中国延龄草属或重楼属药材中单体物质的制备方法及其在制备治疗阿尔茨海默病药物中应用与流程
本发明涉及中国延龄草属或重楼属药材的根及根茎或果实或全草中甾体皂苷类、倍半萜类、苯丙素苷类和酚酸类物质的制备方法及其在制备治疗老年痴呆以及阿尔茨海默病药物中的应用,属于中医药制备技术领域。
背景技术:
阿尔茨海默病(Alzheimer's Disease,AD)是一种神经系统退行性疾病,老年人发病率较高,临床主要表现为记忆力减退、定向障碍和判断推理能力受损等。现在比较公认AD(即老年期痴呆)的分类包括老年性痴呆、血管性痴呆和混合性痴呆。老年斑是AD的主要病理标志,其中Aβ聚集是中心环节,也是AD发病的关键性因素和关键性治疗靶点,干扰Aβ的产生和阻止其聚集是防治AD的有效途径,开发抑制Aβ纤丝形成和聚集的新药对预防和对症治疗AD具有良好的学术与临床应用价值。
中国延龄草属3种药材来源为百合科延龄草属植物延龄草Trillium tschonoskiiMaxim.、吉林延龄草Trillium kamtschaticum Pall.ex Pursh. (或白花延龄草Trillium camschatcense Ker Gawl.)和西藏延龄草Trillium govanianum Wall.ex Royle.的根及根茎或果实或全草。中国重楼属17种药材来源为百合科重楼属植物云南重楼(滇重楼)Paris polyphylla Smith var. yunnanensis (Franch.) Hand.-Mazz.、七叶一枝花(华重楼)Paris polyphylla Smith var. chinensis (Franch.) Hara.、狭叶重楼Paris polyphylla var. stenophylla Franch.、巴山重楼Paris bashanensis F. T. Wang &Tang.、凌云重楼Paris cronquistii (Takht.) H. Li.、金线重楼Paris delavayi Franch.、海南重楼Paris dunniana H. Lév.、球药隔重楼Paris fargesii Franch.、长柱重楼Paris forrestii (Takht.) H.Li.、禄劝花叶重楼Paris luquanensis H. Li.、毛重楼Paris mairei H. Lév.、花叶重楼Paris marmorata Stearn.、四叶重楼Paris quadrifolia L.、皱叶重楼Paris rugosa H. Li & Kurita.、黑籽重楼Paris thibetica Franch.、北重楼Paris verticillata M. Bieb.、南重楼Paris vietnamensis (Takht.) H. Li. 的根茎或全草。化学研究表明,中国延龄草属和重楼属药材中主要含有甾体皂苷类、倍半萜类、苯丙素苷类和酚酸类成分为主,其中中国延龄草属和重楼属中甾体皂苷类成分类型基本一致。发明人体外细胞培养实验中,利用Aβ25-35致PC12细胞损伤模型,采用MTT法检测了各实验组细胞的活力,其中主要是偏诺皂苷类单体、延龄草皂苷类单体、薯蓣皂苷类单体、甾醇皂苷类单体、呋甾烷皂苷类单体、酚酸类单体、倍半萜单体、倍半萜类单体具有抑制Aβ纤丝形成和阻止其聚集的生物活性。因拥有共同的偏诺皂苷元、延龄草皂苷元、薯蓣皂苷元、甾醇皂苷元、呋甾烷皂苷元、苯丙素类、倍半萜类等相同或相似母核结构单元,而在人体中具有类似的治疗功效。上述研究还没有报道,我们通过系统的体外实验研究,首先发现其对由衰老、睡眠障碍、心脑血管疾病(心脏病、高血压、动脉粥样硬化和短暂性脑缺血发作等)、神经系统疾病等导致的淀粉样蛋白Aβ沉积或Aβ纤丝聚集形成的老年痴呆以及阿尔茨海默病疾病具有良好的治疗作用。
技术实现要素:
中国延龄草属或重楼属药材中单体物质的制备方法及其在制备治疗阿尔茨海默病药物中应用。
本发明采用了下述技术方案。
中国延龄草属或重楼属药材提取物的制备方法:将中国延龄草属或重楼属药材的根及根茎或果实或全草粉碎成粗粉,用沸水或30-85%乙醇浸泡1-2小时,4-10倍量溶剂加热回流提取2-4次,每次1-3小时,过滤,合并滤液。上述滤液与45-80℃减压浓缩至0.6-1.2g生药材/ml,0-4℃冷藏过夜,析胶,过滤,滤液再用正丁醇萃取回收得浸膏稀释上大孔吸附树脂或滤液直接上大孔吸附树脂AB-8型或D-101型或HPD-400或各种型号大孔吸附树脂富集,先用水洗脱,依次再用乙醇水由30-85%洗脱得到各洗脱部位,主要收集乙醇水30%、60%、85%的洗脱部位,各洗脱部位在45-80℃减压回收溶剂至乙醇适量浓度,用冷冻干燥法或喷雾干燥进行干燥,收集冻干粉或喷雾干燥粉,即得。
中国延龄草属或重楼属药材的根及根茎或果实或全草中甾体皂苷类、倍半萜类、苯丙素苷类、酚酸类物质中的偏诺皂苷元-3-O-α-L-吡喃鼠李糖基(1→2)-O-β-D-吡喃葡萄糖苷、偏诺皂苷元-3-O-α-L-吡喃鼠李糖基(1→4)-[O-α-L-吡喃鼠李糖基(1→2)]-O-β-D-吡喃葡萄糖苷、偏诺皂苷元-3-O-α-L-吡喃鼠李糖基(1→4)-O-α-L-吡喃鼠李糖基(1→4)-[O-α-L-吡喃鼠李糖基(1→2)]-O-β-D-吡喃葡萄糖苷、偏诺皂苷元-3β-O-β-D-吡喃葡萄糖基(1→6)-[O-α-L-吡喃鼠李糖基(1→2)]-O-β-D-吡喃葡萄糖苷、27-羟基-偏诺皂苷元-3β-O-β-D-吡喃葡萄糖基(1→6)-[O-α-L-吡喃鼠李糖基(1→2)]-O-β-D-吡喃葡萄糖苷、3-乙酰基-偏诺皂苷元、dioscoreanoside I、trillenoside A、deoxytrillenoside A、24-acetyl-deoxytrillenoside A、24-epiacetyl-deoxytrillenoside A、24-methoxy-trillenoside A、trillenoside B、24-acetyl-trillenoside B、24-acetyl-
deoxytrillenoside B、trillenoside C、Epitrillenogenin-24-O-acetate-1-O-[2,3,4-tri-O-
acetyl-α-L-rhamnopyra-nosyl-(1→2)-α-L-arabinopyranoside]、Epitrillenogenin-1-O-
[2,3,4-tri-O-acetyl-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside]、Epitrilleno-
genin-24-O-acetate-1-O-[2,4-di-O-acetyl-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopy-
ranoside]、Epitrillenogenin-1-O-[2,4-di-O-acetyl-α-L-rhamnopyranosyl-(1→2)-α- L-
arabinopyranoside]、Epitrillenogenin-1-O-[4-O-acetyl-α-L-rhamnopyranosyl-(1→2)-α-
L-arabinopyranoside]、薯蓣皂苷元-3-O-α-L-吡喃鼠李糖基(1→2)-O-β-D-葡萄糖苷、薯蓣皂苷元-3-O-α-L-吡喃鼠李糖基(1→4)-[O-α-L-吡喃鼠李糖基(1→2)]-O-β-D-葡萄糖苷、薯蓣皂苷元-3-O-α-L-吡喃鼠李糖基(1→4)-O-α-L-吡喃鼠李糖基(1→4)-[O-α-L-吡喃鼠李糖基(1→2)]-O-β-D-吡喃葡萄糖苷、(23s,24s,25s)-螺甾-5-烯-1β, 3β, 21, 23, 24-五羟基-1β-O-β-D-呋喃芹糖基-(1→3)-O-α-L-吡喃鼠李糖基-(1→2)-[O-β-D-吡喃木糖基(1→3)]-O-α-L-吡喃阿拉伯糖苷、(23s,24s,25s)-螺甾-5-烯-1β, 3β, 23, 24-四羟基-1β-O-β-D-呋喃芹糖基-(1→3)-O-α-L-吡喃鼠李糖基-(1→2)-[O-β-D-吡喃木糖基(1→3)]-O-α-L-吡喃阿拉伯糖苷、(23s,24s,25s)-螺甾-5-烯-1β, 3β, 21, 23, 24-五羟基-1β-O-α-L-吡喃鼠李糖基-(1→2)-[O-β-D-吡喃木糖基(1→3)]-O-α-L-吡喃阿拉伯糖苷、β-脱皮激素、pinnatasterone、∆5,13-20βF,22αF,25αF螺甾烯-3β,21α-二醇、polypodineB、intergristerone B、sileneoside G、26-O-β-D-吡喃葡萄糖基-(25s)-5α-呋甾烷-△20(22)-烯-3β,26-二醇-3-O-α-L-吡喃鼠李糖基(1→4)-[O-α-L-吡喃鼠李糖基(1→2)]-O-β-D-吡喃葡萄糖苷、26-O-β-D-吡喃葡萄糖基-(25s)-5α-呋甾烷-△20(22)-烯-3β, 26-二醇-3-O-α-L-吡喃鼠李糖基(1→4)-O-β-D-吡喃葡萄糖苷、26-O-β-D-吡喃葡萄糖基-(25s)-5α-呋甾烷-△5(6),20(22)-二烯-3β, 26-二醇-3-O-α-L-吡喃鼠李糖基(1→4)-O-β-D-吡喃葡萄糖苷、26-O-β-D-吡喃葡萄糖基-(25R)-Δ5(6)、17(20)-二烯-16,22-二酮-呋甾烷-3β,26-二醇-3-O-α-L-吡喃鼠李糖基(1→4)-O-α-L-吡喃鼠李糖基(1→4)[O-α-L-吡喃鼠李糖基(1→2)-]-O-β-D-吡喃葡萄糖苷、26-O-β-D-吡喃葡萄糖基-(25R)-Δ5(6)、17(20)-二烯-16-羟基-22-酮-呋甾烷-3β,26-二醇-3-O-α-L-吡喃鼠李糖基(1→2) [O-α-L-吡喃鼠李糖基(1→4)-]-O-β-D-吡喃葡萄糖苷、26-O-β-D-吡喃葡萄糖基-(25R)-Δ5(6)、17(20)-二烯-16,22-二酮-呋甾烷-3β,26-二醇-3-O-α-L-吡喃鼠李糖基(1→2)[O-α-L-吡喃鼠李糖基(1→4)-]-O-β-D-吡喃葡萄糖苷、26-O-β-D-吡喃葡萄糖基-(25R)-Δ5(6)、17(20) -二烯-16,22-二酮-呋甾烷-3β,26-二醇-3-O-α-L-吡喃鼠李糖基(1→2)-O-β-D-吡喃葡萄糖苷、3β-O-α-L-rhamnopyranosyl-(1→4)-[O-α-L-rhamno-
