.97-3.91(m,3h),3.80-3.61(m,7h),3.55-3.47(m,3h),2.40(s,3h),1.25(s,9h);
13
c nmr(100mhz,cdcl3)δ165.9,165.7,165.6,165.5,150.0,138.7,138.6,138.5,138.4,137.8,137.7,137.2,133.5,133.4,132.3,130.4,130.3,130.1,130.0,128.8,128.7,128.6,128.5,128.4,128.3,128.2,128.1,128.0,127.8,127.7,127.6,127.5,127.4,127.3,125.6,98.7,98.5,98.3,86.9,79.1,78.4,78.3,77.8,77.4,75.3,75.2,75.1,74.3,74.0,73.8,72.4,72.2,71.9,71.5,71.3,71.2,71.0,68.7,68.5,66.4,65.9,65.7,62.0,34.6,31.5,20.6;hrms(esi)m/z calcd for c
119h120o24
sna[m+na]
+
1987.7788;found 1987.7794.
[0072]
(2)环甘露四糖骨架22的制备:
[0073]
将甘露四糖硫苷线性前体17(39.3mg,0.02mmol)溶于无水ch2cl2(20ml),加入
agotf(15.4mg,0.06mmol)和新活化的ms(100mg),室温搅拌30min。室温下加入ibr的ch2cl2溶液(0.4ml,0.1m),室温反应3h。tlc显示反应完全后,反应液用硅藻土滤除分子筛,滤液用饱和na2s2o3溶液洗涤,ch2cl2萃取3次,合并有机相,依次用饱和nahco3溶液和饱和nacl溶液洗涤,无水na2so4干燥。滤除干燥剂,浓缩,粗品经硅胶柱层析(petroleum ether/etoac=5/1)纯化,得到白色固体,即环甘露四糖骨架22(27.8mg,78%)。
[0074]
(3)环甘露四糖27的制备:步骤同实施例3。
[0075]
实施例5
[0076]
环甘露八糖28的制备方法,包括以下步骤:
[0077]
(1)制备甘露八糖炔烯酸酯线性前体13:
[0078]
(a)化合物8的制备:将化合物3(260.0mg,0.065mmol)和(z)-3-碘代丙烯酸(15.4mg,0.078mmol)溶于无水ch2cl2(5ml),加入ms(50mg),室温搅拌30min,然后加入nis(17.5mg,0.078mmol)和tfoh的ch2cl2溶液(0.1ml,0.1m),室温搅拌30min,tlc显示反应完全后,反应液用et3n淬灭,硅藻土过滤,滤液用饱和na2s2o3溶液洗涤,ch2cl2萃取3次,合并有机相,饱和nacl溶液洗涤,无水na2so4干燥,滤除干燥剂,浓缩,粗品经硅胶柱层析(petroleum ether/etoac=3/1)纯化,得到白色固体(217.0mg,83%)。将上述得到的白色固体(160.0mg,0.04mmol)溶于无水ch3cn/thf(8ml,v/v,3/1),加入pd(pph3)2cl2(2.8mg,0.004mmol)和cui(1.0mg,0.005mmol)。反应液用氩气置换空气5次,室温下依次加入et3n(11μl,0.08mmol)和1-己炔(5.3μl,0.048mmol),室温搅拌1h。tlc显示反应完全后,反应液用饱和nh4cl溶液淬灭,ch2cl2萃取3次,合并有机相,用饱和nacl溶液洗涤,无水na2so4干燥。滤除干燥剂,浓缩,粗品经硅胶柱层析(petroleum ether/etoac=3/1)纯化得黄色固体,即化合物8(137.0mg,86%)。1h nmr(400mhz,cdcl3)δ8.16(m,16h),7.76-7.74(m,2h),7.67-7.65(m,2h),7.50-7.49(m,21h),7.38-7.23(m,20h),7.20-.99(m,69h),6.38(d,j=1.6hz,1h),6.25(dt,j=2.4,11.2hz,1h),5.99(d,j=11.2hz,1h),5.84(br s,5h),5.79(br s,1h),5.73(m,1h),5.09-5.05(m,6h),4.93-4.87(m,7h),4.82-4.73(m,8h),4.61(d,j=10.8hz,1h),4.54(d,j=11.2hz,1h),4.49-4.30(m,13h),4.28-4.17(m,3h),4.07-3.92(m,16h),3.79-3.37(m,25h),2.42-2.31(m,2h),1.48-1.42(m,2h),1.33-1.26(m,2h),1.09(s,9h),0.82(t,j=7.2hz,3h);
13
c nmr(150mhz,cdcl3)δ165.8,165.7,165.6,162.3,138.9,138.7,138.6,138.4,137.9,137.8,137.8,137.7,136.1,135.8,133.7,133.6,133.5,133.3,130.2,130.1,130.0,129.8,129.7,128.9,128.8,128.5,128.4,128.3,128.2,128.0,1279,127.8,127.7,127.6,127.5,127.4,127.3,127.2,126.1,126.0,106.4,98.7,98.6,91.7,78.4,78.4,78.2,78.2,75.5,75.4,75.2,74.0,73.9,73.8,73.7,73.4,72.7,72.0,71.5,71.4,71.3,71.1,68.9,68.6,68.5,68.0,66.0,65.9,65.7,65.6,62.6,30.5,27.0,22.1,20.0,19.5,13.8;hrms(maldi-tof)m/z calcd for c
241h238o50
sina[m+na]
+
3982.5748;found3984.5271.
