一种天然产物Ovatodiolides的合成方法与流程
本发明提供了一种天然产物ent-ovatodiolide及isoovatodiolide的合成方法,本发明属于药物技术领域。
背景技术:
ovatodiolides是一种大环二萜类化合物。具有显著的抗肿瘤活性,其结构特征是一个含有两个不饱和酯的二萜类化合物。但是其绝对构型一致没有得到确定(p.s.manchand,j.f.blount,j.org.chem.1977,42,3824-3828),为后续的药物化学研究造成很多不便,本方法通过全合成简洁高效完成了ent-ovatodiolide和isoovatodiolide的合成,并确定了天然产物ovatodiolide和isoovatodiolid的绝对立体构型。
技术实现要素:
本本发明提供了一种天然产物ent-ovatodiolide及isoovatodiolide的合成方法。
为了实现本发明的上述目的,本发明提供如下的技术方案:
一种如式(i)所示的天然产物ent-ovatodiolide及isoovatodiolide的合成方法
本发明通过关键的mukaiyamaaldol反应,串联烯丙基硼/内酯化反应和串联rom/rcm反应克级规模合成得到ent-ovatodiolide和isoovatodiolide。
附图说明
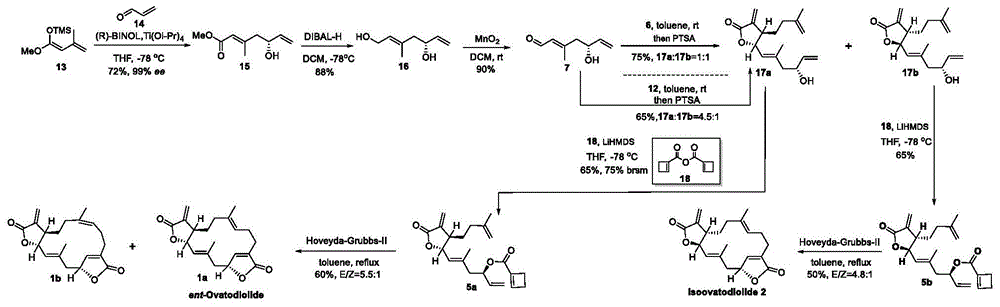
图1天然产物ent-ovatodiolide和isoovatodiolide的合成
图2化合物6和12的合成
具体实施方式
为了理解本发明,下面以实施例进一步说明本发明,但不意于限制本发明的保护范围。
实施例:
具体合成路线见图1和图2,具体步骤如下:
1制备化合物9
-10℃下向8(0.5mthf,1.00l,500.0mmol,2.0eq)的溶液中添加炔丙基氯(18.4ml,250.0mmol,1.0eq)。恢复到室温搅拌2小时。然后将反应再次冷却到-10℃,氯甲酸甲酯(77.4ml,1.00mol,4.0eq)缓慢添加到反应混合物中。反应的颜色变黄,将反应物恢复到室温并搅拌过夜。加入饱和nh4cl(100ml)淬灭反应。水层用etoac(3×300ml)萃取。将合并的有机层na2so4干燥,过滤,真空浓缩。通过柱色谱法(0.6%etoac的己烷溶液)纯化,得化合物9(21.6g,60%),为无色油状物。
rf=0.50(hexanes/etoac=70/1,uv&pma)
ir(neat)1711,1641,1258cm-1
1hnmr(400mhz,cdcl3)δ4.74(d,j=23.8hz,2h),3.72(s,3h),2.44(t,j=7.3hz,2h),2.25(t,j=7.2hz,2h),1.71(s,3h)
13cnmr(101mhz,cdcl3)δ154.2,143.0,111.5,89.1,73.1,52.6,35.4,22.3,17.4
hrms-esi(m/z):calcd.forc9h12o2na[m+na]+:175.0735,found175.0732.
