一种稳定、抗干扰能力强的血清腺苷脱氨酶检测试剂及检测方法与流程
本发明涉及血清腺苷脱氨酶检测技术领域,特别涉及一种血清腺苷脱氨酶检测试剂,还涉及使用此检测试剂的检测方法。
背景技术:
腺苷脱氨酶(ADA)是人体嘌吟核苷酸代谢中的重要酶类,广泛分布于人体各组织中,其中以胸腺、脾和其他淋巴组织中含量最高,而肝、肺、肾和骨胳肌等含量较低;血清中的ADA主要来自肝脏,是反映肝损伤的敏感指标,可作为肝功能常规检查项目之一,依据该酶活性增高或降低反映肝细胞损伤和恢复程度;慢性溶血患者红细胞ADA活性显著升高;肿瘤患者血清及组织中ADA活性均升高;结核性脑膜炎患者CSF-ADA明显升高。
目前,腺苷脱氨酶的测定方法有波氏显色法、氨气敏电极法、酶法、酶偶联法等;波氏显色法试剂简单、易于操作,但该法易受外源性铵根离子的影响;氨气敏电极法虽然抗干扰能力较强,但难以控制电极的响应信号和响应时间,不适于临床大批量样品的测定;酶法较灵敏、可实现自动化分析,但重复性和准确度较差;酶偶联法结果准确、简便实用,但易受抗坏血酸和胆红素等物质干扰。
鉴于此,本发明在酶偶联法的基础上,优化反应体系,添加胆红素氧化酶和抗坏血酸氧化酶,可以有效避免胆红素和抗坏血酸的干扰,大大增强试剂的抗干扰能力,并且采用新的Trinder反应色原物质HTBA(3-羟基-2,4,6-三碘苯甲酸),显著增强了试剂的稳定性和准确度;此外,通过添加稳定剂如丙三醇、聚乙二醇6000、甘露醇、海藻糖、BSA、乙二醇等可有效提高试剂的稳定性;且优选的新型非离子表面活性剂烷基糖苷(APG)的加入可防止反应体系浑浊,增强底物的稳定性,提高试剂的抗干扰能力;该试剂操作简便快速,适用于自动化分析,是一种更加稳定、抗干扰能力强的血清腺苷脱氨酶(ADA)试剂。
技术实现要素:
本发明的目的是提供一种用于检测血清腺苷脱氨酶(ADA)的试剂及使用该试剂检测血清腺苷脱氨酶活性的方法。该试剂盒采用酶偶联法,可以有效检测血清腺苷脱氨酶的活性,抗干扰能力强,稳定性好等优点。
基本原理:
ADA(腺苷脱氨酶)催化底物腺苷脱氨生成次黄嘌呤核苷,次黄嘌呤核苷再经 PNP(嘌呤核苷磷酸化酶)作用生成次黄嘌呤。次黄嘌呤在XOD(黄嘌呤氧化酶)作用下,生成尿酸和过氧化氢(H2O2)。过氧化氢(H2O2) 在过氧化物酶存在的情况下,可与3-羟基-2,4,6-三碘苯甲酸(HTBA)和4-氨基氨替吡啉(4-AA)反应产生有色醒类化合物,有色醒类化合物的生成速率与ADA(腺苷脱氨酶)的活性相关。通过测定550nm波长处吸光度的上升速率,即有色醒类化合物生成速率来测定腺苷脱氨酶的活性。
本发明是通过以下步骤得到的:
一种血清腺苷脱氨酶检测试剂,包括试剂R1和试剂R2,所述试剂R1和试剂R2的组成如下:
试剂R1中含有
缓冲液··························100mmol/L,
4-氨基氨替吡啉··················5mmol/L,
PNP(嘌呤核苷磷酸化酶)···········1.5KU/L,
XOD(黄嘌呤氧化酶)···············3.1KU/L,
POD(过氧化物酶)·················5KU/L,
胆红素氧化酶····················3KU/L,
抗坏血酸氧化酶··················3.5KU/L,
丙三醇··························4ml/L,
聚乙二醇6000····················5g/L,
乙二醇··························6ml/L,
甘露醇··························25g/L,
海藻糖··························15g/L,
BSA·····························1g/L,
烷基糖苷(APG)···················1g/L,
防腐剂··························0.5g/L;
2)试剂R2的组分为:
缓冲液··························100mmol/L,
腺苷····························14mmol/L,
HTBA(3-羟基-2,4,6-三碘苯甲酸)···5mmol/L,
丙三醇··························4ml/L,
聚乙二醇6000····················5g/L,
乙二醇··························6ml/L,
甘露醇··························25g/L,
海藻糖··························15g/L,
BSA·····························1g/L,
烷基糖苷(APG)···················1g/L,
防腐剂··························0.5g/L。
所述的血清腺苷脱氨酶检测试剂,试剂R1中缓冲液为25℃,pH为8.0的HEPES缓冲液。
所述的血清腺苷脱氨酶检测试剂,试剂R2中缓冲液为25℃,pH为4.0的Tris缓冲液。
所述的血清腺苷脱氨酶检测试剂,所述防腐剂为NaN3。
所述的血清腺苷脱氨酶检测试剂来检测血清腺苷脱氨酶含量的检测方法,使用全自动生化分析仪利用速率法进行测定,检测主波长为550nm。
所述的检测方法,R1试剂和R2试剂的比例为2:1。
本发明的有益效果:
1)添加胆红素氧化酶和抗坏血酸氧化酶,可以有效避免胆红素和抗坏血酸的干扰,大大增强试剂的抗干扰能力;
2)采用新的Trinder反应色原物质HTBA(3-羟基-2,4,6-三碘苯甲酸),显著增强了试剂的稳定性和准确度;
3)新型非离子表面活性剂烷基糖苷(APG)的加入可防止反应体系浑浊,增强底物的稳定性,提高试剂的抗干扰能力;
4)优化反应体系并添加丙三醇、聚乙二醇6000、甘露醇、海藻糖、BSA、乙二醇等多种稳定剂,可以显著改善试剂的稳定性;