[0079]
(b)甘露八糖炔烯酸酯线性前体13的制备:将化合物8(307.0mg,0.078mmol)溶于thf(2ml),滴加hf
·
py(0.4ml),室温条件下搅拌过夜。反应液用饱和nahco3溶液淬灭,乙酸乙酯萃取,合并有机层,饱和nacl溶液洗涤,无水na2so4干燥,过滤,浓缩,硅胶柱层析(petroleum ether/etoac=3/1)纯化得白色固体,即甘露八糖炔烯酸酯线性前体13
(251.7mg,87%)。(251.7mg,87%)。1h nmr(600mhz,cdcl3)δ8.18-8.15(m,14h),8.08(d,j=7.2hz,2h),7.59(t,j=7.2hz,1h),7.51-7.45(m,23h),7.34(d,j=12.2hz,2h),7.30-7.28(m,4h),7.23-7.06(m,74h),6.38(br s,1h),6.26(dt,j=2.4,11.4hz,1h),6.00(d,j=12.0hz,1h),5.84-5.83(m,5h),5.80(br s,1h),5.77(br s,1h),5.73(br s,1h),5.09(s,1h),5.04(m,6h),4.89-4.88(m,8h),4.85(d,j=11.4hz,1h),4.82-4.78(m,6h),4.72(d,j=10.8hz,1h),4.59(d,j=10.8hz,1h),4.54(d,j=11.4hz,1h),4.47(d,j=11.4hz,1h),4.45-4.40(m,8h),4.37(d,j=9.6hz,1h),4.35-4.32(m,4h),4.18(dd,j=3.0,8.4hz,1h),4.07-3.92(m,18h),3.82-3.70(m,8h),3.65-3.58(m,8h),3.53(dt,j=3.0,9.6hz,1h),3.48(d,j=11.4hz,1h),3.45-3.42(m,4h),2.41-2.33(m,2h),1.49-1.44(m,2h),1.34-1.29(m,2h),0.83(t,j=7.2hz,3h);
13
c nmr(150mhz,cdcl3)δ165.7,165.6,162.3,138.7,138.6,138.5,138.4,137.8,137.7,133.6,133.5,133.4,130.1,130.0,129.8,128.9,128.8,128.7,128.5,128.4,128.3,128.2,128.0,127.9,127.8,127.7,127.6,127.5,127.4 127.3,127.2,126.2,126.0,106.5,98.7,98.6,98.3,91.7,78.4,78.2,77.8,75.5,75.4,75.3,75.2,74.0,73.9,73.8,73.7,73.4,72.3,72.0,71.5,71.4,71.2,71.0,68.7,68.6,68.5,68.0,66.0,65.9,65.8,65.6,62.0,30.5,22.1,20.0,13.8;hrms(maldi-tof)m/z[m+na]
+
calcd for 3744.4570;found 3744.7039.
[0080]
(2)环甘露八糖骨架23的制备:
[0081]
将甘露八糖炔烯酸酯线性前体13(93.0mg,0.025mmol)溶于无水ch2cl2(25ml),加入新活化的ms(100mg)),室温搅拌30min。加入新鲜制备的ph3pauotf的ch2cl2溶液(50μl,0.1m)和tfoh的ch2cl2溶液(25μl,0.1m),室温搅拌3h,反应液用et3n淬灭,硅藻土滤除分子筛,浓缩,粗品经硅胶柱层析(petroleum ether/etoac=3/1)纯化得白色固体,即环甘露八糖骨架23(82.1mg,92%)。1h nmr(600mhz,cdcl3)δ8.14-8.12(m,16h),7.52-7.51(m,24h),7.21-7.16(m,40h),7.15-7.08(m,40h),5.78(dd,j=1.8,3.0hz,8h),5.05(d,j=1.2hz,8h),4.81(d,j=11.4hz,8h),4.77(d,j=10.8hz,8h),4.43(d,j=11.4hz,8h),4.39(d,j=11.4hz,8h),3.99(dd,j=3.0,9.6hz,8h),3.88(t,j=9.6hz,8h),3.72-3.68(m,16h),3.52(d,j=10.8hz,8h);
13
c nmr(150mhz,cdcl3)δ165.7,138.7,137.8,133.4,130.1,130.0,128.8,128.5,128.4,127.8,127.6,98.3(1j
c1,h1
=171.5hz),78.3,75.0,74.1,71.5,71.4,68.6,65.7;hrms(maldi-tof)m/z[m+na]
+
calcd for c
216h208o48
na 3592.3733;found 3592.7759.