2制备化合物6
0℃条件下向hmpa(141.0ml,808.1mmol,6.0eq)的无水甲苯(250ml)溶液中,逐滴加入dibal-h(1.5mintoluene,134.7ml,200.2mmol,1.5eq)。0℃下将混合物搅拌2小时,然后加入化合物9(20.5g,134.7mmol)。将反应混合物在0℃搅拌8小时。然向体系中加入化合物10(27.6ml,160.2mmol,1.2eq)在室温下搅拌过夜。用乙醚(300ml)稀释,1m盐酸(50ml)溶液淬灭反应。然后用1mhcl(3×60ml)、饱和碳酸氢钠(60ml)、水(2×60ml)、盐水(30ml)依次洗涤有机层。经na2so4干燥,过滤,减压浓缩。通过柱色谱法(3.3%etoac的己烷溶液)纯化,得到化合物6(24.2g,60%)为无色油状物。
rf=0.63(hexanes/etoac=10:1,uv&pma)
ir(neat)2979,2948,1720,1643,1353,1324,1200,1145,884cm-1
1hnmr(400mhz,cdcl3)δ5.93(t,j=7.1hz,1h),4.71(d,j=8.8hz,2h),3.71(s,3h),2.73–2.48(m,2h),2.11(t,j=7.6hz,2h),1.83(s,2h),1.72(s,3h),1.23(s,12h)
13cnmr(101mhz,cdcl3)δ168.4,145.3,142.9,128.1,110.5,83.4,51.3,37.5,27.9,24.8,22.5
hrms-esi(m/z):calcd.forc16h27bo4na[m+na]+:317.1900,found317.1896.
3制备化合物12
室温下向6(500mg,1.70mmol的丙酮(30ml)溶液中加入0.1m醋酸铵溶液(36.9ml)、高碘酸钠(1.10g,5.10mmol,3.0eq)。室温下搅拌过夜。减压除去丙酮,用乙醚(3×25ml)萃取。将所得有机相经na2so4干燥并部分旋蒸,直到剩下30ml乙醚。向该溶液中加入化合物11(419mg,1.70mmol)的乙醚溶液(21ml)和硫酸镁(566mg)。搅拌过夜后,反应混合物通过硅藻土过滤,进行真空浓缩。通过硅胶柱色谱法(3%etoac的己烷溶液)纯化,得到化合物12(581mg,81%)为无色油状物。
rf=0.73(hexanes/etoac=10/1,uv&pma)
[α]22d=+22.9°(c=1.0,chcl3)
ir(neat)2962,1721,1641,1262,1033,760,702cm-1
1hnmr(400mhz,cdcl3)δ7.39(d,j=7.3hz,2h),7.32(t,j=7.4hz,2h),7.27(d,j=7.8hz,1h),5.85(t,j=7.2hz,1h),4.70(d,j=11.3hz,3h),3.29(s,3h),2.63(qd,j=15.5,7.9hz,2h),2.10(dd,j=15.5,6.6hz,3h),1.84(d,j=2.9hz,3h),1.72(s,3h),1.21(s,3h),1.18(d,j=2.8hz,1h),1.14(d,j=4.1hz,1h),1.03(ddd,j=12.2,8.4,5.2hz,1h),0.93(d,j=4.7hz,6h)
13cnmr(101mhz,cdcl3)δ167.9,145.3,142.8,142.0,127.9,127.6,127.3,126.9,110.4,95.9,89.0,52.1,50.8,50.4,49.0,37.5,29.7,27.7,24.9,23.8,22.5,20.9,9.5
hrms-esi(m/z):calcd.forc26h35bo4na[m+na]+:445.2526,found445.2525.
4制备化合物15
向(r)-binol(29.2g,102.0mmol,0.5eq)和cah2(1.36g)的无水thf(200ml)溶液中加入ti(oipr)4(30.2ml,102.0mmol,0.5eq)。在添加过程中颜色从橙色变为棕色和橙色。混合物在室温下搅拌30分钟,然后冷却至-78℃,将丙烯醛14(14.9ml,224.4mmol,1.1eq)的thf(80ml)溶液加入到反应混合物,搅拌10分钟后,加入13溶液(38.0g,204.0mmol)的thf(100ml)溶液。将反应混合物在-78℃下搅拌72小时,然后用nahco3水溶液淬灭反应,用etoac萃取水相(4×350ml)将合并的有机物干燥(na2so4),过滤,真空浓缩,通过柱色谱法(15%etoac的己烷溶液-1.4%meoh的dcm溶液)纯化,得到醇15(25.0g,72%),为无色油状物。
rf=0.30(hexan/etoac=5/1,uv&kmno4)
[α]22d=+14.3°(c=1.0,chcl3);
ir(neat)2960,2925,1719,1708,1642,1260,1018cm-1
1hnmr(400mhz,cdcl3)δ5.96–5.80(m,1h),5.74(s,1h),5.26(d,j=18.0hz,1h),5.13(d,j=10.4hz,1h),4.39–4.22(m,1h),3.67(s,3h),2.34(d,j=6.7hz,2h),2.20(s,3h),1.85(s,1h)
13cnmr(101mhz,cdcl3)δ167.0,156.0,140.1,118.0,115.3,70.7,51.0,48.5,19.1
hrms-esi(m/z):calcd.forc9h14o3na[m+na]+:193.0841,found193.0839.
