一种钴催化的萜烯羟甲基芳基化的方法与流程
[0001]
本发明涉及一种钴催化的萜烯羟甲基芳基化实现三组分反应的方法。具体为,以2-苯基吡啶及类似物和多聚甲醛为原料,在钴催化剂/银盐促进下,可以在萜烯上高选择性的引入羟甲基和芳基实现三组分连续加成反应。本发明有以下优点,多聚甲醛是一种绿色的大宗化学品,简单易得,价格便宜;反应是一种连续加成反应,原子经济性高。
背景技术:
[0002]
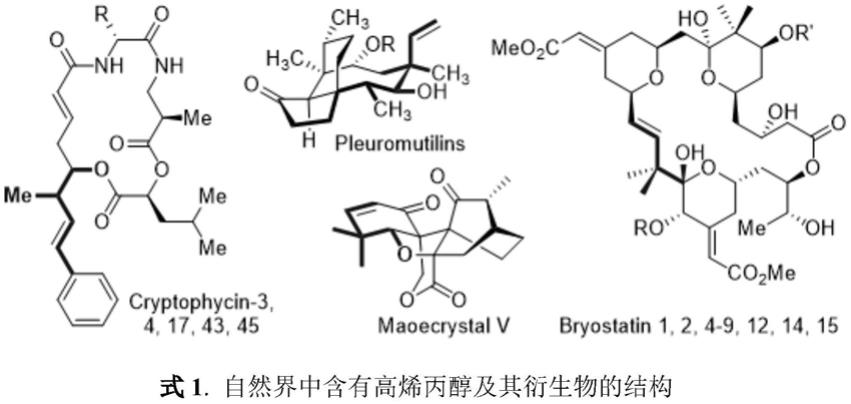
高烯丙醇结构是一类重要的天然产物核心骨架,例如,cryptophycin-3,4,17,43,45等,pleuromutilins,maoecrystal v,bryostatin-1,2,4-9,12,14,15均含有高烯丙醇结构(式1)。因此,探索简单、高效的催化体系来实现在萜烯上引入芳基和羟甲基得到高烯丙醇结构,具有重要的研究意义。
[0003][0004]
通过文献检索发现(式2),ellman小组在2016年报道了2苯基吡啶、1-苯基-2-丙烯基-1-酮和乙醛酸乙酯,在[cp*(rhcl2)2]和agsbf6存在下,可以实现三组分连续加成反应(j.a.boerth,j.a.ellman,chem.sci.2016,7,1474-1479.)在该工作的基础上,2016年,ellman小组发展了一种co(iii)催化的芳基和共轭二烯和醛类化合物的三组分连续加成反应(j.a.boerth,s.maity,s.k.williams,b.q.mercado,j.a.ellman,nat.catal.2018,1,673-679.)。赵东兵小组将这种反应扩展到了萜烯,不过他的文章只报道了两个例子,而且方法的产率不好,萜烯的种类也不足,且醛类也是带有取代基团的吸电子的醛(r.li,c.w.ju,d.zhao,chem.commun.2019,55,695-698.)。因此,选择合适简单的原料(萜烯、醛)来提高反应的适用范围显得尤为重要。
[0005][0006]
萜烯是生物体内许多重要化合物的前体,对于萜烯的衍生可以模拟生物体内物质的转化途径,加深对于生命体的理解。本专利开发出了一种钴催化的萜烯羟甲基芳基化实现三组分反应的方法,可以实现对萜烯的修饰。
技术实现要素:
[0007]
本发明目的在于以2-苯基吡啶及类似物、萜烯(或取代的萜烯)和简单化学品多聚甲醛为原料,发展了一种钴催化剂/银盐/添加剂体系,可以在萜烯上引入芳基和羟甲基。
[0008]
本发明是通过以下技术方案实现的:
[0009]
本发明目的在于以2-苯基吡啶及类似物、萜烯(或取代的萜烯)和简单化学品多聚甲醛为原料,在钴催化剂/银盐/添加剂作用下,可以在萜烯上引入芳基和羟甲基实现三组分连续加成反应,反应式如下所示:
[0010][0011]
具体操作步骤如下:
[0012]
在氩气或氮气气氛下,依次加入钴催化剂、银盐、添加剂、2-苯基吡啶及类似物1,多聚甲醛,然后加入一定量溶剂溶解,最后加入萜烯2,在一定温度下反应,点板监测反应体系,反应结束后,旋干溶剂,柱层析(流动相:石油醚/乙酸乙酯=4/1,v/v)得到目标产物4。
[0013]
反应物1上的导向基dg可以是嘧啶、苯并喹啉、带有取代基(c1-c4烷基、三氟甲基、c1-c4烷氧基)的吡啶中的一种;ar可以是带有c1-c4烷基、卤素(f、cl、br、i中的一种或二种以上)、乙酰基、三氟甲基、c1-c4烷氧基、羟基中的一种或二种以上取代的苯基、萘基及噻吩基中的一种或二种以上;萜烯可以是异戊二烯、c10-c45萜烯或取代萜烯(取代基为c1-c4烷基)中的一种或二种以上。