pyranosyl-(1→2)]-O-β-D-glucopyranosylhomo-aro-cholest-5-ene-26-O-β-D-glucopyranoside(aethiosidecA)、3β-O-α-L-rhamnopyranosyl-(1→4)-O-α-L-rhamnopyranosyl-
(1→4)-[O-α-L-rhamnopyranosyl-(1→2)]-O-β-D-glucopyranosylhomo-aro-cholest-5-ene-26-O-β-D-glucopyranoside(parispseudosideA)、3β-O-α-L-rhamnopyranosyl-(1→4)-
[O-α-L-rhamnopyranosyl-(1→2)]-O-β-D-glucopyranosylhomo-16′-hydroxyl-aro-cholest-5-ene-26-O-β-D-glucopyranoside(16′-hydroxyl-parispseudosideB)、延龄草倍半萜苷C或7,11-二甲基-3-亚甲基-1,6-十二碳二烯-10,11-二羟基-10-O-β-D-吡喃葡萄糖苷、延龄草倍半萜苷A或7,11-二甲基-3-亚甲基-1,6-十二碳二烯-10,11-二羟基-10-O-β-D-吡喃葡萄糖基-(1→4)-O-β-D-吡喃葡萄糖苷、7,11-二甲基-3-亚甲基-10,11-二羟基-1,6-十二碳二烯二醇、2,6,10-三甲基-2,10-二羟基-6-十二碳烯二醇、3,7,11-三甲基-3,9,11-三羟基-1,6-十二碳二烯三醇、7,11-二甲基-3-羟甲基-1,6-十二碳二烯-10,11,15-三醇-10-O-间苯酚、trillium acid A、trillium acid B、trillium acid C、methyl ferulorate、regaloside A、3-O-feruloylsucrose、heronioside A的制备方法:将权利要求1-11所得中国延龄草属或重楼属药材的根及根茎或果实或全草提取物上硅胶柱,上样后依次用三氯甲烷:甲醇或二氯甲烷:甲醇或三氯甲烷:甲醇:水或二氯甲烷:甲醇:水梯度洗脱,以上述中国延龄草属或重楼属药材的根及根茎或果实或全草中甾体皂苷类、倍半萜类、苯丙素苷类、酚酸类的相应化合物单体作为对照品TLC或HPLC检测,合并相应流份。上述流份于45-80℃减压浓缩蒸干,用适量蒸馏水或适当溶剂混悬或溶解,上反相硅胶ODS-C18柱色谱或SephadexLH-20柱色谱或制备高效液相色谱技术分离纯化,流动相为甲醇水5-100%或乙腈水5-100%的等度或梯度洗脱,洗脱液用冷冻干燥法或喷雾干燥进行干燥,收集冻干粉或喷雾干燥粉,用95%乙醇结晶并重结晶,即得。
前述方法制备的中国延龄草属或重楼属药材的根及根茎或果实或全草中甾体皂苷类、倍半萜类、苯丙素苷类、酚酸类物质,其有效成分为偏诺皂苷元-3-O-α-L-吡喃鼠李糖基(1→2)-O-β-D-吡喃葡萄糖苷、偏诺皂苷元-3-O-α-L-吡喃鼠李糖基(1→4)-[O-α-L-吡喃鼠李糖基(1→2)]-O-β-D-吡喃葡萄糖苷、偏诺皂苷元-3-O-α-L-吡喃鼠李糖基(1→4)-O-α-L-吡喃鼠李糖基(1→4)-[O-α-L-吡喃鼠李糖基(1→2)]-O-β-D-吡喃葡萄糖苷、偏诺皂苷元-3β-O-β-D-吡喃葡萄糖基(1→6)-[O-α-L-吡喃鼠李糖基(1→2)]-O-β-D-吡喃葡萄糖苷、27-羟基-偏诺皂苷元-3β-O-β-D-吡喃葡萄糖基(1→6)-[O-α-L-吡喃鼠李糖基(1→2)]-O-β-D-吡喃葡萄糖苷、3-乙酰基-偏诺皂苷元、dioscoreanoside I、trillenoside A、deoxytrillenoside A、24-acetyl-deoxytrillen-
oside A、24-epiacetyl-deoxytrillenoside A、24-methoxy-trillenoside A、trillenoside B、24-acetyl-trillenoside B、24-acetyl-deoxytrillenoside B、trillenoside C、Epitrilleno-
genin-24-O-acetate-1-O-[2,3,4-tri-O-acetyl-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside]、Epitrillenogenin-1-O-[2,3,4-tri-O-acetyl-α-L-rhamnopyranosyl-(1→2)-α-L-
arabinopyranoside]、Epitrillenogenin-24-O-acetate-1-O-[2,4-di-O-acetyl-α-L-rhamno-
pyranosyl-(1→2)-α-L-arabinopyranoside]、Epitrillenogenin-1-O-[2,4-di-O-acetyl-α-L-
rhamnopy-ranosyl-(1→2)-α-L-arabinopyranoside]、Epitrillenogenin-1-O-[4-O-acetyl-
α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside]、薯蓣皂苷元-3-O-α-L-吡喃鼠李糖基(1→2)-O-β-D-葡萄糖苷、薯蓣皂苷元-3-O-α-L-吡喃鼠李糖基(1→4)-[O-α-L-吡喃鼠李糖基(1→2)]-O-β-D-葡萄糖苷、薯蓣皂苷元-3-O-α-L-吡喃鼠李糖基(1→4)-O-α-L-吡喃鼠李糖基(1→4)-[O-α-L-吡喃鼠李糖基(1→2)]-O-β-D-吡喃葡萄糖苷、(23s,24s,25s)-螺甾-5-烯-1β, 3β, 21, 23, 24-五羟基-1β-O-β-D-呋喃芹糖基-(1→3)-O-α-L-吡喃鼠李糖基-(1→2)-[O-β-D-吡喃木糖基(1→3)]-O-α-L-吡喃阿拉伯糖苷、(23s,24s,25s)-螺甾-5-烯-1β, 3β, 23, 24-四羟基-1β-O-β-D-呋喃芹糖基-(1→3)-O-α-L-吡喃鼠李糖基-(1→2)-[O-β-D-吡喃木糖基(1→3)]-O-α-L-吡喃阿拉伯糖苷、(23s,24s,25s)-螺甾-5-烯-1β, 3β, 21, 23, 24-五羟基-1β-O-α-L-吡喃鼠李糖基-(1→2)-[O-β-D-吡喃木糖基(1→3)]-O-α-L-吡喃阿拉伯糖苷、β-脱皮激素、pinnatasterone、∆5,13-20βF,22αF,25αF螺甾烯-3β,21α-二醇、polypodineB、intergristerone B、sileneoside G、26-O-β-D-吡喃葡萄糖基-(25s)-5α-呋甾烷-△20(22)-烯-3β,26-二醇-3-O-α-L-吡喃鼠李糖基(1→4)-[O-α-L-吡喃鼠李糖基(1→2)]-O-β-D-吡喃葡萄糖苷、26-O-β-D-吡喃葡萄糖基-(25s)-5α-呋甾烷-△20(22)-烯-3β, 26-二醇-3-O-α-L-吡喃鼠李糖基(1→4)-O-β-D-吡喃葡萄糖苷、26-O-β-D-吡喃葡萄糖基-(25s)-5α-呋甾烷-△5(6),20(22)-二烯-3β, 26-二醇-3-O-α-L-吡喃鼠李糖基(1→4)-O-β-D-吡喃葡萄糖苷、26-O-β-D-吡喃葡萄糖基-(25R)-Δ5(6)、17(20)-二烯-16,22-二酮-呋甾烷-3β,26-二醇-3-O-α-L-吡喃鼠李糖基(1→4)-O-α-L-吡喃鼠李糖基(1→4)[O-α-L-吡喃鼠李糖基(1→2)-]-O-β-D-吡喃葡萄糖苷、26-O-β-D-吡喃葡萄糖基-(25R)-Δ5(6)、17(20)-二烯-16-羟基-22-酮-呋甾烷-3β,26-二醇-3-O-α-L-吡喃鼠李糖基(1→2) [O-α-L-吡喃鼠李糖基(1→4)-]-O-β-D-吡喃葡萄糖苷、26-O-β-D-吡喃葡萄糖基-(25R)-Δ5(6)、17(20)-二烯-16,22-二酮-呋甾烷-3β,26-二醇-3-O-α-L-吡喃鼠李糖基(1→2)[O-α-L-吡喃鼠李糖基(1→4)-]-O-β-D-吡喃葡萄糖苷、26-O-β-D-吡喃葡萄糖基-(25R)-Δ5(6)、17(20) -二烯-16,22-二酮-呋甾烷-3β,26-二醇-3-O-α-L-吡喃鼠李糖基(1→2)-O-β-D-吡喃葡萄糖苷、3β-O-α-L-rhamnopyranosyl-(1→4)-[O-α-L-rhamno-
pyranosyl-(1→2)]-O-β-D-glucopyranosylhomo-aro-cholest-5-ene-26-O-β-D-glucopyranoside(aethiosidecA)、3β-O-α-L-rhamnopyranosyl-(1→4)-O-α-L-rhamnopyranosyl-
(1→4)-[O-α-L-rhamnopyranosyl-(1→2)]-O-β-D-glucopyranosylhomo-aro-cholest-5-ene-26-O-β-D-glucopyranoside(parispseudosideA)、3β-O-α-L-rhamnopyranosyl-(1→4)-