5制备化合物16
在-78℃下向15(23.0g,133.5mmol)的无水dcm(500ml)溶液中加入dibal-h(30分钟内滴加1.5m甲苯溶液,178.1ml,267.0mmol,2.0eq)。在-78℃下搅拌1小时,通过加入h2o(10.7ml),15%naoh(10.7ml)和h2o(26.7ml)淬灭反应。然后将混合物回热至室温,硅藻土过滤,分离有机层,用饱和食盐水(20ml)洗涤,用na2so4干燥,过滤并进行真空浓缩。通过硅胶柱色谱法(50%etoac/己烷)纯化所得物,得到二醇16(20.5g,88%),为浅黄色油状物。
rf=0.20(hexan/etoac=1/1,uv&kmno4)
[α]22d=+4.9°(c=1.0,chcl3)
ir(neat)3335,3084,2960,2927,1666,1643,1013,990,922,798cm-1
1hnmr(400mhz,cdcl3)δ5.87(ddd,j=17.1,10.4,5.8hz,1h),5.63–5.31(m,1h),5.25(dt,j=17.2,1.4hz,1h),5.10(dt,j=10.4,1.3hz,1h),4.32–3.95(m,3h),2.42(d,j=34.2hz,2h),2.21(ddd,j=22.7,13.7,6.7hz,2h),1.71(s,3h)
13cnmr(101mhz,cdcl3)δ140.7,135.8,127.3,114.7,59.1,47.6,16.5
hrms-esi(m/z):calcd.forc8h14o2na[m+na]+:165.0891,found165.0888.
6制备化合物7
在室温下向16(17.0g,119.5mmol)的无水dcm(300ml)溶液中加入活性mno2(52.0g,597.7mmol,5.0eq)。在室温下搅拌过夜后,将反应混合物通过硅藻土过滤,减压浓缩。通过硅胶柱色谱法(50%etoac的己烷溶液)纯化,得到醛7(15.3g,89%)为红色油状物。
rf=0.44(hexan/etoac=1/1,uv&kmno4)
[α]23d=+26.7°(c=1.0,chcl3)
ir(neat)3428,2917,2849,1665,1439,1260,1198,991,771cm-1
1hnmr(400mhz,cdcl3)δ9.96(d,j=8.0hz,1h),6.29–5.53(m,2h),5.27(d,j=17.2hz,1h),5.14(d,j=10.4hz,1h),4.60–4.18(m,1h),2.41(d,j=6.7hz,2h),2.21(d,j=1.0hz,3h),2.16(d,j=4.1hz,1h)
13cnmr(101mhz,cdcl3)δ191.3,160.3,140.0,129.6,115.7,71.0,48.0,18.2
hrms(ftms+pei)(m/z):calcd.forc8h12o2[m-h]-:139.0759,found139.0757.
7制备化合物17a和17b
在室温下,向7(5.30g,37.6mmol,1.3eq)的无水甲苯(20ml)溶液中加入(z-6-甲基-2-((4,4,5,5-四甲基-1,3,2-二氧杂硼杂环戊烷-2-基)甲基)庚-2,6-二烯酸甲酯(8.00g,29.0mmol)的无水甲苯溶液。将混合物放置在室温下搅拌7天,然后将ptsa(498mg,2.90mmol,0.1eq)加入溶液中。混合物在室温下搅拌30分钟后,用饱和nahco3(35ml)水溶液淬灭反应。水相用etoac(3×30ml)萃取。将合并的有机层用na2so4干燥,过滤并减压浓缩。通过硅胶柱色谱法(6%etoac的己烷溶液)纯化,得到17a(3.00g,37.5%)无色油状物和17b(3.00g,37.5%)无色油状物。.