[0082]
(3)环甘露八糖28的制备:
[0083]
将环甘露八糖骨架23(47.2mg,0.013mmol)溶于ch2cl2/meoh的混合溶剂(1ml,v/v,1/1)中,加入甲醇钠(47mg,0.87mmol),室温搅拌72h。反应液用氢离子树脂调节ph至中性,过滤,浓缩,硅胶柱层析(ch2cl2/meoh=60/1)纯化得白色皂化产物(31.3mg,88%)。将上述皂化产物(27.3mg,0.01mmol)溶于thf/h2o的混合溶剂中(3.3ml,v/v,10/1),加入10% pd/c(27.3mg),氢气置换5次,室温反应48h后,tlc监测显示原料反应完全,过滤,浓缩,经lh-20凝胶柱层析(h2o)纯化得白色固体,即环甘露八糖28(10.0mg,77%)。1h nmr(400mhz,d2o)δ4.89(s,8h),3.98(dd,j=1.6,3.2hz,8h),3.93-3.89(m,16h),3.82-3.78(m,16h),3.70(t,j=9.6hz,8h);
13
c nmr(150mhz,d2o)δ
99.1,70.9,70.7,69.9,66.6,65.5;hrms(esi)m/z[m+na]
+
calcd for c
48h80o40
na 1319.4124;found 1319.4126.
[0084]
实施例6
[0085]
环甘露八糖28的制备方法,包括以下步骤:
[0086]
(1)甘露八糖硫苷线性前体18的制备:
[0087]
将化合物3(2.85g,0.71mmol)溶于无水thf(4ml),加入hf
·
py(6ml),室温搅拌5.5h。tlc显示反应完全后,反应液用饱和nahco3溶液淬灭,etoac萃取4次,合并有机相,用饱和nacl溶液洗涤,无水na2so4干燥,滤除干燥剂,浓缩,粗品经硅胶柱层析(petroleum ether/etoac=3/1)纯化,得到白色泡沫状固体,即甘露八糖硫苷线性前体18(2.22g,83%)。83%)。1h nmr(400mhz,cdcl3)δ8.18-8.13(m,13h),8.08(d,j=7.6hz,2h),7.58(t,j=7.6hz,1h),7.52-7.44(m,23h),7.36-7.34(m,2h),7.31-7.08(m,82h),5.96(s,1h),5.84-5.78(m,7h),5.52(s,1h),5.05-5.02(m,7h),4.94-4.87(m,8h),4.81-4.78(m,6h),4.72(d,j=11.2hz,1h),4.60(dd,j=2.8,11.2hz,2h),4.47-4.31(m,15h),4.15-3.91(m,17h),3.79-3.42(m,23h),2.39(s,3h),1.24(s,9h);
13
c nmr(100mhz,cdcl3)δ165.9,165.7,165.6,165.5,150.0,138.7,138.6,138.5,137.8,137.7,137.6,137.2,133.5,132.3,130.4,130.3,130.2,130.1,130.0,128.8,128.7,128.6,128.5,128.3,128.2,128.1,128.0,127.9,127.8,127.7,127.6,127.5,127.4,127.3,127.2,125.6,98.7,98.6,98.3,86.9,79.2,78.4,77.8,77.4,75.4,75.3,75.2,74.3,74.0,73.9,72.4,72.2,71.9,71.5,71.3,71.2,71.1,68.7,68.5,66.3,65.9,65.6,62.0,34.6,31.5,20.6;hrms(maldi-tof)m/z calcd for c
227h224o48
sna[m+na]
+
3772.4705;found 3771.0032.