[O-α-L-rhamnopyranosyl-(1→2)]-O-β-D-glucopyranosylhomo-16′-hydroxyl-aro-cholest-5-ene-26-O-β-D-glucopyranoside(16′-hydroxyl-parispseudosideB)、延龄草倍半萜苷C或7,11-二甲基-3-亚甲基-1,6-十二碳二烯-10,11-二羟基-10-O-β-D-吡喃葡萄糖苷、延龄草倍半萜苷A或7,11-二甲基-3-亚甲基-1,6-十二碳二烯-10,11-二羟基-10-O-β-D-吡喃葡萄糖基-(1→4)-O-β-D-吡喃葡萄糖苷、7,11-二甲基-3-亚甲基-10,11-二羟基-1,6-十二碳二烯二醇、2,6,10-三甲基-2,10-二羟基-6-十二碳烯二醇、3,7,11-三甲基-3,9,11-三羟基-1,6-十二碳二烯三醇、7,11-二甲基-3-羟甲基-1,6-十二碳二烯-10,11,15-三醇-10-O-间苯酚、trillium acid A、trillium acid B、trillium acid C、methyl ferulorate、regaloside A、3-O-feruloylsucrose、heronioside A中的一种或多种,或其该物质中的皂苷元的第21位和第27位的转化羟甲基的羟基或皂苷元所含羟基或所有糖基的醇羟基被乙酰基、脂肪链烷烃基、芳香族取代基、酰胺基中的任意一种取代,或者是水解后会产生相应皂苷元的物质,可用于制备治疗老年痴呆以及阿尔茨海默病的药物,所述药物包括口服液、胶囊、片剂、泡腾片和注射剂等各种已知剂型或可接受剂型,所述药物还包括各种单方和复方制剂。
所涉及的老年痴呆以及阿尔茨海默病是由衰老、睡眠障碍、心脑血管疾病(心脏病、高血压、动脉粥样硬化和短暂性脑缺血发作等)、精神系统疾病导致的淀粉样蛋白Aβ沉积或Aβ纤丝聚集形成的老年痴呆以及阿尔茨海默病疾病中的任意一种。
本发明的优点是:提供了中国延龄草属或重楼属药材的根及根茎或果实或全草中甾体皂苷类、倍半萜类、苯丙素苷类、酚酸类物质的提取与制备方法,所得物质可用于制备治疗异质性及多因性老年痴呆以及阿尔茨海默病或制备各类应用药物的口服液、胶囊、片剂、泡腾片、粉针剂、水针剂、注射剂或各种剂型制剂的原料药。
中国延龄草属或重楼属药材的根及根茎或果实或全草中甾体皂苷类、倍半萜类、苯丙素苷类、酚酸类物质在体内外对由衰老、睡眠障碍、心脑血管疾病(心脏病、高血压、动脉粥样硬化和短暂性脑缺血发作等)、精神系统疾病导致的淀粉样蛋白Aβ沉积或Aβ纤丝聚集形成的老年痴呆以及阿尔茨海默病具有良好的治疗作用和较强的生物活性。
中国延龄草属或重楼属药材的根及根茎或果实或全草中甾体皂苷类、倍半萜类、苯丙素苷类、酚酸类物质的制备方法主要通过从天然药物中提取,为纯天然组分与单体,具有良好的生物相容性。其中,单体化合物26-O-β-D-吡喃葡萄糖基-(25R)-Δ5(6)、17(20)-二烯-16,22-二酮-呋甾烷-3β,26-二醇-3-O-α-L-吡喃鼠李糖基(1→4)-O-α-L-吡喃鼠李糖基(1→4)[O-α-L-吡喃鼠李糖基(1→2)-]-O-β-D-吡喃葡萄糖苷、26-O-β-D-吡喃葡萄糖基-(25R)-Δ5(6)、17(20)-二烯-16-羟基-22-酮-呋甾烷-3β,26-二醇-3-O-α-L-吡喃鼠李糖基(1→2) [O-α-L-吡喃鼠李糖基(1→4)-]-O-β-D-吡喃葡萄糖苷、3β-O-α-L-rhamnopyranosyl-(1→2)-O-β-D-glucopyranosylhomo-16′-hydroxyl-
aro-cholest-5-ene-26-O-β-D-glucopyranoside(16′-hydroxyl-parispseudosideB)、7,11-二甲基-3-羟甲基-1,6-十二碳二烯-10,11,15-三醇-10-O-间苯酚、trillium acid A、trillium acid B、trillium acid C为新化合物。
附图说明
图1 为24h和48h后不同浓度的Aβ25-35对PC12细胞活力的影响;
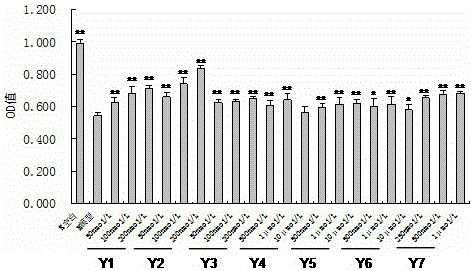
图2为不同浓度的偏诺皂苷类化合物Y1~Y7对受损的PC12细胞的保护作用;
图3为不同浓度的延龄草皂苷类化合物Y8~Y14对受损的PC12细胞的保护作用。
图4为不同浓度的延龄草皂苷类化合物Y15~Y21对受损PC12细胞的保护作用。
图5为不同浓度的薯蓣皂苷类化合物Y22~Y27对受损的PC12细胞的保护作用。
图6为不同浓度的甾醇类化合物Y28~Y33对受损的PC12细胞的保护作用。
图7为不同浓度的呋甾烷皂苷类化合物Y34~Y40对受损PC12细胞的保护作用。
图8为不同浓度的倍半萜类单体化合物Y44~Y49对受损PC12细胞的保护作用。
图9为不同浓度的呋甾烷皂苷类化合物Y41~Y43对受损PC12细胞的保护作用。
图10不同浓度的酚酸类化合物Y50~Y52对受损PC12细胞的保护作用。
图11不同浓度的苯丙素类化合物Y53~Y56对受损PC12细胞的保护作用。
具体实施方式
下面结合实施例对本发明作进一步描述:
实施例1
中国延龄草属或重楼属药材的根及根茎或果实或全草提取物及60-85%乙醇洗脱部位所含偏诺皂苷类物质中偏诺皂苷元-3-O-α-L-吡喃鼠李糖基(1→2)-O-β-D-吡喃葡萄糖苷、偏诺皂苷元-3-O-α-L-吡喃鼠李糖基(1→4)-[-O-α-L-吡喃鼠李糖基(1→2)]-O-β-D-吡喃葡萄糖苷、偏诺皂苷元-3-O-α-L-吡喃鼠李糖基(1→4)-O-α-L-吡喃鼠李糖基(1→4)-[ O-α-L-吡喃鼠李糖基(1→2)]-O-β-D-吡喃葡萄糖苷、偏诺皂苷元-3β-O-β-D-吡喃葡萄糖基(1→6)-[O-α-L-吡喃鼠李糖基(1→2)]-O-β-D-吡喃葡萄糖苷、27-羟基-偏诺皂苷元-3β-O-β-D-吡喃葡萄糖基(1→6)-[O-α-L-吡喃鼠李糖基(1→2)]-O-β-D-吡喃葡萄糖苷、3-乙酰基-偏诺皂苷元、dioscoreanoside I的制备方法:将中国延龄草属或重楼属药材的根及根茎或果实或全草粉碎成粗粉,用60%乙醇浸泡1小时,10倍量溶剂加热回流提取3次,每次2小时,过滤,合并滤液。上述滤液与65℃减压浓缩至1.0g生药材/ml,0-4℃冷藏过夜,析胶,过滤,滤液再用正丁醇等有机溶剂萃取,回收有机溶剂,得浸膏加水混悬,上AB-8型号大孔吸附树脂富集,依次用水、30%乙醇、60%乙醇和85%乙醇洗脱,得到各洗脱部位,用冷冻干燥法进行干燥,收集冻干粉,即得。其中60- 85%的洗脱部位以甾体皂苷类成分为主,甾体皂苷总含量大于90%,即得中国延龄草属或重楼属药材的根及根茎或果实或全草提取物或60-85%乙醇洗脱部位。将上述中国延龄草属或重楼属药材的根及根茎或果实或全草提取物既60-85%乙醇的洗脱部位的浸膏加入等量的100-200目硅胶拌匀,50℃减压挥干溶剂,研匀,干法上样,上常压硅胶柱(200-300目,硅胶量为浸膏的30倍),上样后依次用三氯甲烷:甲醇:水梯度洗脱,主要收集三氯甲烷:甲醇:水为65:35:1的流份,以上述中国延龄草属或重楼属药材的根及根茎或果实或全草中甾体皂苷类的偏诺皂苷类物质中的单体皂苷作为对照品TLC或HPLC检测,合并相应流份。上述流份于65℃减压浓缩蒸干,用适量蒸馏水混悬,上反相硅胶ODS-C18柱色谱,依次用乙醇水由0-50%洗脱,再用乙醇水50-95%洗脱,主要收集60%-85%洗脱液用冷冻干燥法进行干燥,收集冻干粉,用95%乙醇结晶并重结晶,即得。采用上述方法制备中国延龄草属或重楼属药材的根及根茎或果实或全草中偏诺皂苷类物质的得率为0.3-2.8g/ kg,经HPLC检测纯度为97-98.2%;色谱条件UItimateXB-C18色谱柱(4.6mm×150mm,3μm),流动相为乙腈-水(10%-100%梯度洗脱),检测波长为203nm,流速为1.0ml/min,柱温30℃,进样量20μl。
实施例2
中国延龄草属或重楼属药材的根及根茎或果实或全草提取物及60-85%乙醇洗脱部位所含延龄草皂苷类物质中trillenoside A、deoxytrillenoside A、24-acetyl-
deoxytrill-enoside A、24-epiacetyl-deoxytrillenoside A、24-methoxy-trillenoside A、trillenoside B、24-acetyl-trillenoside B、24-acetyl-deoxytrillenoside B、trillenoside C、Epitrillenogenin-24-O-acetate-1-O-[2,3,4-tri-O-acetyl-α-L-rhamnopyra-nosyl-(1
→2)-α-L-arabinopyranoside]、Epitrillenogenin-1-O-[2,3,4-tri-O-acetyl-α-L-rhamnopy-
ranosyl-(1→2)-α-L-arabinopyranosi-de]、Epitrillenogenin-24-O-acetate-1-O-[2,4-di-O-
acetyl-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside]、Epitrillenogenin-1-O-
[2,4-di-O-acetyl-α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside]、Epitrillenogen-