[0014]
所用钴催化剂为下述中的一种或二种以上:co2(co)8、cp*co(co)i2、[cp*coi2]2、cp*co(mecn)3[sbf6]2,其中,催化剂与2-苯基吡啶及类似物的摩尔比为0.001-1,优选范围为0.01-0.2。
[0015]
所用银盐为下述中的一种或二种以上:六氟锑酸银(agsbf6)、四氟硼酸银(agbf4)、三氟甲烷磺酸银(agotf)、双三氟甲磺酸亚胺银(agntf2)、乙酸银(agoac)、碳酸银(ag2co3)、氧化银(ag2o)、氟化银(agf),其中,银盐与2-苯基吡啶及类似物的摩尔比为0.001-1,优选范围为0.01-0.2。
[0016]
所用的添加剂为下述中的一种或二种以上:樟脑磺酸、对甲苯磺酸、苯磺酸、三氟
乙酸、甲酸、乙酸、丙二酸、三氟甲磺酸、苯甲酸、邻苯二甲酸、对苯二甲酸、金刚烷甲酸、磷酸二苯酯、脯氨酸、碳酸铯、碳酸钾、碳酸钠、乙酸钠、特戊酸钠、苯甲酸钠、苯甲酸钾、四(3,5-二(三氟甲基)苯基)硼酸钠(nabar
f4
),其中,添加剂与2-苯基吡啶及类似物的摩尔比为0.01-2,优选范围为0.1-1.2。
[0017]
所用溶剂为,以甲醇、乙醇、异丙醇、叔丁醇、乙腈、氯苯、环己烷、四氢呋喃、1,4-二氧六环、二氯甲烷、二氯乙烷、乙酸乙酯、n,n-二甲基甲酰胺、二甲亚砜中的一种或二种以上为溶剂,2-苯基吡啶及类似物优选浓度范围0.01-1.5mol/l。
[0018]
萜烯(或取代的萜烯)用量是2-苯基吡啶及类似物摩尔量的0.5-10倍之间;反应温度在25-120℃之间;反应时间在0.5-36h之间。
[0019]
本发明具有如下优点:
[0020]
本发明有以下优点,多聚甲醛是一种绿色的大宗化学品,简单易得,价格便宜;反应是一种连续加成反应,原子经济性高。因此,本专利在合成高烯丙醇类化合物方面有着潜在的应用前景。
具体实施方式
[0021]
下面将以具体的实施例来对本发明加以说明,但本发明的保护范围不局限于这些实例。
[0022]
1.co催化2-苯基吡啶及类似物和萜烯(或取代的萜烯)、多聚甲醛的反应
[0023]
在2.0ml封管中,依次加入co催化剂(5mol%,相对于2-苯基吡啶及类似物的用量)、银盐(10mol%,相对于2-苯基吡啶及类似物的用量)、添加剂(40mol%,相对于2-苯基吡啶及类似物的用量)、多聚甲醛(3.0equiv,相对于2-苯基吡啶及类似物的用量)、2-苯基吡啶1a(0.2mmol,31.0mg),用0.5ml溶剂溶解,然后加入萜烯2a(2.0equiv,相对于2-苯基吡啶及类似物的用量),在50℃反应16h,结束后加入均三甲氧基苯作为内标,1h nmr检测目标产物4a收率。
[0024][0025]
表1.催化剂、银盐、添加剂和溶剂等因素对反应的影响
[0026][0027][0028]
由表1结果可以看出,不加钴催化剂或者银盐,催化反应都无法顺利进行,不加入添加剂时,目标产物收率可以达到29%。添加剂,如(pho)2p(o)oh和phco2na,反应几乎不进行,碱类如,pivona、naoac对于反应收率稍有提高。acoh可以明显提高产率,通过对acoh用量的筛选发现,40%的acoh作为添加剂时,收率可以提高到90%。
[0029]
2.底物类型
[0030]