[0088]
(2)环甘露八糖骨架23的制备:
[0089]
将甘露八糖硫苷线性前体18(37.4mg,0.01mmol)溶于无水ch2cl2(10ml),加入agotf(7.7mg,0.03mmol)和新活化的ms(50mg),室温搅拌30min。室温下加入ibr的ch2cl2溶液(0.1ml,0.2m),室温反应3h,tlc显示反应完全后,反应液用硅藻土滤除分子筛,滤液用饱和na2s2o3溶液洗涤,ch2cl2萃取3次,合并有机相,依次用饱和nahco3溶液和饱和nacl溶液洗涤,无水na2so4干燥,滤除干燥剂,浓缩,粗品经硅胶柱层析(petroleum ether/etoac=3/1)纯化得白色固体,即环甘露八糖骨架23(32.1mg,90%)。
[0090]
(3)环甘露八糖28的制备:步骤同实施例5。
[0091]
实施例7
[0092]
环甘露十六糖29的制备方法,包括以下步骤:
[0093]
(1)制备甘露十六糖炔烯酸酯线性前体14:
[0094]
(a)化合物9的制备:将化合物4(377.8mg,0.05mmol)和(z)-3-碘代丙烯酸(14.8mg,0.075mmol)溶于无水ch2cl2(2.5ml),加入agotf(39.0mg,0.15mmol)和ms(100mg),室温搅拌30min。加入ibr的ch2cl2溶液(1ml,0.1m),室温搅拌2h。tlc显示反应完全后,反应液用et3n淬灭,硅藻土过滤,滤液用ch2cl2稀释,饱和na2s2o3溶液洗涤,ch2cl2萃取3次,合并有机相,饱和nacl溶液洗涤,无水na2so4干燥,滤除干燥剂,浓缩,粗品经硅胶柱层析(petroleum ether/etoac=3/1
→
2/1)纯化,得到白色固体(340.7mg,90%);将得到的白色
固体(48.0mg,0.006mmol)溶于无水ch3cn/thf(2ml,v/v,1/1),加入pd(pph3)2cl2(2.0mg,0.003mmol)和cui(1.0mg,0.005mmol)。反应液用氩气置换空气5次,室温下依次加入et3n(3.0μl,0.022mmol)和1-己炔(2.0μl,0.018mmol),室温搅拌1h。tlc显示反应完全后,反应液用饱和nh4cl溶液淬灭,ch2cl2萃取3次,合并有机相,用饱和nacl溶液洗涤,无水na2so4干燥。滤除干燥剂,浓缩,粗品经硅胶柱层析(petroleum ether/etoac=3/1
→
2/1)纯化,得到白色固体,即化合物9(42.5mg,94%)。白色固体,即化合物9(42.5mg,94%)。1h nmr(400mhz,cdcl3)δ8.19-8.18(m,32h),7.77(d,j=6.8hz,2h),7.67(d,j=6.8hz,2h),7.53-7.50(m,42h),7.41-7.27(m,16h),7.23-6.98(m,156h),6.39(d,j=2.0hz,1h),6.27(dt,j=2.4,11.6hz,1h),6.01(d,j=11.2hz,1h),5.86(br s,12h),5.81(br s,1h),5.75(m,1h),5.11-5.06(m,15h),4.95-4.87(m,15h),4.84-4.75(m,16h),4.63(d,j=11.2hz,1h),4.56(d,j=10.8hz,1h),4.50-4.42(m,16h),4.39-4.19(m,16h),4.08-3.93(m,32h),3.83-3.39(m,49h),2.41-2.35(m,2h),1.51-1.44(m,2h),1.36-1.33(m,2h),1.11(s,9h),0.84(t,j=7.2hz,3h);
13
c nmr(150mhz,cdcl3)δ165.8,165.7,162.3,138.9,138.7,138.6,138.4,137.8,137.7,136.1,135.8,133.7,133.6,133.5,133.4,130.2,130.0,129.8,129.8,129.7,128.8,128.5,128.3,128.2,128.0,127.9,127.8,127.7,127.6,127.5,127.4,127.3,127.2,126.2,126.0,106.4,98.7,98.6,91.7,78.4,75.5,75.4,75.2,74.0,73.9,73.7,73.4,72.7,72.0,71.5,71.0,68.9,68.5,68.0,65.9,30.5,27.0,22.1,20.0,19.5,13.7;hrms(maldi-tof)m/z calcd for c
457h447o98
si[m+h]
+
7529.9685;found 7529.6162.
[0095]
(b)甘露十六糖炔烯酸酯线性前体14的制备:将化合物9(90.3mg,0.012mmol)溶于thf(5ml),滴加hf
·
py(0.5ml),室温条件下搅拌过夜,将反应液用饱和nahco3溶液淬灭,乙酸乙酯萃取,合并有机层,饱和nacl溶液洗涤,无水na2so4干燥。过滤,浓缩,硅胶柱层析(petroleum ether/etoac=2/1)得到白色固体,即甘露十六糖炔烯酸酯线性前体14(68.0mg,78%)。(68.0mg,78%)。1h nmr(400mhz,cdcl3)δ8.19-8.17(m,30h),8.07(d,j=8.0hz,2h),7.59(t,j=7.6hz,2h),7.51-7.50(m,46h),7.33-7.29(m,8h),7.20-7.08(m,152h),6.39(br s,1h),6.27(d-like,j=11.6hz,1h),6.01(d,j=11.6hz,1h),5.85-5.78(m,15h),5.74(br s,1h),5.10(s,1h),5.05(s,14h),4.90-4.78(m,31h),4.73(d,j=11.6hz,1h),4.60(d,j=11.2hz,1h),4.55(d,j=10.8hz,1h),4.49-4.32(m,30h),4.19(d-like,j=6.4hz,1h),4.08-3.92(m,32h),3.80-3.41(m,48h),2.43-2.33(m,2h),1.51-1.45(m,2h),1.34-1.30(m,2h),0.84(t,j=7.2hz,3h);
13
c nmr(150mhz,cdcl3)δ165.7,165.6,165.5,162.3,138.7,138.6,138.5,138.4,137.8,137.7,137.6,133.6,133.5,133.4,130.2,130.1,130.0,129.8,128.9,128.8,128.7,128.5,128.4,128.3,128.2,128.1,128.0,127.9,127.8,127.7,127.6,127.5,127.4,127.3,127.2,126.2,126.0,106.4,98.7,98.6,98.3,91.7,78.4,78.2,77.8,75.5,75.4,75.3,75.2,74.0,73.9,73.8,73.7,73.4,72.3,72.0,71.5,71.4,71.3,71.0,70.8,68.7,68.6,68.5,68.0,66.0,65.9,65.8,65.6,62.0,30.5,22.1,20.0,13.8;hrms(maldi-tof)m/z[m+na]
+
calcd for c
441h428o98
na 7319.1668;found 7317.2080.