in-1-O-[4-O-acetyl-α-L-rhamnopyranosyl-(1→2)-α-L- arabinopyranoside]的制备方法:将中国延龄草属或重楼属药材的根及根茎或果实或全草粉碎成粗粉,用70%乙醇浸泡2.0小时,12倍量溶剂加热回流提取3次,每次2小时,过滤,合并滤液。上述滤液与70℃减压浓缩至1g生药材/ml,0-4℃冷藏过夜,析胶,过滤,滤液上D101型号大孔吸附树脂富集,依次用水、30%乙醇、60%乙醇和85%乙醇洗脱,得到各洗脱部位,用喷雾干燥法进行干燥,收集喷雾干粉,即得。其中60- 85%的洗脱部位以甾体皂苷类成分为主,甾体皂苷总含量大于90%,即得中国延龄草属或重楼属药材的根及根茎或果实或全草提取物或60-85%乙醇洗脱部位。将上述中国延龄草属或重楼属药材的根及根茎或果实或全草提取物既60-85%乙醇的洗脱部位的浸膏加入等量的100-200目硅胶拌匀,50℃减压挥干溶剂,研匀,干法上样,上常压硅胶柱(200-300目,硅胶量为浸膏的25倍),上样后依次用三氯甲烷:甲醇:水梯度洗脱,主要收集三氯甲烷:甲醇:水为60:30:1的洗脱流份,以上述中国延龄草属或重楼属药材的根及根茎或果实或全草中甾体皂苷类的延龄草皂苷中单体皂苷作为对照品TLC或HPLC检测,合并相应流份。上述流份于65℃减压浓缩蒸干,用适量蒸馏水混悬,上SephadexLH-20柱色谱,依次用乙醇水由0-50%洗脱,再用乙醇水50-95%洗脱,洗脱液用喷雾干燥进行干燥,收集喷雾干燥粉,用95%乙醇结晶并重结晶,即得。采用上述方法制备中国延龄草属或重楼属药材的根及根茎或果实或全草中延龄草皂苷类物质为0.3-1.5g/kg,经HPLC检测纯度为97-98.7%;色谱条件UItimateXB-C18色谱柱(4.6mm×150mm,3μm),流动相为乙腈-水(10%-100%梯度洗脱),检测波长为203nm,流速为1.0ml/min,柱温30℃,进样量20μl。
实施例3
中国延龄草属或重楼属药材的根及根茎或果实或全草提取物及60-85%乙醇洗脱部位所含薯蓣皂苷类物质中薯蓣皂苷元-3-O-α-L-吡喃鼠李糖基(1→2)-O-β-D-吡喃葡萄糖苷、薯蓣皂苷元-3-O-α-L-吡喃鼠李糖基(1→4)-[O-α-L-吡喃鼠李糖基(1→2)]-O-β-D-吡喃葡萄糖苷、薯蓣皂苷元-3-O-α-L-吡喃鼠李糖基(1→4)-O-α-L-吡喃鼠李糖基(1→4)-[O-α-L-吡喃鼠李糖基(1→2)]-O-β-D-吡喃葡萄糖苷、(23s,24s,25s)-螺甾-5-烯-1β, 3β, 21, 23, 24-五羟基-1β-O-β-D-呋喃芹糖基-(1→3)-O-α-L-吡喃鼠李糖基-(1→2)-[O-β-D-吡喃木糖基(1→3)]-O-α-L-吡喃阿拉伯糖苷、(23s,24s,25s)-螺甾-5-烯-1β, 3β, 23, 24-四羟基-1β-O-β-D-呋喃芹糖基-(1→3)-O-α-L-吡喃鼠李糖基-(1→2)-[O-β-D-吡喃木糖基(1→3)]-O-α-L-吡喃阿拉伯糖苷、(23s,24s,25s)-螺甾-5-烯-1β, 3β, 21, 23, 24-五羟基-1β-O-α-L-吡喃鼠李糖基-(1→2)-[O-β-D-吡喃木糖基(1→3)]-O-α-L-吡喃阿拉伯糖苷的制备方法:将中国延龄草属或重楼属药材的根及根茎或果实或全草粉碎成粗粉,用85%乙醇浸泡1.0小时,8倍量溶剂加热回流提取3次,每次2小时,过滤,合并滤液。上述滤液与70℃减压浓缩至1g生药材/ml,0-4℃冷藏过夜,析胶,过滤,滤液上HPD400型号大孔吸附树脂富集,依次用水、30%乙醇、60%乙醇和85%乙醇洗脱,得到各洗脱部位,用喷雾干燥法进行干燥,收集喷雾干粉,即得。其中 60-85%的洗脱部位以甾体皂苷类成分为主,甾体皂苷总含量大于90%,即得中国延龄草属或重楼属药材的根及根茎或果实或全草提取物或60-85%乙醇洗脱部位。将上述中国延龄草属或重楼属药材的根及根茎或果实或全草提取物既60-85%乙醇的洗脱部位的浸膏加入等量的100-200目硅胶拌匀,50℃减压挥干溶剂,研匀,干法上样,上常压硅胶柱(200-300目,硅胶量为浸膏的20倍),上样后依次用三氯甲烷:甲醇:水梯度洗脱,主要收集三氯甲烷:甲醇:水为65:35:1的洗脱流份,以上述中国延龄草属或重楼属药材的根及根茎或果实或全草甾体皂苷中薯蓣皂苷类物质的单体皂苷作为对照品TLC或HPLC检测,合并相应流份。上述流份于65℃减压浓缩蒸干,用制备高效液相色谱技术分离纯化,用乙醇水50-95%洗脱,洗脱液经适当浓缩用喷雾干燥进行干燥,收集喷雾干燥粉,用95%乙醇结晶并重结晶,即得。采用上述方法制备中国延龄草属或重楼属药材的根及根茎或果实或全草中所含薯蓣皂苷类物质的得率为0.3-2.8g/kg,经HPLC检测纯度为97-98.8%;色谱条件 UItimateXB-C18色谱柱(4.6mm×150mm,3μm),流动相为乙腈-水(10%-100%梯度洗脱),检测波长为203nm,流速为1.0ml/min,柱温30℃,进样量20μl。
实施例4
中国延龄草属或重楼属药材的根及根茎或果实或全草提取物及60-85%乙醇洗脱部位所含呋甾烷皂苷类物质中26-O-β-D-吡喃葡萄糖基-(25s)-5α-呋甾烷-△20(22)-烯-3β,26-二醇-3-O-α-L-吡喃鼠李糖基(1→4)-[O-α-L-吡喃鼠李糖基(1→2)]-O-β-D-吡喃葡萄糖苷、26-O-β-D-吡喃葡萄糖基-(25s)-5α-呋甾烷-△20(22)-烯-3β, 26-二醇-3-O-α-L-吡喃鼠李糖基(1→4)-O-β-D-吡喃葡萄糖苷、26-O-β-D-吡喃葡萄糖基-(25s)-5α-呋甾烷-△5(6),20(22)-二烯-3β, 26-二醇-3-O-α-L-吡喃鼠李糖基(1→4)-O-β-D-吡喃葡萄糖苷、26-O-β-D-吡喃葡萄糖基-(25R)-Δ5(6)、17(20)-二烯-16,22-二酮-呋甾烷-3β,26-二醇-3-O-α-L-吡喃鼠李糖基(1→4)-O-α-L-吡喃鼠李糖基(1→4)[O-α-L-吡喃鼠李糖基(1→2)-]-O-β-D-吡喃葡萄糖苷、26-O-β-D-吡喃葡萄糖基-(25R)-Δ5(6)、17(20)-二烯-16-羟基-22-酮-呋甾烷-3β,26-二醇-3-O-α-L-吡喃鼠李糖基(1→2) [O-α-L-吡喃鼠李糖基(1→4)-]-O-β-D-吡喃葡萄糖苷、26-O-β-D-吡喃葡萄糖基-(25R)-Δ5(6)、17(20)-二烯-16,22-二酮-呋甾烷-3β,26-二醇-3-O-α-L-吡喃鼠李糖基(1→2)[O-α-L-吡喃鼠李糖基(1→4)-]-O-β-D-吡喃葡萄糖苷、26-O-β-D-吡喃葡萄糖基-(25R)-Δ5(6)、17(20) -二烯-16,22-二酮-呋甾烷-3β,26-二醇-3-O-α-L-吡喃鼠李糖基(1→2)-O-β-D-吡喃葡萄糖苷、3β-O-α-L-rhamnopyranosyl-(1→4)-[O-α-L-rhamno-
pyranosyl-(1→2)]-O-β-D-glucopyranosylhomo-aro-cholest-5-ene-26-O-β-D-glucopyra-noside(aethiosidecA)、3β-O-α-L-rhamnopyranosyl-(1→4)-O-α-L-rhamnopyranosyl-
(1→4)-[O-α-L-rhamnopyranosyl-(1→2)]-O-β-D-glucopyranosylhomo-aro-cholest-5-ene-26-O-β-D-glucopyranoside(parispseudosideA)、3β-O-α-L-rhamnopyranosyl-(1→2)- O-β-D-glucopyranosylhomo-16′-hydroxyl-aro-cholest-5-ene-26-O-β-D-glucopyranoside(16′-hydroxylparispseudosideB)的制备方法:将中国延龄草属或重楼属药材的根及根茎或果实或全草粉碎成粗粉,用75%乙醇浸泡1.0小时,10倍量溶剂加热回流提取3次,每次2小时,过滤,合并滤液。上述滤液与55℃减压浓缩至0.8g生药材/ml,0-4℃冷藏过夜,析胶,过滤,滤液上HPD300型号大孔吸附树脂富集,依次用水、30%乙醇、60%乙醇和85%乙醇洗脱,得到各洗脱部位,用喷雾干燥法进行干燥,收集喷雾干粉,即得。其中 60-85%的洗脱部位以甾体皂苷类成分为主,甾体皂苷总含量大于90%,即得中国延龄草属或重楼属药材的根及根茎或果实或全草提取物或60-85%乙醇洗脱部位。将上述中国延龄草属或重楼属药材的根及根茎或果实或全草提取物既60-85%乙醇的洗脱部位的浸膏加入等量的100-200目硅胶拌匀,50℃减压挥干溶剂,研匀,干法上样,上常压硅胶柱(200-300目,硅胶量为浸膏的20倍),上样后依次用三氯甲烷:甲醇:水梯度洗脱,主要收集三氯甲烷:甲醇:水为65:35:1的洗脱流份,以上述中国延龄草属或重楼属药材的根及根茎或果实或全草中甾体皂苷类的呋甾烷皂苷类物质中的单体皂苷作为对照品TLC或HPLC检测,合并相应流份。上述流份于65℃减压浓缩蒸干,用制备高效液相色谱技术分离纯化,用乙醇水50-95%洗脱,洗脱液经适当浓缩用喷雾干燥进行干燥,收集喷雾干燥粉,用95%乙醇结晶并重结晶,即得。采用上述方法制备中国延龄草属或重楼属药材的根及根茎或果实或全草中所含呋甾烷皂苷类物质的得率为0.5-1.6g/kg,经HPLC检测纯度为98-99%;色谱条件 UItimateXB-C18色谱柱(4.6mm×150mm,3μm),流动相为乙腈-水(10%-100%梯度洗脱),检测波长为203nm,流速为1.0ml/min,柱温30℃,进样量20μl。
实施例5