compound17a:
rf=0.48(hexane/etoac=1/1,uv&i2,pma)
[α]25d=-11.8°(c=1.0,chcl3)
ir(neat)3077,2927,2856,1762,1451,1267,1142,1035,988,958cm-1
1hnmr(400mhz,cdcl3)δ6.28(d,j=2.9hz,1h),5.85(ddd,j=16.8,10.4,6.0hz,1h),5.60(d,j=2.5hz,1h),5.32(d,j=8.3hz,1h),5.26(d,j=17.2hz,1h),5.13(d,j=10.4hz,1h),4.85(dd,j=9.1,5.9hz,1h),4.77(s,1h),4.68(s,1h),4.27(d,j=6.2hz,1h),2.74(ddd,j=8.3,5.8,3.0hz,1h),2.28(d,j=6.6hz,2h),2.06(t,j=7.8hz,2h),1.84(t,j=4.1hz,3h),1.81–1.75(m,1h),1.72(s,3h),1.69(d,j=6.8hz,1h)
13cnmr(101mhz,cdcl3)δ170.3,144.4,140.3,140.2,139.4,126.2,121.7,115.2,111.0,79.7,77.5,77.2,76.8,70.8,47.5,45.5,34.5,30.8,22.5,17.5
hrms-esi(m/z):calcd.forc17h24o3na[m+na]+:299.1623,found299.1620.compound17b:
rf=0.51(hexane/etoac=1/1,uv&i2,pma)
[α]26d=+54.7°(c=1.0,chcl3)
ir(neat)2929,2857,1761,1447,1266,1132,990,961cm-1
1hnmr(400mhz,cdcl3)δ6.27(d,j=1.9hz,1h),5.96–5.66(m,1h),5.59(s,1h),5.47–5.18(m,2h),5.13(d,j=10.4hz,1h),4.86(dd,j=8.9,5.8hz,1h),4.76(s,1h),4.68(s,1h),4.28(s,1h),2.86–2.57(m,1h),2.37–2.21(m,2h),2.07(t,j=7.9hz,2h),1.83(s,3h),1.78(dd,j=23.0,7.4hz,1h),1.72(s,3h),1.68–1.61(m,1h)
13cnmr(101mhz,cdcl3)δ170.4,144.5,140.4,140.2,139.3,126.1,121.8,115.1,111.0,76.8,70.8,47.39,45.6,34.5,30.9,22.5,17.4
hrms-esi(m/z):calcd.forc17h24o3na[m+na]+:299.1623,found299.1622.
8制备化合物5a
向17a(2.40g,8.6mmol)的无水thf(30ml)溶液中加入lihmds(1.0m,11.3ml,11.3mmol,1.3eq)的thf溶液,在-78℃下,,将混合物搅拌30分钟。然后加入化合物18的thf(10ml)。在-78℃下搅拌45分钟后,将混合物用饱和nh4cl水溶液(35ml)淬灭,用etoac(3×30ml)萃取水层。将合并的有机相用na2so4干燥过滤并在减压下浓缩。通过硅胶柱色谱法(4%etoac的己烷溶液)纯化,得到5a(2.10g,65%)浅黄色油状物。
rf=0.52(hexane/etoac=4/1,uv&i2,pma)
[α]23d=-46.5°(c=0.99,chcl3)
ir(neat)2926,2854,1718,1648,1115,749,693cm-1
1hnmr(400mhz,cdcl3)δ6.78(s,1h),6.26(d,j=2.4hz,1h),5.79(ddd,j=17.0,10.5,6.2hz,1h),5.58(d,j=2.0hz,1h),5.45(dd,j=13.1,6.6hz,1h),5.33–5.22(m,2h),5.18(d,j=10.2hz,1h),4.82(dd,j=9.1,5.8hz,1h),4.76(s,1h),4.67(s,1h),2.81–2.60(m,3h),2.52–2.44(m,2h),2.39(dt,j=23.0,6.7hz,2h),2.03(t,j=7.8hz,2h),1.82(s,3h),1.76(dd,j=14.5,6.5hz,1h),1.73–1.64(m,4h)
13cnmr(101mhz,cdcl3)δ170.3,161.4,147.2,144.5,139.3,138.8,138.6,135.9,126.5,121.7,117.2,111.0,79.6,72.3,45.5,44.4,34.5,30.9,29.2,27.3,22.5,17.6s13
hrms-esi(m/z):calcd.forc22h32no4[m+nh4]+:374.2331,found374.2330.
