一种稳定性强的单试剂液态血氨(AMM)检测试剂的制作方法
本发明涉及一种血氨(AMM)检测技术领域,特别涉及一种稳定性强的单试剂的液态血氨(AMM)检测试剂。
背景技术:
体内各组织各种氨基酸分解代谢产生的氨以及由肠管吸收进来的氨进入血液,形成血氨。血氨正常值18-72μmol/L。血氨的产生分为内源性产生和外源性获取,内源性产生主要是体内代谢产生的氨称为内源性氨,主要来自氨基酸的脱氨基作用,部分来自肾小管上皮细胞中谷氨酰胺分解产生的氨。胺类的分解也可产生氨。外源性获取则由消化道吸收人体内的氨称为外源性氨。它包括肠道内未被消化的蛋白质和未被吸收的氨基酸,经肠道细菌作用产生的氨。血中尿素扩散到肠道,经细菌尿素酶作用水解生成的氨。
血氨增高是肝性脑病临床特征之一,他的检测结果的准确性对于肝性脑病、儿科Reye综合征等的准确诊断有非常重要的意义。血氨在人体中主要以NH4+和NH3两种形式存在,血液中血氨的主要来源主要是肠道产生的氨和肾脏分泌的氨。医学中检测血液中氨的方法非常多,比如去蛋白波氏比色法、氨电极法、扩散法、离子交换层析法、酶法和干化学法等方法。其中扩散法和波氏比色法灵敏度较低,准确性和精密度满足不了临床的要求,离子交换层析法则需要非常长的时间的处理,氨电极法准确性和精密度非常好,但是检测结果的稳定性受到多种因素的影响,并且电极需要经常的保养和更换,检测的成本和操作要求较高,无法在普通医院中普及。目前使用的较多的为干化学法和酶法,这两种方法可以直接应用在半自动和全自动生化分析仪器上,成本较低,操作相对较低,但是干化学法需要将干粉在使用前进行手工处理,这无疑增加了操作的繁琐性,也增加操作的误差,并且干粉一旦被溶解后保存时间较短,试剂中关键组分容易衰减,无法保证检测结果的稳定性和准确性,而液体的酶两点动力学法则不需要做前期的工作,直接可以配合仪器使用,但是目前市场出现的酶两点法试剂为了保证试剂的稳定性,多为四试剂或者为三试剂,因此客户在使用的时候必须将试剂按照比例调配混匀,试剂一旦在混合之后多不稳定,也必须要尽快使用,不然的话试剂会发生衰减,影响结果的准确性,因此发明一种准确性强又稳定的酶两点法单试剂,能够大大简化操作的流程,方便临床的应用,有很大的应用前景。
技术实现要素:
本发明的目的是提供一种稳定性强的单试剂的液态血氨(AMM)检测试剂以及检测方法。
基本原理:
谷氨酸脱氢酶两点法检测血氨的基本原理为:在乳酸脱氢酶(LDH)的作用下,血清中干扰氨测定的丙酮酸在预反应中被除去。氨在谷氨酸脱氢酶的作用下与α-KG 和 NADH 反应,与同样处理的校准液比较,可计算出血清中氨的含量。
第一步: 乳酸脱氢酶
丙酮酸 + NADH + H+ L-乳酸+NAD+
第二步: GLDH
α-酮戊二酸+氨+NADH 谷氨酸+NAD+ +H2O。
本发明是通过以下步骤得到的:
一种稳定性强的单试剂液态血氨试剂试剂组成如下:
缓冲液···························································································································50mmol/L,
LDH(乳酸脱氢酶)································································································5KU/L-10KU/L
重金属离子螯合剂·······································································································3g/L-10g/L
离子平衡剂·····················································································································2g/L-5g/L
防腐剂··························································································································0.1g/L-1g/L
α-酮戊二酸(α-KG)················································································7mmol/L-12 mmol/L
NADH.NA2(还原性辅酶I二钠盐)····················································0.18mmol/L-0.36 mmol/L
表面活性剂···············································································································1ml/L-5ml/L
保护剂1·······················································································································3g/L-5 g/L
保护剂2························································································································2g/L-5g/L
保护剂3························································································································2g/L-5g/L
所述的一种稳定性强的单试剂的血氨检测试剂,试剂R中缓冲液为25℃,PH=8.1的,浓度为50mmol/L的TES(N-三(羟甲基)甲基-2-氨基乙磺酸)缓冲液;
所述的一种稳定性强的单试剂的血氨检测试剂,所述的重金属离子螯合剂为环已烷二胺四醋酸(CDTA);
所述的一种稳定性强的单试剂的血氨检测试剂,所述离子平衡剂为氯化钾;
所述的一种稳定性强的单试剂的血氨检测试剂,所述防腐剂为氯霉素;
所述的一种稳定性强的单试剂的血氨检测试剂,所述表面活性剂为Emulgen A90;
所述的一种稳定性强的单试剂的血氨检测试剂,所述的保护剂1为ADP.NA2;
所述的一种稳定性强的单试剂的血氨检测试剂,所述的保护剂2为聚乙烯醇AH-26;
所述的一种稳定性强的单试剂的血氨检测试剂,所述的保护剂3为黄原胶;
所述的一种稳定性强的单试剂的血氨检测试剂,使用全自动生化分析仪利用终点法进行测定,检测主波长为340nm;
所述的试剂为液态单试剂。
本发明的创新处:
1)本试剂建立的是谷氨酸脱氢酶两点法动力学法的单试剂,有效的简化了使用的操作,且保证了产品有效成分的稳定性;
2)本发明缓冲体系的建立优先选择了TES(N-三(羟甲基)甲基-2-氨基乙磺酸)作为缓冲液,在起到基本的缓冲能力之外,能够对NADH起到较好的保护作用;
3)在试剂中选择了环已烷二胺四醋酸(CDTA)作为重金属里的螯合剂,能够较好的螯合重金属对GLDH和LDH两种酶的影响,同时还不会对GLDH的催化活性产生抑制,并且能够较好的去除重金属离子的对NADH稳定性的影响;
4)在试剂中优先选择了氯化钾作为例子平衡剂,能够对体系产生良好的平衡作用;
5)在试剂R1中加入了环己二胺四乙酸(CDTA),能够有效的螯合重金属离子,并且能够较好的提高试剂的准确性;
6)本发明在试剂中添加了Emulgen A90表面活性剂作为乳化剂,能够非常好的提高检测结果的重复性并且对酶的催化作用有增效作用;
7)本发明优选ADP.NA2、聚乙烯醇AH-26、黄原胶三种物质作为添加保护剂,对GLDH、LDH和NADH三种物质具有非常好的保护作用。
附图说明
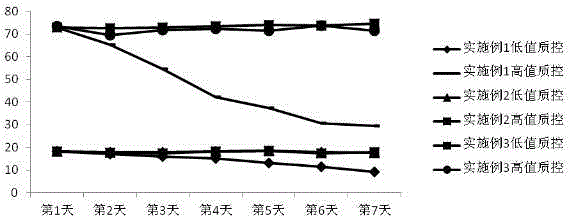
图1 为15天开瓶稳定性实验验证结果曲线变化图,
图2 为37℃ 7天热稳定性实验验证结果曲线变化图。
具体实施方式
下面结合具体实施例对本发明进行进一步说明:
实施例1
一种现有的三试剂的血氨检测试剂:
三试剂:
试剂1a(R1a):
Tirs缓冲液 PH8.0 50mmol/L
LDH 2KU/L
EDTA 5.3mmol/L
试剂1b(R1b):
α-KG 18.0mmol/L
NADH 0.18mmol/L