中国延龄草属3种药材的根及根茎或果实或全草提取物及30-85%乙醇洗脱部位所含倍半萜类物质中的延龄草倍半萜苷C或7,11-二甲基-3-亚甲基-1,6-十二碳二烯-10,11-二羟基-10-O-β-D-吡喃葡萄糖苷、延龄草倍半萜苷A或7,11-二甲基-3-亚甲基-1,6-十二碳二烯-10,11-二羟基-10-O-β-D-吡喃葡萄糖基-(1→4)-O-β-D-吡喃葡萄糖苷、7,11-二甲基-3-亚甲基-10,11-二羟基-1,6-十二碳二烯二醇、2,6,10-三甲基-2,10-二羟基-6-十二碳烯二醇、3,7,11-三甲基-3,9,11-三羟基-1,6-十二碳二烯三醇、7,11-二甲基-3-羟甲基-1,6-十二碳二烯-10,11,15-三醇-10-O-间苯酚的制备方法:将中国延龄草属3种药材的根及根茎或果实或全草粉碎成粗粉,用50%乙醇浸泡1.0小时,7倍量溶剂加热回流提取3次,每次2小时,过滤,合并滤液。上述滤液与55℃减压浓缩至1g生药材/ml,0-4℃冷藏过夜,析胶,过滤,滤液上AB-8型号大孔吸附树脂富集,依次用水、30%乙醇、60%乙醇和85%乙醇洗脱,得到各洗脱部位,用喷雾干燥法进行干燥,收集喷雾干粉,即得。其中 60%的洗脱部位主要含有倍半萜类成分,即得中国延龄草属3种药材的根及根茎或果实或全草提取物或60%乙醇洗脱部位。将上述中国延龄草属3种药材的根及根茎或果实或全草提取物既60%乙醇的洗脱部位的浸膏加入等量的100-200目硅胶拌匀,50℃减压挥干溶剂,研匀,干法上样,上常压硅胶柱(200-300目,硅胶量为浸膏的20倍),上样后依次用三氯甲烷:甲醇梯度洗脱,主要收集三氯甲烷:甲醇为12:1至8:1的洗脱流份,以上述中国延龄草属3种药材的根及根茎或果实或全草中倍半萜类物质中的单体作为对照品TLC或HPLC检测,合并相应流份。上述流份于65℃减压浓缩蒸干,用制备高效液相色谱技术分离纯化,用乙醇水50-95%洗脱,洗脱液经适当浓缩用喷雾干燥进行干燥,收集喷雾干燥粉,用95%乙醇结晶并重结晶,即得。采用上述方法制备中国延龄草属3种药材的根及根茎或果实或全草中所含倍半萜类物质的得率为0.1-0.9g/kg,经HPLC检测纯度为97-98.5%;色谱条件 UItimateXB-C18色谱柱(4.6mm×150mm,3μm),流动相为乙腈-水(10%-100%梯度洗脱),检测波长为205nm,流速为1.0ml/min,柱温30℃,进样量20μl。
实施例6
中国延龄草属3种药材的根及根茎或果实或全草提取物及30-85%乙醇洗脱部位所含苯丙素类物质中的methyl ferulorate、regaloside A、3-O-feruloylsucrose、heronioside A或酚酸类物质中的trillium acid A、trillium acid B、trillium acid C的制备方法:将中国延龄草属3种药材的根及根茎或果实或全草粉碎成粗粉,用30%乙醇浸泡1.0小时,6倍量溶剂加热回流提取3次,每次2小时,过滤,合并滤液。上述滤液与50℃减压浓缩至1g生药材/ml,0-4℃冷藏过夜,析胶,过滤,滤液上D101型号大孔吸附树脂富集,依次用水、30%乙醇、60%乙醇和85%乙醇洗脱,得到各洗脱部位,用喷雾干燥法进行干燥,收集喷雾干粉,即得。其中 30%的洗脱部位主要含有苯丙素类和酚酸类成分,即得中国延龄草属3种药材的根及根茎或果实或全草提取物或30%乙醇洗脱部位。将上述中国延龄草属3种药材的根及根茎或果实或全草提取物既30%乙醇的洗脱部位的浸膏加入等量的100-200目硅胶拌匀,50℃减压挥干溶剂,研匀,干法上样,上常压硅胶柱(200-300目,硅胶量为浸膏的20倍),上样后依次用三氯甲烷:甲醇梯度洗脱,主要收集三氯甲烷:甲醇为12:1或5:1的洗脱流份,以上述中国延龄草属3种药材的根及根茎或果实或全草中苯丙素类或酚酸类物质中的上述单体作为对照品TLC或HPLC检测,合并相应流份。上述流份于50℃减压浓缩蒸干,用制备高效液相色谱技术分离纯化,用乙醇水50-95%洗脱,洗脱液经适当浓缩用喷雾干燥进行干燥,收集喷雾干燥粉,用95%乙醇结晶并重结晶,即得。采用上述方法制备中国延龄草属3种药材的根及根茎或果实或全草中所含苯丙素类或酚酸类物质的得率为0.1-0.6g/kg 或0.1-0.3g/kg,经HPLC检测纯度为98-98.9%;色谱条件 UItimateXB-C18色谱柱(4.6mm×150mm,3μm),流动相为乙腈-水(10%-100%梯度洗脱),检测波长为205nm,流速为1.0ml/min,柱温30℃,进样量20μl。
实施例7
中国延龄草属或重楼属药材的根及根茎或果实或全草中甾体皂苷类、倍半萜类、苯丙素苷类和酚酸类单体物质的结构确证与波谱数据信息。
化合物 1:无色针晶(甲醇),分子式为C39H62O13,ESI-MS[M+H]+峰m/z:739。Liebermann-Burchard反应和Molish反应均呈阳性为甾体皂苷。1H-NMR(DMSO-d6,400 MHz) δ: 0.73(3H,s,CH3-18),0.74(3H,d,J=6.0 Hz,CH3-27),0.78(3H,d, J=6.8 Hz,CH3-21),0.95(3H,brs,CH3-19),1.08(3H,d,J=6.0 Hz,Rha-CH3-6″),5.34 (1H, d,J=4.4 Hz,H-6),5.03 (1H,d,J=0.8 Hz,Rha-H-1″),4.93(1H,d, J=6.0 Hz,Glc-H-1′);13C-NMR (DMSO-d6, 100 MHz)δ:36.9(C-1),30.8(C-2),77.7(C-3), 37.6(C-4), 140.3(C-5),121.3(C-6),31.5(C-7),31.2(C-8),51.9 (C-9),36.3(C-10),22.4(C-11),31.4(C-12),43.6(C-13),49.5(C-14),29.7(C-15),88.9(C-16),88.3(C-17),16.6(C-18),17.7(C-19),44.3(C-20),17.1(C-21),108.7(C-22),31.6(C-23),28.0(C-24),29.0(C-25),65.8(C-26),9.3(C-27),98.1(C-1′),76.5(C-2′),76.3(C-3′),71.9(C-4′),76.0(C-5′),60.9(C-6′),100.0(C-1″),70.6(C-2″),70.5(C-3″),70.2(C-4″),67.9(C-5"),19.0(C-6″)。化合物1为重楼皂苷Ⅵ即偏诺皂苷元-3-O-α-L-吡喃鼠李糖基-(1→2)-O-β-D-吡喃葡萄糖苷(pennogenin-3-O-α-L-rhamnopyranosyl-(1→2)-O-β-D-glucopyranoside)。
化合物 2:白色针晶(甲醇),mp259-261℃,分子式为 C45H71O17,ESI-MS [M+H]+峰m/z:884。Liebermann-Burchard反应和Molish反应均呈阳性为甾体皂苷。1H-NMR(DMSO-d6,400 MHz)δ: 0.73(3H,s,CH3-18),0.74(3H,d,J=6.0 Hz,CH3-27),0.78
(3H,d, J=6.8 Hz,CH3-21),0.95(3H,brs,CH3-19),1.08(3H,d,J=6.0 Hz,Rha-CH3-6″),1.10
(3H,d,J=6.0 Hz,Rha-CH3-6″′),5.33 (1H, d,J=4.4 Hz,H-6),5.02 (1H,brs,Rha-H-1″), 4.92
(1H,d, J=6.4 Hz,Glc-H-1′),4.70(1H, d, J=2.0 Hz,Rha-H-1″′);13C-NMR (DMSO-d6, 100 MHz)δ:36.8(C-1),30.8(C-2),76.9(C-3),37.6(C-4),140.2(C-5),121.3(C-6),31.5(C-7),31.2(C-8),51.9(C-9),36.3(C-10),22.4(C-11),31.4(C-12),43.6(C-13),49.5(C-14),29.7(C-15),88.9(C-16),88.3(C-17),16.6(C-18),17.8(C-19),44.3(C-20),17.1(C-21),108.7(C-22),31.6(C-23),28.0(C-24),29.0(C-25),65.8(C-26),9.3(C-27),98.1(Glc-C-1′),76.2(C-2′),76.1 (C-3′),71.9(C-4′),75.2(C-5′),60.0(C-6′),100.3(Rha-C-1″),70.7(C-2″),70.5(C-3″),70.4(C-4″),67.9(C-5″),19.0(C-6″),100.5(Rha-C-1″′),71.9(C-2″′),70.5
(C-3″′),76.7(C-4″′),68.6(C-5″′),17.7(C-6″′)。化合物2为偏诺皂苷元-3-O-α-L-吡喃鼠李糖基(1→4)-[O-α-L-吡喃鼠李糖基(1→2)]-O-β-D-吡喃葡萄糖苷(pennogenin-
3-O-α-L-rhamnopyranosyl-(1→4)-[O-α-L-rhamnopyranosyl-(1→2)]-O-β-D-glucopyran-oside)。
化合物 3:白色针晶(甲醇),mp259-261℃,C51H80O21 ,ESI-MS [M+H]+ 峰m/z: 1029。Liebermann-Burchard反应和Molish反应均呈阳性为甾体皂苷。1H-NMR(DMSO-d6,400 MHz)δ: 0.73(3H,s,CH3-18),0.74(3H,d,J=6.0 Hz,CH3-27),0.78
(3H,d, J=6.8 Hz,CH3-21),0.95(3H,brs,CH3-19),1.08(3H,d,J=6.0 Hz,Rha-CH3-6″),1.10