9制备化合物5b
向17b(2.40g,8.60mmol)的无水thf(30ml)溶液中加入lihmds(1.0m,11.3ml,11.3mmol,1.3eq)的thf溶液,在-78℃下,将混合物搅拌30分钟。然后加入化合物18的thf(10ml)。在-78℃下搅拌45分钟后,用饱和nh4cl水溶液(35ml)淬灭混合物分离各层,用etoac(3×30ml)萃取水层。将合并的有机层用na2so4干燥过滤并在减压下浓缩。通过硅胶柱色谱法(4%etoac的己烷溶液)纯化,得到5b(2.10g,65%),为浅黄色油状物。
rf=0.52(hexane/etoac=4/1,uv&i2,pma)
[α]26d=+42.7°(c=0.2,chcl3)
ir(neat)2935,1765,1717,1445,1249,1117,963cm-1
1hnmr(400mhz,cdcl3)δ6.77(s,1h),6.26(d,j=2.7hz,1h),5.80(ddd,j=16.9,10.5,6.0hz,1h),5.56(d,j=2.4hz,1h),5.51–5.41(m,1h),5.32–5.21(m,2h),5.17(d,j=10.5hz,1h),4.86–4.80(m,1h),4.75(s,1h),4.67(s,1h),2.80–2.55(m,3h),2.50–2.39(m,3h),2.38–2.27(m,
1h),2.08–1.95(m,2h),1.82(d,j=1.0hz,3h),1.79–1.72(m,1h),1.71(s,3h),1.69–1.62(m,1h)
13cnmr(101mhz,cdcl3)δ170.2,161.2,146.8,144.4,139.2,138.7,138.6,135.9,126.5,121.6,116.9,110.9,79.5,71.8,45.6,44.7,34.3,30.8,29.2,27.2,22.4,17.2
hrms-esi(m/z):calcd.forc22h32no4[m+nh4]+:374.2331,found374.2326.
10制备化合物1a
将酯5a(2.00g,5.60mmol,1.0eq)和hoveyda-grubbsii催化剂(369mg,0.56mmol,0.1eq)的无水甲苯(3.7l)溶液在稳定的氩气流保护下脱气35分钟。将所得混合物加热至120℃并在氩气氛下搅拌3小时。将所得溶液在减压条件下直接浓缩,然后纯化,在硅胶上进行色谱法(20%etoac的己烷-60%etoac的己烷溶液),得到化合物1a和1b为5.5:1的混合物(1.10g,60%,淡绿色固体),其中可结晶得到纯的白化合物1a和纯的非对映异构体1b(白色固体)。
ent-ovatodiolide(1a):
rf=0.31(hexane/etoac=5/3,uv&i2,pma)
[α]26d=-22.9°(c=1.0,chcl3)
ir(neat)2929,2862,1755,1640,1266,1118,1053,1032,1013cm-1
1hnmr(400mhz,cdcl3)δ7.00(s,1h),6.21(d,j=1.7hz,1h),5.59(d,j=1.5hz,1h),5.15(d,j=10.2hz,1h),5.10(s,1h),4.85(dd,j=15.9,8.0hz,2h),2.86(dd,j=14.3,3.8hz,1h),2.83–2.78(m,1h),2.59–2.49(m,1h),2.48–2.44(m,1h),2.44–2.37(m,1h),2.28(dd,j=14.4,3.7hz,1h),2.25–2.18(m,1h),2.18–2.09(m,1h),2.09–1.99(m,1h),1.73(s,3h),1.69–1.62(m,2h),1.61(s,3h)
13cnmr(101mhz,cdcl3)δ173.1,170.5,147.4,139.9,134.7,134.5,131.4,129.3,125.1,122.9,78.9,78.1,42.9,40.5,36.5,33.4,25.1,23.9,19.5,15.3
hrms-esi(m/z):calcd.forc20h24o4na[m+na]+:351.1572,found351.1570.compound1b:
[α]29d=-31.8°(c=0.19,chcl3)
ir(neat)1751,1636,1558,1507,1491,1456,1418,1395,1372,1260,1219,1017,772cm-1
1hnmr(400mhz,cdcl3)δ6.95(s,1h),6.22(d,j=1.8hz,1h),5.65(d,j=1.6hz,1h),5.23–5.01(m,3h),4.68(dd,j=10.0,2.8hz,1h),2.83(dd,j=14.1,3.6hz,1h),2.77–2.64(m,1h),2.56–2.33(m,4h),2.27(dd,j=14.1,3.7hz,1h),2.16–2.02(m,1h),2.02–1.90(m,1h),1.90–1.77(m,2h),1.73(s,3h),1.66(s,3h)
13cnmr(101mhz,cdcl3)δ173.4,170.4,146.8,140.2,135.4,134.1,133.4,128.6,127.5,122.6,79.7,78.6,46.0,40.6,32.9,30.4,26.3,26.0,22.9,19.5,15.3
hrms-esi(m/z):calcd.forc20h24o4na[m+na]+:351.1572,found351.1571.