在手套箱中,向2.0ml封管中,依次加入co(co)i2(5mol%,4.8mg)、agsbf6(10mol%,6.9mg)、多聚甲醛(3.0equiv,18.0mg)acoh(40mol%,4.8mg)和2-苯基吡啶及类似物1(0.2mmol),用0.5ml dioxane溶解,然后加入萜烯2(2.0equiv),在50℃反应16h,结束后,水洗,二氯甲烷萃取,旋干,柱层析分离,流动相为石油醚/乙酸乙酯=4/1,v/v。
[0031]
[0032]
2h),7.29
–
7.21(m,1h),6.49(d,j=16.2hz,1h),5.95(d,j=16.2hz,1h),3.35(s,2h),2.70(brs,1h),1.04(s,6h).
13
c nmr(100mhz,cdcl3)δ159.1,149.1,138.8,138.4,136.7,136.3,129.8,128.8,128.7,127.2,126.9,124.5,121.9,71.5,39.1,24.0.hrms calculated for c
17
h
20
no[m+h]
+
254.1539,found 254.1534.
[0033]
2h),1.26(s,6h).
13
c nmr(100mhz,cdcl3)δ147.8,147.4,139.1,136.2,136.1,135.2,134.7,128.6,128.4,128.0,127.9,127.8,127.7,125.7,121.0,71.6,38.8,24.9.hrms calculated for c
19
h
20
no[m+h]
+
278.1539,found 278.1538.
[0034]
1h),1.12(s,6h).
13
c nmr(100mhz,cdcl3)δ166.5,157.0,138.5,138.2,135.8,130.8,130.7,130.1,127.7,127.3,118.7,71.4,39.2,24.1.hrms calculated for c
16
h
19
n2o[m+h]
+
255.1492,found 255.1495.
[0035]
(d,j=16.2hz,1h),5.91(d,j=16.2hz,1h),3.76(s,3h),3.26(s,2h),2.13(brs,1h),0.96(s,6h).
13
c nmr(100mhz,cdcl3)δ153.5,149.2,141.2,137.9,136.6,136.0,130.1,128.4,128.0,126.8,125.7,123.3,118.4,71.3,55.6,39.0,23.8.hrms calculated for c
18
h
22
no2[m+h]
+
284.1645,found 284.1647.
[0036]
1h),6.48(d,j=16.3hz,1h),5.94(d,j=16.2hz,1h),3.36(s,2h),2.65(brs,1h),2.40(s,3h),1.04(s,6h).
13
c nmr(100mhz,cdcl3)δ158.8,148.8,147.4,138.5,138.4,136.8,129.7,129.2,128.5,127.2,126.9,125.4,122.9,71.6,39.1,24.0,21.1.hrms calculated for c
18
h
22
no[m+h]
+
268.1696,found 268.1700.
[0037]
6.46(d,j=16.2hz,1h),6.03(d,j=16.2hz,1h),3.39(s,2h),2.19(brs,1h),1.06(s,6h).