[0096]
(2)环甘露十六糖骨架24的制备:
[0097]
将甘露十六糖炔烯酸酯线性前体14(29.2mg,0.004mmol)溶于无水甲苯(4ml),加
入新活化的ms(50mg),室温搅拌30min。0℃下加入新鲜制备的ph3pauotf的ch2cl2溶液(60μl,0.04m)和tfoh的ch2cl2溶液(20μl,0.02m),逐渐升至室温反应3h,tlc显示反应完全后,将反应液用et3n淬灭,硅藻土滤除分子筛,浓缩,硅胶柱层析(petroleum ether/etoac=3/1)纯化,得到白色固体24(14.8mg,52%)。1h nmr(600mhz,cdcl3)δ8.15-8.13(m,32h),7.50-7.45(m,48h),7.18-7.05(m,160h),5.79(t,j=1.8hz,16h),5.04(s,16h),4.84(d,j=11.4hz,16h),4.76(d,j=10.8hz,16h),4.40(d,j=11.4hz,16h),4.37(d,j=11.4hz,16h),4.02(dd,j=3.0,9.6hz,16h),3.93(t,j=9.6hz,16h),3.80(d-like,j=8.4hz,16h),3.67(d,j=9.6hz,16h),3.56(d,j=10.8hz,16h);
13
c nmr(150mhz,cdcl3)δ165.7,138.6,137.8,133.5,130.1,130.0,128.8,128.5,128.4,128.3,127.8,127.6,127.5,98.7(1j
c1,h1
=171.6hz),78.2,75.3,74.0,71.4,71.3,68.6,66.1;hrms(maldi-tof)m/z[m+na]
+
calcd for c
432h416o96
na 7166.9738;found 7164.9404.
[0098]
(3)环甘露十六糖29的制备:
[0099]
将得到的环甘露十六糖骨架24(44.0mg,0.0062mmol)溶于ch2cl2/meoh的混合溶剂(2ml,v/v,1/1)中,加入甲醇钠(108mg,2mmol),室温搅拌72h。反应液用氢离子树脂调节ph至中性,过滤,浓缩,硅胶柱层析((ch2cl2/meoh=50/1)纯化得白色皂化产物(30.0mg,88%)。将上述皂化产物(20.0mg,0.0037mmol)溶于thf/h2o的混合溶剂中(7.7ml,v/v,10/1),加入10% pd/c(23mg),氢气置换5次,室温反应48h后,tlc监测显示原料反应完全,过滤,浓缩,依次用氯离子树脂(h2o)及反相c18硅胶柱层析(meoh/h2o=10/90)纯化,得到白色固体,即环甘露十六糖29(8.4mg,88%)。1h nmr(600mhz,d2o)δ4.92(d,j=0.6hz,16h),4.00(dd,j=1.2,3.0hz,16h),3.94(dd,j=5.4,11.4hz,16h),3.89-3.86(m,16h),3.85(dd,j=3.0,9.6hz,16h),3.80(d,j=10.2hz,16h),3.73(t,j=9.6hz,16h);
13
c nmr(150mhz,d2o)δ99.3,70.8,70.7,70.0,66.6,65.5;hrms(maldi-tof)m/z[m+na]
+
calcd for c
96h160o80
na 2615.8349;found 2615.7881.
[0100]
实施例8
[0101]
环甘露十六糖29的制备方法,包括以下步骤:
[0102]
(1)甘露十六糖硫苷线性前体19的制备:
[0103]
将化合物4(1.28g,0.17mmol)溶于无水thf(4ml),加入hf
·
py(5ml),室温搅拌10h。tlc显示反应完全后,反应液用饱和nahco3溶液淬灭,etoac萃取3次,合并有机相,用饱和nacl溶液洗涤,无水na2so4干燥,滤除干燥剂,浓缩,粗品经硅胶柱层析(petroleum ether/etoac=2/1)纯化,得到白色泡沫状固体,即甘露十六糖硫苷线性前体19(1.15g,90%)。90%)。1h nmr(400mhz,cdcl3)δ8.18-8.12(m,28h),8.08(d,j=7.2hz,2h),7.58(t,j=7.2hz,2h),7.52-7.44(m,46h),7.36-7.06(m,165h),5.96(s,1h),5.84-5.77(m,15h),5.52(s,1h),5.04-5.02(m,15h),4.94-4.86(m,15h),4.83-4.77(m,14h),4.72(d,j=11.6hz,1h),4.59(dd,j=3.2,11.2hz,2h),4.47-4.31(m,30h),4.15-3.91(m,33h),3.79-3.40(m,49h),2.39(s,3h),1.24(s,9h);
13
c nmr(100mhz,cdcl3)δ165.7,150.1,138.6,138.4,137.7,137.6,137.3,133.5,130.1,130.0,128.8,128.7,128.6,128.5,128.3,128.2,128.1,127.9,127.6,127.5,127.4,127.3,127.2,127.1,98.7,78.4,77.8,77.4,75.4,75.3,75.2,74.3,73.8,72.4,72.2,71.9,71.4,71.3,71.2,71.0,
68.7,68.5,65.8,34.6,31.5,20.6;hrms(maldi-tof)m/z calcd for c
443h433o96
s[m+h]
+
7325.3010;found 7324.5054.