11制备化合物2
将酯5b(332mg,0.930mmol,1.0eq)和grubbs-hoveydaii催化剂(58.4mg,0.0930mmol,0.1eq)的无水甲苯(620ml)溶液在稳定的氩气流保护下脱气35分钟。将所得混合物加热至120℃并在氩气下搅拌3小时。将所得溶液在减压条件下直接浓缩,然后纯化,通过硅胶柱色谱法(20%etoac的己烷-60%etoac的己烷溶液)得到化合物2(166mg,50%,白色固体)。
rf=0.34(hexane/etoac=5/3,uv&i2,pma)
[α]25d=-305.9°(c=0.5,chcl3)
ir(neat)2961,2925,1741,1261,1219,1094,1021,799,772cm-1
1hnmr(400mhz,cdcl3)δ6.89(s,1h),6.25(d,j=2.0hz,1h),5.60(d,j=1.5hz,1h),5.17(s,1h),5.07(d,j=9.8hz,1h),4.88(dd,j=9.8,2.3hz,1h),4.82(t,j=6.5hz,1h),2.83(dd,j=14.4,3.5hz,1h),2.69–2.54(m,2h),2.51(dd,j=14.4,3.5hz,2h),2.37–2.27(m,2h),2.24–2.16(m,1h),2.16–2.06(m,1h),1.72(s,3h),1.71–1.64(m,2h),1.62(s,3h)
13cnmr(101mhz,cdcl3)δ173.6,170.3,148.8,140.1,136.3,133.7,128.1,125.2,122.5,80.8,77.5,43.2,41.7,36.0,32.6,25.3,24.1,18.5,15.7
hrms-esi(m/z):calcd.forc20h24o4na[m+na]+:351.1572,found351.1570。
- 还没有人留言评论。精彩留言会获得点赞!
- 天然产物活性成分的加压逆循环提取装置的制造方法
- 一种海洋天然产物Haterumadienone的合成方法
- 天然产物(-)-(6S,2’R)-cryptocaryalactone的合成方法
- 一种天然产物抽提过程强化方法
- 从天然资源产生产物的制作方法
- 一种用于糖尿病防治的天然产物组合及其应用
- 一种天然产物6′-hydroxy justicidin B在制备治疗慢性粒细胞白血病药物中的应用
- 一种海洋天然产物(r,z)-24-甲基-二十五碳-16-丁烯-2,4-二炔-1,6-二醇及其对映体的 ...的制作方法
- 一种海洋天然产物(+)-(4e,15z)-4,15-二十二碳二烯-1-炔-3-醇及其对映体的合成方法
- 一种无糖天然产物保健粉的制备方法
- 天然产物活性成分的加压逆循环提取装置的制造方法
- 一种海洋天然产物Haterumadienone的合成方法
- 天然产物(-)-(6S,2’R)-cryptocaryalactone的合成方法
- 一种天然产物抽提过程强化方法
- 从天然资源产生产物的制作方法
- 一种用于糖尿病防治的天然产物组合及其应用
- 一种天然产物6′-hydroxy justicidin B在制备治疗慢性粒细胞白血病药物中的应用
- 一种海洋天然产物(r,z)-24-甲基-二十五碳-16-丁烯-2,4-二炔-1,6-二醇及其对映体的 ...的制作方法
- 一种海洋天然产物(+)-(4e,15z)-4,15-二十二碳二烯-1-炔-3-醇及其对映体的合成方法
- 一种无糖天然产物保健粉的制备方法