13
c nmr(100mhz,cdcl3)δ160.3,150.2,139.7,138.7(q,j=33.9hz),137.0,136.8,129.9,129.4,128.2,127.5,127.3,124.2(q,j=273.4hz),120.3(q,j=3.6hz),117.3(q,j=3.5hz),71.5,39.1,23.8.
19
f nmr(376mhz,cdcl3)δ-64.77.hrms calculated for c
18
h
19
f3no[m+h]
+
322.1413,found 322.1412.
[0038]
hz,1h),6.16(d,j=16.2hz,1h),5.96(d,j=16.2hz,1h),3.27(s,2h),2.84(s,1h),0.95(s,6h).
13
c nmr(100mhz,cdcl3)δ161.3(d,j=246.1hz),158.8,153.3,149.3,140.2,139.5(d,j=2.8hz),136.2,129.7(d,j=9.2hz),126.9(d,j=3.2hz),126.3(d,j=2.7hz),122.5,122.0(d,j=3.1hz),114.2(d,j=22.9hz),71.4,39.1,23.7.
19
f nmr(376mhz,cdcl3)δ-117.20.hrms calculated for c
17
h
19
fno[m+h]
+
272.1445,found 272.1448.
[0039]
=16.2hz,1h),3.29(s,2h),2.68(brs,1h),0.96(s,6h).
13
c nmr(100mhz,cdcl3)δ163.8(dd,j=248.6hz),161.7(dd,j=248.4hz),152.5,149.4,141.2,140.9(d,j=4.4hz),140.8(d,j=4.4hz),136.3,126.4(d,j=2.7hz),126.3(t,j=2.9hz),122.6,108.9(d,j=3.4hz),108.8(d,j=3.4hz)102.6(d,j=25.8hz),102.3(d,j=25.8hz),71.3,39.1,23.6.
19
f nmr(376mhz,cdcl3)δ-109.96(d,j=8.1hz),-112.85(d,j=8.1hz).hrms calculated for c
17
h
18
f2no[m+h]
+
290.1351,found 290.1349.
[0040]
6.87(d,j=8.2hz,1h),5.97(d,j=16.2hz,1h),5.89(d,j=16.2hz,1h),3.72(s,3h),3.24(s,2h),2.99(brs,1h),0.91(s,6h).
13
c nmr(100mhz,cdcl3)δ156.9,155.6,148.6,139.4,138.5,136.4,129.5,127.5,127.3,126.8,122.2,118.7,109.7,71.4,55.8,39.0,23.7.hrms calculated for c
18
h
22
no2[m+h]
+
284.1645,found 284.1643.
[0041]
7.26
–
7.21(m,1h),7.17(d,j=7.8hz,1h),7.17(d,j=7.8hz,1h),6.45(d,j=16.2hz,1h),5.92(d,j=16.2hz,1h),3.34(s,2h),2.63(brs,1h),2.38(s,3h),1.03(s,6h).
13
c nmr(100mhz,cdcl3)δ159.2,149.1,138.3,137.9,137.0,136.2,133.8,130.4,129.4,128.6,126.7,124.5,121.8,71.6,39.0,24.0,21.1.hrms calculated for c
18
h
22
no[m+h]
+
268.1696,found 268.1695.
[0042]
7.80(td,j=7.7,1.7hz,1h),7.62(d,j=8.2hz,1h),7.52(d,j=7.8hz,1h),7.30(dd,j=7.1,5.3hz,1h),6.50(d,j=16.3hz,1h),6.13(d,j=16.2hz,1h),3.38(s,2h),2.78(brs,1h),2.62(s,3h),1.04(s,6h).
13
c nmr(100mhz,cdcl3)δ197.5,158.1,149.2,141.38,141.36,138.5,136.6,135.7,130.2,128.3,127.6,127.0,124.7,122.4,71.4,39.3,26.6,23.8.hrms calculated for c
19
h
22
no2[m+h]
+
296.1645,found 296.1645.
[0043]
(d,j=4.9hz,1h),6.40(d,j=16.2hz,1h),5.96(d,j=16.2hz,1h),3.35(s,2h),2.75(brs,1h),1.02(s,6h).