[0104]
(2)环甘露十六糖骨架24的制备:
[0105]
将甘露十六糖硫苷线性前体19(60.0mg,0.008mmol)溶于无水ch2cl2(8ml),加入agotf(6.2mg,0.024mmol)和新活化的ms(100mg),室温搅拌30min。0℃下加入ibr的ch2cl2溶液(0.28ml,0.057m),逐渐升至室温反应3h。tlc显示反应完全后,反应液用硅藻土滤除分子筛,滤液用饱和na2s2o3溶液洗涤,ch2cl2萃取3次,合并有机相,依次用饱和nahco3溶液和饱和nacl溶液洗涤,无水na2so4干燥,滤除干燥剂,浓缩,粗品经硅胶柱层析(petroleum ether/etoac=3/1)纯化得白色固体,即环甘露十六糖骨架24(40.5mg,71%)。
[0106]
(3)环甘露十六糖29的制备:步骤同实施例7。
[0107]
实施例9
[0108]
环甘露糖三十二糖30的制备方法,包括以下步骤:
[0109]
(1)甘露三十二糖炔烯酸酯线性前体15的制备:
[0110]
(a)化合物10的制备:将化合物5(200.0mg,0.014mmol)和(z)-3-碘代丙烯酸(4.0mg,0.02mmol)溶于无水ch2cl2(5ml),加入agotf(10.7mg,0.042mmol)和活化的ms(100mg),室温搅拌30min。加入ibr的ch2cl2溶液(0.1ml,0.28m),室温搅拌30min。tlc显示反应完全后,反应液用硅藻土滤除分子筛,滤液用ch2cl2稀释,饱和na2s2o3溶液洗涤,ch2cl2萃取3次,合并有机相,依次用饱和nahco3溶液和饱和nacl溶液洗涤,无水na2so4干燥,滤除干燥剂,浓缩,粗品经硅胶柱层析(petroleum ether/etoac=2/1
→
1.8/1)纯化得相应的白色泡沫状固体(170.0mg,83%)。将上述得到的糖基碘代丙烯酸酯(170.0mg,0.0116mmol)溶于ch3cn/thf(4ml,v/v,1/1),加入pd(pph3)2cl2(0.8mg,0.0012mmol)和cui(0.2mg,0.0012mmol),氩气置换空气3次,加入et3n(3.2μl,0.0232mmol)和1-己炔(1.5μl,0.0139mmol),室温搅拌3h。tlc显示反应完全后,反应液用饱和nh4cl溶液淬灭,ch2cl2萃取,合并有机层,饱和nacl溶液洗涤,无水na2so4干燥,过滤,浓缩,硅胶柱层析(petroleum ether/etoac=2/1
→
1.9/1)得白色泡沫状固体,即化合物10(159mg,93%)。1.9/1)得白色泡沫状固体,即化合物10(159mg,93%)。1h nmr(600mhz,cdcl3)δ8.19-8.16(m,64h),7.76(d,j=7.8hz,2h),7.66(d,j=7.2hz,2h),7.55-7.48(m,90h),7.41-7.27(m,18h),7.22-7.07(m,314h),6.39(d,j=1.8hz,1h),6.27(dt,j=2.4,11.4hz,1h),6.01(d,j=11.4hz,1h),5.85(br s,29h),5.80(br s,1h),5.74(d,j=2.4hz,1h),5.10(s,1h),5.05(br s,30h),4.93(d,j=10.8hz,2h),4.90-4.87(m,29h),4.83-4.79(m,29h),4.75(d,j=11.4hz,2h),4.62(d,j=10.8hz,1h),4.55(d,j=10.8hz,1h),4.49-4.41(m,32h),4.39-4.32(m,29h),4.28-4.23(m,3h),4.19(dd,j=3.0,9.0hz,1h),4.03-3.94(m,63h),3.73-3.39(m,96h),2.42-2.35(m,2h),1.48-1.44(m,2h),1.33-1.31(m,2h),1.10(s,9h),0.84(t,j=7.2hz,3h);
13
c nmr(150mhz,cdcl3)δ165.8,165.7,162.3,138.9,138.7,138.6,138.4,137.8,137.7,137.6,136.1,135.8,133.7,133.6,133.5,133.3,130.2,130.0,129.8,129.7,128.8,128.6,128.5,128.3,128.2,128.0,127.9,127.8,127.7,127.6,127.5,127.4,127.2,126.2,126.0,106.4,98.7,91.7,78.4,78.3,78.2,75.5,75.4,75.2,73.9,73.8,73.7,73.4,72.7,72.0,71.5,71.2,71.0,70.8,68.9,68.5,68.0,66.0,65.8,65.6,63.4,62.6,30.5,27.0,
22.1,20.0,19.5,13.8;hrms(maldi-tof)m/z calcd for c
889h862o194
sik[m+k]
+
14706.6992;found 14705.8716.