(3H,d,J=6.0 Hz,Rha-CH3-6″′),1.12(3H,d,J=6.0 Hz,Rha-CH3-6″′′),5.32 (1H, d,J=4.0 Hz,H-
6),5.06 (1H,brs,Rha-H-1″″), 5.02 (1H,brs,Rha-H-1″), 4.86(1H,d, J=4.0 Hz,Glc-H-1′),4.67(1H, d, J=1.2 Hz,Rha-H-1″′);13C-NMR (DMSO-d6, 100 MHz)δ:36.9(C-1),30.8(C-2),77.8(C-3),37.6(C-4),140.2(C-5),121.3(C-6),31.5(C-7),31.2(C-8),51.9(C-9),36.3
(C-10),22.4(C-11),31.4(C-12),43.6(C-13),49.5(C-14),29.7(C-15),88.9(C-16),88.3(C-17),16.6(C-18),17.8(C-19),44.3(C-20),17.1(C-21),108.7(C-22),31.6(C-23),28.0(C-24),29.0(C-25),66.7(C-26),9.3(C-27),98.2(Glc-C-1′),76.2(C-2′),76.0 (C-3′),71.9(C-4′),75.3(C-5′),65.8(C-6′),100.0(Rha-C-1″),70.7(C-2″),70.6(C-3″),70.4(C-4″),67.9(C-5″),19.0(C-6″),100.3(Rha-C-1″′),71.9(C-2″′),70.6(C-3″′),77.0(C-4″′),68.9(C-5″′),17.7(C-6″′) ,101.0(Rha-C-1″′′),71.3(C-2″′′),71.3(C-3″′′),76.0(C-4″′′),69.7(C-5″′′),18.1(C-6″′′)。化合物3为重楼皂苷Ⅶ即偏诺皂苷元-3-O-α-L-吡喃鼠李糖基-(1→4)-O-α-L-吡喃鼠李糖基(1→4)-[O-α-L-吡喃鼠李糖基-(1→2)]-O-β-D-吡喃葡萄糖苷(pennogenin-3-O-α-L-rhamnopy- ranosyl-(1→4)-O-α-L-rhamnopyranosyl-
(1→4)-O-[α-L-rhamnopyranosyl-(1→2)]-O-β-D-glucopyranoside)。
化合物4:白色针状结晶(甲醇),分子式为C45H72O18, FAB-MS m/z: 923[M+Na]+。1H-NMR(MeOD,400 MHz)δ :0.79(3H, d, J=6.0 Hz, CH3-27), 0.82(3H, s, CH3-18), 0.88(3H, d, J=7.2 Hz, CH3-21), 1.04(3H, brs, CH3-19), 1.22(3H, d, J=6.0 Hz, Rha-6″-CH3), 5.38(1H, d, J=4.8 Hz, H-6), 5.20(1H, d, J=1.4 Hz, Rha-1″-H), 4.48(1H, d, J=7.6 Hz, Glc-1′″-H), 4.36(1H, d, J=7.6 Hz, Glc-1′-H); 13C-NMR(MeOD,100 MHz)δ :38.6(C-1), 30.8(C-2), 79.2(C-3), 39.6(C-4), 141.9(C-5), 122.6(C-6), 33.3(C-7), 33.2(C-8), 51.4(C-9), 38.0(C-10), 21.7(C-11), 32.5(C-12), 45.9(C-13), 53.9(C-14), 32.1(C-15), 90.5(C-16), 91.3(C-17), 17.5(C-18), 18.0(C-19), 45.5(C-20), 9.1(C-21), 111.0(C-22), 32.9(C-23), 29.5(C-24), 31.3(C-25), 67.7(C-26), 17.5(C-27), 100.8(Glc C-1′), 79.6(C-2′), 78.8(C-3′), 71.6(C-4′), 76.8(C-5′), 69.7(C-6′), 102.1(Rha C-1″), 72.4(C-2″), 72.2(C-3″), 73.9(C-4″), 69.6(C-5″), 19.9(C-6″), 104.8(Glc C-1′″), 75.1(C-2′″), 78.0(C-3′″), 71.6(C-4′″), 78.0(C-5′″), 62.8(C-6′″)。化合物4为偏诺皂苷元-3β-O-β-D-吡喃葡萄糖基(1→6)-[O-α-L-吡喃鼠李糖基(1→2)]-O-β-D-吡喃葡萄糖苷(trikamsteroside B)或(pennogenin-3β-O-β-D-glucopyranosyl(1→6)-[O-α-L-rhamnopyranosyl(1→2)]-O-β-D-glucopyranoside)。
化合物5:白色针状结晶(甲醇),分子式为C45H72O19, FAB-MS m/z: 939[M+Na]+。1H-NMR(MeOD,400 MHz)δ :0.82(3H, s, CH3-18), 0.89(3H, d, J=7.2 Hz, CH3-21), 1.04(3H, brs, CH3-19), 1.22(3H, d, J=6.0 Hz, Rha-6″-CH3), 5.38(1H, d, J=4.8 Hz, H-6), 5.19(1H, d, J=1.2 Hz, Rha-1″-H), 4.48(1H, d, J=7.6 Hz, Glc-1′″-H), 4.36(1H, d, J=8.0 Hz, Glc-1′-H); 13C-NMR(MeOD,100 MHz)δ :38.6(C-1), 30.8(C-2), 79.2(C-3), 39.6(C-4), 141.9(C-5), 122.6(C-6), 33.3(C-7), 33.2(C-8), 51.4(C-9), 38.0(C-10), 21.7(C-11), 32.1(C-12), 45.9(C-13), 53.9(C-14), 32.1(C-15), 90.6(C-16), 91.3(C-17), 17.5(C-18), 18.0(C-19), 45.6(C-20), 9.2(C-21), 111.2(C-22), 32.9(C-23), 23.9(C-24), 39.2(C-25), 65.1(C-26), 64.3(C-27), 100.8(Glc C-1′), 79.6(C-2′), 78.8(C-3′), 71.6(C-4′), 76.8(C-5′), 69.8(C-6′), 102.1(Rha C-1″), 72.4(C-2″), 72.2(C-3″), 73.9(C-4″), 69.6(C-5″), 19.9(C-6″), 104.8(Glc C-1′″), 75.1(C-2′″), 78.0(C-3′″), 71.6(C-4′″), 78.0(C-5′″), 62.8(C-6′″)。化合物5为27-羟基-偏诺皂苷元-3β-O-β-D-吡喃葡萄糖基(1→6)-[O-α-L-吡喃鼠李糖基(1→2)]-O-β-D-吡喃葡萄糖苷(27-hydroxy-trikamsteroside B)或(27-hydroxy-penno-
genin-3β-O-β-D-glucopyranosyl(1→6)-[O-α-L-rhamnopyrano-syl(1→2)]-O-β-D-glucopyranoside)。
化合物6:无色针状结晶(甲醇)。1H-NMR(CDCl3,400 MHz)δ:0.79(3H, d, J=6.0 Hz, CH3-27), 0.88(3H, d, J=6.4 Hz, CH3-21), 1.00(3H, s, CH3-18), 1.25(3H, brs, CH3-19), 1.98(3H, s, -OCOCH3), 5.34(1H, brs, H-6); 13C-NMR(CDCl3,100 MHz)δ:38.2(C-1), 33.4(C-2), 72.0(C-3), 39.8(C-4), 141.9(C-5), 123.3(C-6), 32.2(C-7), 33.0(C-8), 51.4(C-9), 38.7(C-10), 24.1(C-11), 32.6(C-12), 45.2(C-13), 54.3(C-14), 30.6(C-15), 91.5(C-16), 92.3(C-17), 15.6(C-18), 18.5(C-19), 45.9(C-20), 9.5(C-21), 111.4(C-22), 31.5(C-23), 26.4(C-24), 35.7(C-25), 68.2(C-26), 18.4(C-27), 175.8(C-OCOCH3), 20.7(CH3-OCOCH3)。化合物6为3-乙酰基-偏诺皂苷元(3-O-acetyl-pennogenin)。
化合物7:白色针状结晶(甲醇),分子式为C57H92O27, FAB-MS m/z: 1231[M+
Na]+。1H-NMR(MeOD,400 MHz)δ :0.79(3H, d, J=6.0 Hz, CH3-27), 0.82(3H, s, CH3-18), 0.88(3H, d, J=7.2 Hz, CH3-21), 1.04(3H, brs, CH3-19), 1.21(3H, d, J=6.0 Hz, Rha-6″-CH3), 1.24(3H, d, J=6.0 Hz, Rha-6′″-CH3), 1.28(3H, d, J=6.0 Hz, Rha-6′′′′-CH3), 5.37(1H, d, J=5.2 Hz, H-6), 5.19(1H, d, J=1.2 Hz, Rha-1″-H), 5.17(1H, d, J=2.0 Hz, Rha-1′″-H), 4.83