试剂2(R2):
GLDH 25.0KU/L
实施例2
所述的一种稳定性强的单试剂的血氨检测试剂组成如下:
TES(N-三(羟甲基)甲基-2-氨基乙磺酸)缓冲液·····················································50mmol/L,
LDH(乳酸脱氢酶)··············································································································5KU/L
CDTA······································································································································3g/L
氯化钾·····································································································································2g/L
氯霉素··································································································································0.1g/L
α-酮戊二酸(α-KG)····································································································7mmol/L
NADH.NA2(还原性辅酶I二钠盐)···········································································0.18mmol/L
Emulgen A90·························································································································1ml/L
ADP.NA2··························································································································3mmol/L
聚乙烯醇AH-26·····················································································································2g/L
黄原胶·····································································································································2g/L
实施例3
1)本实施例描述的是一种关键原材料增加的稳定性强的单试剂的血氨检测试剂组成如下:
TES(N-三(羟甲基)甲基-2-氨基乙磺酸)缓冲液···················································50mmol/L,
LDH(乳酸脱氢酶)··········································································································10KU/L
CDTA··································································································································10g/L
氯化钾···································································································································5g/L
氯霉素···································································································································1g/L
α-酮戊二酸(α-KG)·······························································································12mmol/L
NADH.NA2(还原性辅酶I二钠盐)········································································0.36mmol/L
Emulgen A90·····················································································································5ml/L
ADP.NA2······················································································································5mmol/L
聚乙烯醇AH-26·················································································································5g/L
黄原胶···································································································································5g/L
2)校准品和质控品:
校准品:自制标准,其中AMM的含量为30µmol/L
质控品:自制高值质控:靶值:73.6µmol/L,靶值范围:58.88-88.32µmol/L
自制低值质控:靶值:18.0µmol/L,靶值范围:14.4-21.6µmol/L
3)试剂稳定性验证具体操作方法:
(1)关于试剂的稳定性验证,将分为试剂的15天开平稳定性和7天37℃热稳定性验证。首先将本实施例中的检测试剂和实施例1中的检测试剂,按照配方配置好,分别取相同的两组,一组做15天开瓶稳定性,将试剂开瓶放置在仪器的2-8℃冷藏箱中(15天不取出),作为15天开瓶稳定性检测,另一组做37℃热稳定性,封闭放置在37℃恒温水浴锅中(每天仅在检测的时候取出,检测完毕后,依然封口放回37℃水浴锅中,连续7天),作为7天37℃热稳定性验证。将试剂同时在日立7180全自动生化分析仪器上,按照如下表1方法进行检测,并在仪器上建立标准曲线。分别取高、低质控,各自平均分成15份,4℃储存,每天高、低值质控各取一个,并且跟踪检测结果,其跟踪监测趋势如附图1和附图2
(2)试剂检测使用方法:
本实施例描述的所述的一种稳定性强的单试剂的血氨检测试剂,采用全自动生化分析仪,如日立7180全自动分析仪等,将实施例2和实施例3利用两点动力学法,按照表1中的要求进行测定;实施例1使用双试剂终点法进行操作,首先将试剂1(a)和试剂1(b)按照要求比例混匀后形成使用的R1,然后按照表2中的检测方法进行操作。
表1 实施例2和3试剂检测方法
计算:一种稳定性强的单试剂的血氨检测试剂(µmol/L)=(∆A测定÷∆A标准)×C标准。
表2 实施例1试剂检测方法
计算:现有的三试剂的血氨检测试剂(µmol/L)=(∆A测定÷∆A标准)×C标准。
(3)通过图1和图2中的曲线变化明显能够发现,实施例2和实施3中的单试剂明显比实施例1中的多试剂混合后要稳定很多,从而证明了本发明的对于产品的稳定性的效果。
- 还没有人留言评论。精彩留言会获得点赞!