13
c nmr(100mhz,cdcl3)δ157.7,149.3,139.7,139.5,136.5,135.2,132.8,129.7,128.6,128.2,127.5,124.5,122.4,71.5,39.1,23.9.hrms calculated for c
17
h
19
clno[m+h]
+
288.1150,found 288.1152.
[0044]
(m,1h),7.01(d,j=2.7hz,1h),6.93(dd,j=8.6,2.7hz,1h),6.40(d,j=16.2hz,1h),5.86(d,j=16.2hz,1h),3.84(d,j=2.1hz,3h),3.34(s,2h),2.48(brs,1h),1.02(s,6h).
13
c nmr(100mhz,cdcl3)δ158.85,158.77,149.1,139.5,137.0,136.3,129.3,128.1,128.0,124.6,122.0,114.9,114.6,71.6,55.4,39.0,24.0.hrms calculated for c
18
h
22
no2[m+h]
+
284.1645,found 284.1636.
[0045]
2h),7.25
–
7.18(m,1h),7.15(d,j=7.7hz,1h),6.50(d,j=16.2hz,1h),5.94(d,j=16.2hz,1h),3.37(s,2h),2.68(brs,1h),2.40(s,3h),1.05(s,6h).
13
c nmr(100mhz,cdcl3)δ159.1,149.1,138.5,138.4,136.5,136.3,135.7,129.8,129.2,128.1,127.5,124.4,121.6,71.6,39.1,24.0,21.3.hrms calculated for c
18
h
22
no[m+h]
+
268.1696,found 268.1695.
[0046]
7.33(d,j=8.2hz,1h),7.27(t,j=6.0hz,1h),6.42(d,j=16.2hz,1h),6.00(d,j=16.2hz,1h),3.36(s,2h),2.63(brs,1h),1.04(s,6h).
13
c nmr(100mhz,cdcl3)δ157.9,149.2,140.1,138.7,137.1,136.5,131.4,130.1,129.7,127.6,124.4,122.9,122.2,71.5,39.2,23.9.hrms calculated for c
17
h
19
brno[m+h]
+
332.0645,found 332.0647.
[0047]
7.40(d,j=8.2hz,1h),7.34
–
7.23(m,2h),6.43(d,j=16.2hz,1h),6.00(d,j=16.2hz,1h),3.37(s,2h),2.62(brs,1h),1.04(s,6h).
13
c nmr(100mhz,cdcl3)δ157.9,149.2,140.0,138.4,136.7,136.5,134.6,131.2,127.7,127.2,126.7,124.4,122.1,71.5,39.2,23.9.hrms calculated for c
17
h
19
clno[m+h]
+
288.1150,found 288.1154.
[0048]
7.20(ddd,j=7.4,4.9,0.9hz,1h),7.03(d,j=2.6hz,1h),6.88(dd,j=8.5,2.6hz,1h),6.52(d,j=16.2hz,1h),5.96(d,j=16.2hz,1h),3.87(s,3h),3.37(s,2h),2.75(brs,1h),1.05(s,6h).
13
c nmr(100mhz,cdcl3)δ159.9,158.8,149.0,138.8,138.2,136.3,131.3,131.2,129.2,124.3,121.4,113.0,112.0,71.6,55.4,39.1,24.0.hrms calculated for c
18
h
22
no2[m+h]
+
284.1645,found 284.1648.
[0049]
7.34
–
7.28(m,1h),6.48(d,j=16.3hz,1h),6.06(d,j=16.2hz,1h),3.38(s,2h),2.63(brs,1h),1.05(s,6h).
13
c nmr(100mhz,cdcl3)δ157.7,149.4,141.4,140.5,137.5,136.6,130.9(q,j=32.4hz),130.5,130.3,127.6,124.5,124.1(q,j=272.3hz).123.7(q,j=3.8hz),122.6,71.5,39.2,23.8.
19
f nmr(376mhz,cdcl3)δ-62.59.hrms calculated for c
18
h
19
f3no[m+h]
+
322.1413,found 322.1414.