(1H, d, J=7.6 Hz, Glc-1′′′′′-H), 4.82(1H, s, Rha-1′′′′-H), 4.48(1H, d, J=8.0 Hz, Glc-1′-H); 13C-NMR(MeOD,100 MHz)δ :33.3(C-1), 29.5(C-2), 79.3(C-3), 38.6(C-4), 141.9(C-5), 122.7(C-6), 30.8(C-7), 32.5(C-8), 51.5(C-9), 32.9(C-10), 21.7(C-11), 32.1(C-12), 45.5
(C-13), 53.9(C-14), 31.3(C-15), 90.5(C-16), 91.3(C-17), 17.5(C-18), 19.8(C-19), 45.9
(C-20), 9.1(C-21), 111.0(C-22), 39.5(C-23), 80.9(C-24), 38.0(C-25), 67.7(C-26), 17.5
(C-27), 100.4(Glc-C-1′), 79.4(C-2′), 77.9(C-3′), 79.5(C-4′), 76.7(C-5′), 61.9(C-6′), 102.4
(Rha-C-1″), 72.2(C-2″), 72.9(C-3″), 73.9(C-4″), 69.8(C-5″), 17.9(C-6″), 102.6(Rha-C-1′″), 73.0(C-2′″), 73.7(C-3′″), 79.9(C-4′″), 69.1(C-5′″), 18.6(C-6′″), 103.0(Rha-C-1′′′′), 72.4
(C-2′′′′), 72.5(C-3′′′′), 73.8(C-4′′′′), 70.5(C-5′′′′), 18.0(C-6′′′′), 103.2(Glc-C-1′′′′′), 76.6
(C-2′′′′′), 78.0(C-3′′′′′), 70.7(C-4′′′′′), 79.3(C-5′′′′′), 61.9(C-6′′′′′)。化合物7为dioscorean-
oside I。
化合物8:无色针状结晶,分子式为C47H70O24。Liebermann-Burchard 反应和Molish 反应均呈阳性。FAB-MS m/z 1017[M-H]-。1H-NMR(MeOD,400MHz)δ :0.90(3H, d, J=6.8 Hz, CH3-27), 1.09(3H, s, CH3-19), 1.24(3H, d, J=6.0 Hz, Rha-6″-CH3), 5.60(1H, d, J=5.2 Hz, H-6), 5.30(1H, d, J=1.2 Hz,Rha-1″-H), 5.18(1H, d, J=2.8 Hz, Api-1″″-H), 4.41
(1H, d, J=6.0 Hz, Xyl-1′″-H), 4.30(1H, d, J=6.8 Hz, Ara-1′-H); 13C-NMR(MeOD,100
MHz)δ :85.2(C-1), 36.3(C-2), 67.0(C-3), 43.2(C-4), 139.8(C-5), 126.0(C-6), 30.1(C-7), 32.7(C-8), 48.8(C-9), 42.8(C-10), 26.2(C-11), 29.1(C-12), 179.1(C-13), 139.6(C-14), 207.1(C-15), 83.0(C-16), 50.0(C-17), 14.3(C-19), 42.1(C-20), 61.8(C-21), 114.0(C-22), 72.9(C-23), 80.3(C-24), 37.4(C-25), 65.6(C-26), 12.8(C-27), 101.4(C-1′), 73.2(C-2′), 85.2(C-3′), 69.7(C-4′), 65.4(C-5′), 101.5(C-1″), 71.1(C-2″), 77.9(C-3″), 71.9(C-4″), 69.3(C-5″), 18.6(C-6″), 106.4(C-1′″), 74.4(C-2′″), 82.2(C-3′″), 70.5(C-4′″), 66.9(C-5′″), 112.1(C-1″″), 75.0(C-2″″), 78.1(C-3″″), 73.9(C-4″″), 64.4(C-5″″)。化合物8为trillenoside A。
化合物9:白色针状结晶(甲醇)。1H-NMR(MeOD,400MHz)δ :0.92(3H, d, J=6.4 Hz, CH3-27), 1.09(3H, s, CH3-19), 1.13(3H, d, J=6.8 Hz, CH3-21), 1.24(3H, d, J=6.0 Hz, Rha-
6″-CH3), 5.60(1H, d, J=5.6 Hz, H-6), 5.30(1H, d, J=1.2 Hz,Rha-1″-H), 5.18(1H, d, J=2.8 Hz, Api-1″″-H), 4.41(1H, d, J=6.8 Hz, Xyl-1′″-H), 4.30(1H, d, J=7.2 Hz, Ara-1′-H); 13C-NMR(MeOD,100MHz)δ :85.3(C-1), 33.1(C-2), 67.0(C-3), 43.2(C-4), 139.5(C-5), 126.0(C-6), 30.1(C-7), 32.6(C-8), 48.8(C-9), 42.8(C-10), 26.7(C-11), 29.1(C-12), 179.1
(C-13), 139.4(C-14), 207.5(C-15), 80.3(C-16), 52.6(C-17), 14.2(C-19), 42.7(C-20), 13.6
(C-21), 115.1(C-22), 72.9(C-23), 80.3(C-24), 39.4(C-25), 64.4(C-26), 13.2(C-27), 101.4
(C-1′), 73.0(C-2′), 82.2(C-3′), 69.7(C-4′), 65.6(C-5′), 101.5(C-1″), 71.1(C-2″), 78.0(C-3″), 71.9(C-4″), 69.3(C-5″), 18.6(C-6″), 106.4(C-1′″), 74.4(C-2′″), 82.2(C-3′″), 74.8(C-4′″), 66.9(C-5′″), 112.1(C-1″″), 75.0(C-2″″), 78.1(C-3″″), 74.8(C-4″″), 65.4(C-5″″)。化合物9为deoxytrillenoside A。
化合物10:白色针状结晶(甲醇)。1H-NMR(MeOD,400MHz)δ :0.76(3H, d, J=6.8 Hz, CH3-27), 1.08(3H, s, CH3-19), 1.13(3H, d, J=6.8 Hz, CH3-21), 1.24(3H, d, J=6.0 Hz, Rha-6″-CH3), 2.13(3H, s, 24-OCOCH3),5.60(1H, d, J=5.2 Hz, H-6), 5.30(1H, d, J=1.2 Hz,
Rha-1″-H), 5.18(1H, d, J=2.8 Hz, Api-1″″-H), 4.41(1H, d, J=6.8 Hz, Xyl-1′″-H), 4.30(1H, d, J=7.2 Hz, Ara-1′-H); 13C-NMR(MeOD,100MHz)δ :85.3(C-1), 35.3(C-2), 67.4(C-3), 43.5(C-4), 139.6(C-5), 126.0(C-6), 30.2(C-7), 32.7(C-8), 48.8(C-9), 43.2(C-10), 26.2
(C-11), 29.2(C-12), 179.3(C-13), 139.5(C-14), 208.0(C-15), 82.8(C-16), 52.3(C-17), 14.3(C-19), 42.8(C-20), 13.9(C-21), 113.4(C-22), 72.9(C-23), 80.4(C-24), 37.5(C-25), 62.3(C-26), 12.4(C-27) , 173.6(C-OCOCH3), 21.1(CH3-OCOCH3), 101.4(C-1′), 73.7(C-2′), 85.3(C-3′), 69.7(C-4′), 66.9(C-5′), 101.5(C-1″), 71.1(C-2″), 78.0(C-3″), 71.9(C-4″), 69.3(C-5″), 18.6(C-6″), 106.4(C-1′″), 74.4(C-2′″), 80.3(C-3′″), 70.6(C-4′″), 67.0(C-5′″), 112.1(C-1″″), 75.0(C-2″″), 78.1(C-3″″), 74.8(C-4″″), 65.4(C-5″″)。化合物10为24-acetyl-deoxytrillenoside A。
化合物11:白色针状结晶(甲醇)。1H-NMR(MeOD,400MHz)δ :0.76(3H, d, J=6.8 Hz, CH3-27), 0.93(3H, d, J=6.4 Hz, CH3-21),1.08(3H, s, CH3-19), 1.24(3H, d, J=6.0 Hz, Rha-6″-CH3), 2.07(3H, s, 24-OCOCH3),5.60(1H, d, J=5.2 Hz, H-6), 5.30(1H, d, J=1.2 Hz,Rha-1″-H), 5.18(1H, d, J=2.8 Hz, Api-1″″-H), 4.41(1H, d, J=6.8 Hz, Xyl-1′″-H), 4.30
(1H, d, J=7.2 Hz, Ara-1′-H); 13C-NMR(MeOD,100MHz)δ :85.3(C-1), 35.1(C-2), 68.9