5)试剂的准确度和稳定性良好,抗干扰性强,使用方便,完全可以满足临床需要。
附图说明
图1为两种试剂的相关性曲线图,
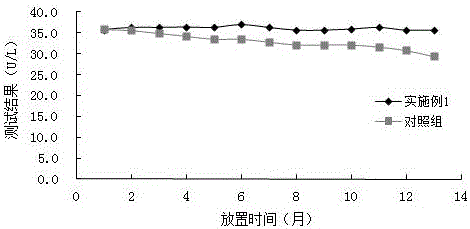
图2为两种试剂效期稳定性曲线图,
图3为实施例1试剂检测方法,
图4为实施例试剂抗干扰性能比较,
图5为实施例1试剂与市场常见并得到认可的血清腺苷脱氨酶测定试剂盒对比检测结果。
具体实施方式
下面结合具体实施例对本发明进行进一步说明。
实施例1
血清腺苷脱氨酶的检测试剂,包括试剂R1和试剂R2:
1)其R1的组成为:
HEPES(4-羟乙基哌嗪乙磺酸)缓冲液(pH=8.0,25℃) ··100mmol/L,
4-氨基氨替吡啉·································5mmol/L,
PNP(嘌呤核苷磷酸化酶)··························1.5KU/L,
XOD(黄嘌呤氧化酶)······························3.1KU/L,
POD(过氧化物酶)································5KU/L,
胆红素氧化酶···································3KU/L,
抗坏血酸氧化酶·································3.5KU/L,
丙三醇·········································4ml/L,
聚乙二醇6000···································5g/L,
乙二醇·········································6ml/L,
甘露醇·········································25g/L,
海藻糖·········································15g/L,
BSA············································1g/L,
烷基糖苷(APG)··································1g/L,
防腐剂NaN3·····································0.5g/L;
2)试剂R2的组分为:
Tris(三羟甲基氨基甲烷)缓冲液(pH=4.0,25℃) ·····100mmol/L,
腺苷···········································14mmol/L,
HTBA(3-羟基-2,4,6-三碘苯甲酸)··················5mmol/L,
丙三醇·········································4ml/L,
聚乙二醇6000···································5g/L,
乙二醇·········································6ml/L,
甘露醇·········································25g/L,
海藻糖·········································15g/L,
BSA············································1g/L,
烷基糖苷(APG)··································1g/L,
防腐剂NaN3·····································0.5g/L;
3)本实施例试剂的使用方法:
本实施例描述的血清腺苷脱氨酶检测试剂,在使用时采用具有双试剂功能的全自动生化分析仪,如日立7180全自动分析仪等,利用速率法进行测定;将R1和R2按照2:1的比例放置到对应的试剂位上,在样品盘的对应位置放置好蒸馏水、标准品和样本,操作如图3。
实施例2
干扰性试验:取新鲜混合血清,分成2等份,然后将每等份再分成5等份,加入不同的干扰物质,使其在血清中的浓度达到图4的要求;然后分别用实施例1所得试剂,与市场常见并认可的血清腺苷脱氨酶(ADA)试剂同时对比测定血清中ADA的活性,对照组测定结果与加入不同干扰物质后各组的测定结果见图4;相对偏差(%)=(干扰样本的测定均值-对照样本的测定均值)/对照样本的测定均值×100%;
由图4可以看出,实施例1试剂在总胆红素≤30mg/dL、甘油三酯≤3000mg/dL、血红蛋白≤800mg/dL、抗坏血酸≤220mg/dL时对测试结果没有明显干扰,而对照组试剂在上述浓度干扰物质存在时,受到明显干扰,这说明通过优化反应缓冲体系、胆红素氧化酶和抗坏血酸氧化酶,及新型非离子表面活性剂烷基糖苷(APG)后,实施例1试剂的抗干扰性能显著提高,远远优于对比试剂。
实施例3
相关性实验:利用实施例1配方配制试剂,与市场常见的国家食品药品监督管理局认可的某公司的腺苷脱氨酶(ADA)试剂盒进行对照检测,同时检测了20个临床血清样本,检测结果如图5所示,并获得了两种试剂的相关性曲线(如图1所示),通过检测结果显示,两个试剂盒的相关系数为0.9996,说明了两者有极大的相关性。
实施例4
试剂的稳定性对比试验:对实施例1中的试剂,均匀分装13组,每组的试剂量R1为20mL,R2为10mL;并且取13组市场常见的国家食品药品监督管理局认可的某公司的腺苷脱氨酶(ADA)试剂盒作对照;放置到2-8℃冰箱中,每月的同一天取出一组试剂检测ADA质控品(靶值为35.7U/L),检测结果如图2所示,实施例1试剂在2-8℃储存条件下比市场常见的腺苷脱氨酶(ADA)测定试剂盒更加稳定;
通过验证,本试剂与同类检测试剂对比相关性好,临床检测样本结果一致,能够达到市场对产品的应用要求,并且抗干扰性能好,是一种更加稳定、良好的腺苷脱氨酶(ADA)检测试剂。
- 还没有人留言评论。精彩留言会获得点赞!