[0050]
1h),7.56(d,j=7.9hz,1h),7.51
–
7.40(m,2h),7.25(ddd,j=7.5,4.4,1.0hz,1h),6.55(d,j=16.1hz,1h),6.04(d,j=16.1hz,1h),3.38(s,2h),2.91(brs,1h),1.06(s,6h).
13
c nmr(100mhz,cdcl3)δ159.1,149.1,139.0,137.1,136.5,135.1,133.4,132.5,129.33,129.28,128.0,127.6,126.7,126.0,125.7,124.6,122.0,71.6,39.2,24.0.hrms calculated for c
21
h
22
no[m+h]
+
304.1696,found 304.1695.
[0051]
5.3hz,1h),7.20
–
7.15(m,1h),6.87(d,j=16.3hz,1h),6.09(d,j=16.3hz,1h),3.43(s,2h),1.90(brs,1h),1.13(s,6h).
13
c nmr(100mhz,cdcl3)δ153.1,149.7,139.0,138.0,137.1,136.5,127.2,126.1,123.1,122.7,121.7,71.6,39.1,24.0.hrms calculated for c
15
h
18
nos[m+h]
+
260.1104,found 260.1103.
[0052]
(petroleum ether/etoac 4/1).1h nmr(400mhz,dmso-d6)δ9.59(s,1h),8.66
–
8.60(m,1h),7.81(td,j=7.7,1.8hz,1h),7.42(d,j=7.9hz,1h),7.36
–
7.28(m,2h),6.99(d,j=2.4hz,1h),6.76(dd,j=8.4,2.4hz,1h),6.44(d,j=16.2hz,1h),6.12(d,j=16.2hz,1h),4.64(s,1h),3.21(s,2h),0.95(s,6h).
13
c nmr(100mhz,dmso-d6)δ158.5,158.0,149.4,139.8,137.6,136.6,131.9,130.4,125.8,125.0,121.9,114.8,112.5,70.7,39.0,24.3.hrms calculated for c
17
h
20
no2[m+h]
+
270.1489,found 270.1485.
[0053]
7.75(td,j=7.7,1.7hz,1h),7.58(d,j=7.8hz,1h),7.51
–
7.40(m,2h),7.29
–
7.24(m,1h),6.54(d,j=16.2hz,1h),6.00(d,j=16.2hz,1h),5.11(td,j=7.1,6.4,3.5hz,1h),3.45(d,j=10.7hz,1h),3.37(d,j=10.7hz,1h),2.90(s,1h),2.00(m,2h),1.69(s,3h),1.59(s,3h),1.50
–
1.32(m,2h),1.06(s,3h).
13
c nmr(100mhz,cdcl3)δ159.0,149.1,138.2,137.0,136.5,135.3,133.4,132.5,131.3,130.2,129.3,128.0,127.6,126.7,126.0,125.8,124.9,124.6,122.0,70.7,42.5,37.9,25.8,22.9,20.4,17.7.hrms calculated for c
26
h
30
no[m+h]
+
372.2322,found 372.2323.
[0054]
1h),7.93(d,j=3.8hz,2h),7.87
–
7.81(m,2h),7.77(td,j=7.7,1.8hz,1h),7.59(d,j=7.9hz,1h),7.52
–
7.42(m,2h),7.31
–
7.22(m,1h),6.56(d,j=16.2hz,1h),6.01(d,j=16.2hz,1h),5.19
–
5.04(m,2h),3.47(d,j=10.7hz,1h),3.38(d,j=10.7hz,1h),2.70(brs,1h),2.02(dq,j=7.3hz,6h),1.67(s,3h),1.59(s,6h),1.53
–
1.43(m,1h),1.38(m,1h),1.08(s,3h).
13
c nmr(100mhz,cdcl3)δ159.1,149.1,138.1,137.0,136.5,135.3,135.0,133.4,132.6,131.4,130.4,129.3,128.0,127.6,126.7,126.0,125.8,124.7,124.6,124.3,122.0,70.7,42.5,39.7,37.9,26.8,25.7,22.7,20.4,17.7,16.0.hrms calculated for c
31
h
38
no[m+h]
+
440.2948,found 440.2947.