[0111]
(b)甘露三十二糖炔烯酸酯线性前体15的制备:
[0112]
将化合物10(111.5mg,0.0076mmol)溶于无水thf(5ml),滴加hf
·
py(0.6ml),室温条件下搅拌过夜。tlc显示反应完全后,反应液用饱和nahco3溶液淬灭,乙酸乙酯萃取3次,合并有机层,饱和nacl溶液洗涤,无水na2so4干燥。滤除干燥剂,浓缩,粗品用硅胶柱层析(petroleum ether/etoac=2/1
→
1.9/1)纯化得白色泡沫状固体,即甘露三十二糖炔烯酸酯线性前体15(92.4mg,84%)。1h nmr(400mhz,cdcl3)δ8.19-8.17(m,64h),8.08(d,j=7.6hz,2h),7.59(t,j=7.6hz,2h),7.51-7.49(m,91h),7.33-7.28(m,10h),7.19-7.06(m,311h),6.39(s,1h),6.26(dt,j=2.0,11.6hz,1h),6.01(d,j=11.6hz,1h),5.85(br s,29h),5.80(br s,1h),5.78(br s,1h),5.74(br s,1h),5.10(s,1h),5.05(br s,30h),4.89-4.78(m,62h),4.72(d,j=11.6hz,2h),4.60(d,j=11.2hz,1h),4.55(d,j=10.8hz,1h),4.46-4.32(m,61h),4.19(dd,j=3.2,8.4hz,1h),4.04-3.92(m,62h),3.80-3.40(m,98h),2.40-2.35(m,2h),1.49-1.44(m,2h),1.34-1.30(m,2h),0.83(t,j=7.2hz,3h);
13
c nmr(150mhz,cdcl3)δ165.7,165.6,165.5,162.3,138.7,138.6,138.5,138.4,138.3,137.8,137.7,137.6,136.4,135.4,133.6,133.5,133.4,130.2,130.0,129.9,129.8,129.0,128.8,128.7,128.6,128.5,128.3,128.2,128.1,128.0,127.9,127.8,127.7,127.6,127.5,127.4,127.3,127.2,127.1,126.5,126.2,126.0,125.2,98.7,98.3,91.7,89.3,78.4,78.3,78.2,77.9,75.5,75.4,75.3,75.2,74.0,73.9,73.7,72.3,72.0,71.5,71.4,71.0,68.7,68.5,68.0,66.0,65.9,65.6,62.0,30.1,22.1,20.0,13.8;hrms(maldi-tof)m/z calcd for c
873h844o194
na[m+na]
+
14463.1508;found 14464.7412.
[0113]
(2)环甘露三十二糖骨架25的制备:
[0114]
将得到的甘露三十二糖炔烯酸酯线性前体15(28.9mg,0.002mmol)溶于无水甲苯(2ml),加入新活化的ms(50mg),室温搅拌30min。-40℃下加入新鲜制备的sphosauntf2的ch2cl2溶液(18μl,0.1m)和tfoh的ch2cl2溶液(20μl,0.01m),逐渐升至0℃搅拌4h。反应液用et3n淬灭,硅藻土滤除分子筛,浓缩,粗品经硅胶柱层析(petroleum ether/etoac=5/2
→
2.3/1)得白色固体,即环甘露三十二糖骨架25(16.0mg,56%)。2.3/1)得白色固体,即环甘露三十二糖骨架25(16.0mg,56%)。1h nmr(600mhz,cdcl3)δ8.16(d,j=6.6hz,64h),7.50-7.45(m,96h),7.18-7.15(m,160h),7.11(t-like,j=7.2hz,64h),7.07-7.05(m,96h),5.83(s,32h),5.04(s,32h),4.86(d,j=12.0hz,32h),4.78(d,j=10.8hz,32h),4.41(d,j=10.8hz,32h),4.33(d,j=11.4hz,32h),4.01(dd,j=2.4,9.6hz,32h),3.96(t,j=9.6hz,32h),3.73(d,j=9.6hz,32h),3.58(d,j=9.6hz,32h),3.44(d,j=10.8hz,32h);
13
c nmr(150mhz,cdcl3)δ65.7,138.6,137.7,133.5,130.1,130.0,128.8,128.5,128.3,127.9,127.5,127.2,98.7(1j
c1,h1
=172.1hz),78.3,75.2,73.9,71.5,71.1,68.5,65.9;hrms(maldi-tof)m/z[m+na]
+
calcd for c
864h832o192
na 14300.5238;found 14301.9531.