[0055]
nmr(400mhz,cdcl3)δ8.69(d,j=4.1hz,1h),7.93(d,j=1.8hz,2h),7.87
–
7.81(m,2h),7.77(td,j=7.7,1.8hz,1h),7.59(d,j=7.9hz,1h),7.53
–
7.41(m,2h),7.30
–
7.22(m,1h),6.56(d,j=16.2hz,1h),6.01(d,j=16.2hz,1h),5.12(dt,j=13.8,7.0hz,3h),3.47(d,j=10.7hz,1h),3.38(d,j=10.7hz,1h),2.87(brs,1h),2.03(m,10h),1.67(s,3h),1.59(s,9h),1.53
–
1.33(m,2h),1.08(s,3h).
13
c nmr(100mhz,cdcl3)δ159.1,149.1,138.1,137.0,136.5,135.3,135.04,134.99,133.4,132.6,131.3,130.5,129.3,128.0,127.6,126.7,126.0,125.8,124.7,124.6,124.4,124.2,122.0,70.7,42.5,39.7,37.9,26.8,26.7,25.7,22.8,20.4,17.7,16.1.hrms calculated for c
36
h
46
no[m+h]
+
508.3574,found 508.3583.
[0056]
(petroleum ether/etoac 5/1).1h nmr(400mhz,cdcl3)δ8.71(d,j=4.2hz,1h),7.94(s,2h),7.85(d,j=9.0hz,2h),7.83
–
7.78(m,1h),7.63(d,j=7.8hz,1h),7.48(p,j=6.9hz,2h),7.34
–
7.27(m,1h),6.57(d,j=16.2hz,1h),6.02(d,j=16.2hz,1h),5.11(t,j=6.6hz,8h),3.49(d,j=10.7hz,1h),3.40(d,j=10.7hz,1h).2.07(m,16h),1.98(m,16h),1.60(s,30h).
13
c nmr(100mhz,cdcl3)δ159.0,149.0,138.1,136.9,136.7,135.3,135.1,135.05,134.96,134.94,134.93,134.91,134.88,133.5,132.6,131.2,130.5,129.3,128.0,127.6,126.7,126.0,125.8,124.6,124.4,124.27,124.26,124.24,124.16,122.0,70.7,42.6,39.8,39.7,37.9,26.8,26.72,26.69,25.7,22.7,20.4,17.7,16.1,16.04,16.02.hrms calculated for c
61
h
86
no[m+h]
+
848.6704,found 848.6703.
[0057]
3、不同条件的实施例
[0058]
对于c45萜烯,在手套箱中,向2.0ml封管中,依次加入co(co)i2(10mol%,4.8mg)、agsbf6(20mol%,6.9mg)、多聚甲醛(3.0equiv,9.0mg)、acoh(40mol%,2.4mg)和2-(2-萘基)吡啶1(0.1mmol),用0.25ml dioxane溶解,然后加入c45萜烯2(2.0equiv),在50℃反应16h,结束后,水洗,二氯甲烷萃取,旋干,柱层析分离,流动相为石油醚/乙酸乙酯=4/1(v/v),相应的产率为30%。
[0059]
在手套箱中,向2.0ml封管中,依次加入co(co)i2(5mol%,4.8mg)、agsbf6(10mol%,6.9mg)、多聚甲醛(3.0equiv,18.0mg)、acoh(10mol%,1.2mg)和2-苯基吡啶1a(0.2mmol,31.0mg),用0.50ml dioxane溶解,然后加入异戊二烯2a(2.0equiv,27.2mg),在50℃反应16h,结束后,水洗,二氯甲烷萃取,旋干,柱层析分离,流动相为石油醚/乙酸乙酯=4/1(v/v),相应的产率为57%。
[0060]
在手套箱中,向2.0ml封管中,依次加入co(co)i2(5mol%,4.8mg)、agsbf6(10mol%,6.9mg)、多聚甲醛(3.0equiv,18.0mg)、acoh(20mol%,2.4mg)和2-苯基吡啶1a
(0.2mmol,31.0mg),用0.50ml dioxane溶解,然后加入异戊二烯2a(2.0equiv,27.2mg),在50℃反应16h,结束后,水洗,二氯甲烷萃取,旋干,柱层析分离,流动相为石油醚/乙酸乙酯=4/1(v/v),相应的产率为72%。
[0061]
在手套箱中,向2.0ml封管中,依次加入co(co)i2(5mol%,4.8mg)、agsbf6(10mol%,6.9mg)、多聚甲醛(3.0equiv,18.0mg)、acoh(30mol%,3.6mg)和2-苯基吡啶1a(0.2mmol,31.0mg),用0.50ml dioxane溶解,然后加入异戊二烯2a(2.0equiv,27.2mg),在50℃反应16h,结束后,水洗,二氯甲烷萃取,旋干,柱层析分离,流动相为石油醚/乙酸乙酯=4/1(v/v),相应的产率为86%。
[0062]
在手套箱中,向2.0ml封管中,依次加入co(co)i2(5mol%,4.8mg)、agsbf6(10mol%,6.9mg)、多聚甲醛(3.0equiv,18.0mg)、acoh(20mol%,2.4mg)和2-苯基吡啶1a(0.2mmol,31.0mg),用0.50ml dioxane溶解,然后加入月桂烯(2.0equiv,54.5mg),在50℃反应16h,结束后,水洗,二氯甲烷萃取,旋干,柱层析分离,流动相为石油醚/乙酸乙酯=4/1(v/v),相应的产率为67%。
[0063]
4、应用例
[0064][0065]
上述制备获得的修饰后萜烯可以在mcpba(1.5equiv)的条件下,在常温下转化为环氧化物9,环氧化物9可以和分子内的羟基反应开环,形成七元环状化合物10。修饰后的萜烯还可以在酸性条件下,直接转化为环状化合物10。
[0066]
在手套箱中,向100ml封管中,依次加入co(co)i2(5mol%,119mg)、agsbf6(10mol%,172mg)、多聚甲醛(3.0equiv,450mg)、acoh(40mol%,120mg)和2-(2-萘基)吡啶1s(0.2mmol,1.026g),用12.5ml dioxane溶解,然后加入月桂烯5a(2.0equiv,1.362g),在50℃反应16h,结束后,水洗,二氯甲烷萃取,旋干,柱层析分离,流动相为石油醚/乙酸乙酯=4/1(v/v),6a的产率为84%。
[0067]
在空气中,向4ml反应瓶中,依次加入6a(0.10mmol,37.2mg)、mcpba(间氯过氧苯甲酸,0.15mmol,25.9mg),用1ml二氯甲烷(dcm)溶解,在常温下反应4h,结束后,水洗,二氯甲烷萃取,旋干,柱层析分离,流动相为石油醚/乙酸乙酯=4/1(v/v),产物9的产率为54%。
[0068]
在空气中,向4ml反应瓶中,加入9(0.10mmol,38.8mg),用1ml二氯甲烷(dcm)和水的混合溶剂(1/1,v/v)溶解,在70℃反应24h,结束后,水洗,二氯甲烷萃取,旋干,柱层析分离,流动相为石油醚/乙酸乙酯=4/1(v/v),产物10的产率为26%。
[0069]
在空气中,向4ml反应瓶中,依次加入6a(0.10mmol,37.2mg)、mcpba(间氯过氧苯甲酸,0.15mmol,25.9mg),hotf(三氟甲磺酸,0.03mmol,4.5mg),用1ml二氯甲烷(dcm)溶解,在常温下反应4h,结束后,水洗,二氯甲烷萃取,旋干,柱层析分离,流动相为石油醚/乙酸乙酯=4/1(v/v),产物10的产率为37%。
相关技术
网友询问留言
已有0条留言
- 还没有人留言评论。精彩留言会获得点赞!
1