[0115]
(3)环甘露三十二糖30的制备:
[0116]
将环甘露三十二糖骨架25(14.3mg,0.001mmol)溶于ch2cl2/meoh的混合溶剂(2ml,
v/v,1/1)中,加入甲醇钠(135mg,2.5mmol),室温搅拌48h。反应液用氢离子树脂调节ph至中性,过滤,浓缩,硅胶柱层析(ch2cl2/meoh=50/1)纯化得白色皂化产物(9.9mg,90%)。将上述皂化产物(6mg,0.55μmol)溶于thf/h2o的混合溶剂中(3.3ml,v/v,10/1),加入10% pd/c(6mg),氢气置换5次,室温反应72h后,tlc监测显示原料反应完全,过滤,浓缩,依次用氯离子树脂(h2o)及反相c18硅胶柱层析(meoh/h2o=10/90)纯化得白色固体,即环甘露三十二糖30(2.0mg,70%)。1h nmr(600mhz,d2o)δ4.89(s,32h),3.98(br s,32h),3.93(dd,j=5.4,11.4hz,16h),3.86-3.82(m,64h),3.77(d,j=10.8hz,32h),3.71(t,j=9.6hz,32h);
13
c nmr(150mhz,d2o)δ99.3,70.8,70.6,70.0,66.6,65.5.
[0117]
实施例10
[0118]
环甘露三十二糖30的制备,包括以下步骤:
[0119]
(1)甘露三十二糖硫苷线性前体20的制备:
[0120]
将化合物5(441mg,0.03mmol)溶于无水thf(4ml),加入hf
·
py(4ml),室温搅拌4h。tlc显示反应完全后,反应液用饱和nahco3溶液淬灭,etoac萃取3次,合并有机相,用饱和nacl溶液洗涤,无水na2so4干燥。滤除干燥剂,浓缩,粗品经硅胶柱层析(petroleum ether/etoac=2/1)纯化得白色泡沫状固体,即甘露三十二糖硫苷线性前体20(377mg,87%)。etoac=2/1)纯化得白色泡沫状固体,即甘露三十二糖硫苷线性前体20(377mg,87%)。1h nmr(600mhz,cdcl3)δ8.18-8.16(m,60h),8.09-8.07(m,4h),7.51-7.47(m,93h),7.31-7.28(m,13h),7.18-7.15(m,170h),7.13-7.11(m,62h),7.08-7.06(m,81h),5.84(s,32h),5.04(s,32h),4.87(d,j=11.4hz,32h),4.79(d,j=10.8hz,32h),4.42(d,j=10.8hz,32h),4.32(d,j=11.6hz,32h),4.03-3.94(m,64h),3.72(d,j=9.6hz,32h),3.57(d,j=9.0hz,32h),3.41(d,j=10.8hz,32h),2.40(s,3h),1.25(s,9h);
13
c nmr(150mhz,cdcl3)δ165.7,138.6,137.7,133.5,130.2,130.0,128.8,128.7,128.6,128.5,128.3,128.2,128.1,127.9,127.5,127.4,127.2,98.7,78.4,75.2,73.8,71.5,71.0,68.5,65.8,31.5,29.9,20.7;hrms(maldi-tof)m/z calcd for c
875h848o192
sna[m+na]
+
14480.6211;found 14478.7246.
[0121]
(2)环甘露三十二糖骨架25的制备:
[0122]
将甘露三十二糖硫苷线性前体20(28.9mg,0.002mmol)溶于无水ch2cl2(2ml),加入agotf(6.2mg,0.024mmol)和新活化的ms(50mg),室温搅拌30min,室温下加入ibr的ch2cl2溶液(0.2ml,0.08m),室温反应4h。tlc显示反应完全后,反应液用硅藻土滤除分子筛,滤液用饱和na2s2o3溶液洗涤,ch2cl2萃取3次,合并有机相,依次用饱和nahco3溶液和饱和nacl溶液洗涤,无水na2so4干燥,滤除干燥剂,浓缩,粗品经硅胶柱层析(petroleum ether/etoac=5/2
→
2.3/1)纯化,得到白色固体,即环甘露三十二糖骨架25(5mg,18%)。
[0123]
(3)环甘露三十二糖30的制备:步骤同实施例9。
[0124]
本发明不局限于上述具体的实施方式,本领域的普通技术人员从上述构思出发,不经过创造性的劳动,所做出的种种变换,均落在本发明的保护范围之内。
相关技术
网友询问留言
已有0条留言
- 还没有人留言评论。精彩留言会获得点赞!
1