(C-3), 43.2(C-4), 139.7(C-5), 126.1(C-6), 30.1(C-7), 32.8(C-8), 48.9(C-9), 46.2(C-10), 26.2(C-11), 28.9(C-12), 178.2(C-13), 139.6(C-14), 206.3(C-15), 82.0(C-16), 52.1(C-17), 14.3(C-19), 42.8(C-20), 13.1(C-21), 112.8(C-22), 72.9(C-23), 80.4(C-24), 39.3(C-25), 62.0(C-26), 12.4(C-27) , 172.7(C-OCOCH3), 21.0(CH3-OCOCH3), 101.4(C-1′), 73.7(C-2′), 85.3(C-3′), 69.7(C-4′), 66.9(C-5′), 101.5(C-1″), 71.1(C-2″), 76.0(C-3″), 71.9(C-4″), 69.3
(C-5″), 18.6(C-6″), 106.4(C-1′″), 74.4(C-2′″), 80.3(C-3′″), 70.5(C-4′″), 67.0(C-5′″), 112.1
(C-1″″), 75.0(C-2″″), 76.9(C-3″″), 70.5(C-4″″), 64.4(C-5″″)。化合物11为24-epiacetyl-
deoxytrillenoside A。
化合物12:白色针状结晶(甲醇)。1H-NMR(MeOD,400MHz)δ :0.90(3H, d, J=6.8 Hz, CH3-27), 1.09(3H, s, CH3-19), 1.24(3H, d, J=6.0 Hz, Rha-6″-CH3), 3.61(3H, s, 24-OCH3),5.60(1H, d, J=5.6 Hz, H-6), 5.30(1H, d, J=1.2 Hz,Rha-1″-H), 5.18(1H, d, J=2.8 Hz, Api-1″″-H), 4.41(1H, d, J=6.8 Hz, Xyl-1′″-H), 4.30(1H, d, J=7.2 Hz, Ara-1′-H); 13C-NMR(MeOD,100MHz)δ :85.3(C-1), 36.3(C-2), 67.1(C-3), 43.2(C-4), 139.8(C-5), 126.1(C-6), 30.2(C-7), 32.7(C-8), 48.8(C-9), 43.2(C-10), 26.2(C-11), 29.1(C-12), 179.2(C-13), 139.6(C-14), 207.0(C-15), 80.4(C-16), 49.9(C-17), 14.3(C-19), 42.8(C-20), 61.9(C-21), 114.0(C-22), 72.9(C-23), 80.3(C-24), 37.4(C-25), 69.7(C-26), 12.8(C-27) , 49.9(24-OCH3), 101.4(C-1′), 73.3(C-2′), 83.0(C-3′), 69.9(C-4′), 65.4(C-5′), 101.5(C-1″), 71.1(C-2″), 78.0(C-3″), 71.9(C-4″), 69.3(C-5″), 18.6(C-6″), 106.4(C-1′″), 74.4(C-2′″), 78.1(C-3′″), 70.6(C-4′″), 66.9(C-5′″), 112.1(C-1″″), 74.4(C-2″″), 78.1(C-3″″), 74.8(C-4″″), 66.9(C-5″″)。化合物12为24-methoxy-trillenoside A。
化合物13:白色针状结晶(甲醇)。1H-NMR(MeOD,400MHz)δ :0.92(3H, d, J=6.4 Hz, CH3-27), 1.09(3H, s, CH3-19), 1.24(3H, d, J=6.0 Hz, Rha-6″-CH3), 5.60(1H, d, J=5.2 Hz, H-6), 5.32(1H, d, J=1.2 Hz,Rha-1″-H), 4.41(1H, d, J=6.8 Hz, Xyl-1′″-H), 4.29(1H, d, J=7.6 Hz, Ara-1′-H); 13C-NMR(MeOD,100MHz)δ :85.3(C-1), 37.4(C-2), 66.9(C-3), 43.2(C-4), 139.7(C-5), 126.1(C-6), 30.2(C-7), 32.8(C-8), 48.8(C-9), 42.9(C-10), 26.1(C-11), 29.1(C-12), 179.2(C-13), 139.6(C-14), 207.0(C-15), 82.2(C-16), 49.3(C-17), 14.2(C-19), 49.8(C-20), 61.9(C-21), 114.7(C-22), 74.8(C-23), 76.1(C-24), 39.2(C-25), 65.7(C-26), 13.2(C-27), 101.5(C-1′), 74.2(C-2′), 85.4(C-3′), 69.3(C-4′), 67.1(C-5′), 101.7(C-1″), 72.3(C-2″), 72.0(C-3″), 74.3(C-4″), 70.6(C-5″), 18.5(C-6″), 106.5(C-1′″), 74.4(C-2′″), 78.0(C-3′″), 71.1(C-4′″), 69.7(C-5′″)。化合物13为Trillenoside B。
化合物14:白色针状结晶(甲醇)。1H-NMR(MeOD,400MHz)δ :0.88(3H, d, J=6.8 Hz, CH3-27), 1.09(3H, s, CH3-19), 1.24(3H, d, J=6.0 Hz, Rha-6″-CH3), 2.08(3H, s, 24-
OCOCH3),5.60(1H, d, J=4.8 Hz, H-6), 5.32(1H, d, J=1.2 Hz,Rha-1″-H), 4.41(1H, d, J=6.8 Hz, Xyl-1′″-H), 4.30(1H, d, J=7.6 Hz, Ara-1′-H); 13C-NMR(MeOD,100MHz)δ :85.3(C-1), 37.2(C-2), 66.9(C-3), 43.2(C-4), 140.2(C-5), 126.0(C-6), 30.8(C-7), 32.8(C-8), 46.2(C-9), 42.8(C-10), 26.2(C-11), 30.1(C-12), 178.2(C-13), 139.5(C-14), 206.8(C-15), 80.3(C-16), 48.6(C-17), 18.6(C-19), 21.0(C-20), 61.1(C-21), 112.1(C-22), 75.0(C-23), 77.9(C-24), 39.3(C-25), 65.4(C-26), 14.2(C-27),172.7(C-OCOCH3),21.0(CH3-OCOCH3),101.4(C-1′),
73.9(C-2′),85.3(C-3′), 69.7(C-4′), 69.3(C-5′), 101.5(C-1″), 72.9(C-2″), 71.9(C-3″), 74.4
(C-4″), 71.1(C-5″), 18.6(C-6″), 106.4(C-1′″), 74.8(C-2′″), 78.1(C-3′″), 71.3(C-4′″), 70.5
(C-5′″)。化合物14为24-acetyl-trillenoside B。
化合物15:白色针状结晶(甲醇)。1H-NMR(MeOD,400MHz)δ :0.76(3H, d, J=6.8 Hz, CH3-27), 0.93(3H, d, J=6.4 Hz, CH3-21), 1.09(3H, s, CH3-19), 1.24(3H, d, J=6.0 Hz, Rha-6″-CH3), 2.07(3H, s, 24-OCOCH3),5.60(1H, d, J=5.6 Hz, H-6), 5.31(1H, d, J=1.2 Hz,Rha-1″-H), 4.40(1H, d, J=6.8 Hz, Xyl-1′″-H), 4.28(1H, d, J=7.6 Hz, Ara-1′-H); 13C-NMR(MeOD,100MHz)δ :85.7(C-1), 37.8(C-2), 67.3(C-3), 43.6(C-4), 140.6(C-5), 126.4(C-6), 31.2(C-7), 33.2(C-8), 46.5(C-9), 43.5(C-10), 26.5(C-11), 30.5(C-12), 179.7(C-13), 139.9(C-14), 207.9(C-15), 83.4(C-16), 50.5(C-17), 18.9(C-19), 21.5(C-20), 26.5(C-21), 114.5(C-22), 74.7(C-23), 76.3(C-24), 39.7(C-25),64.8(C-26),14.6(C-27),
174.0(C-OCOCH3),21.4(CH3-OCOCH3),101.9(C-1′),72.7(C-2′),85.8(C-3′), 69.3(C-4′),
67.5(C-5′), 102.1(C-1″), 72.4(C-2″), 71.5(C-3″), 73.6(C-4″), 70.1(C-5″), 18.4(C-6″), 106.9(C-1′″), 74.1(C-2′″), 78.3(C-3′″), 71.0(C-4′″), 69.6(C-5′″)。化合物15为24-acetyl-deoxytrillenoside B。
化合物16:无色针状结晶,分子式为C37H54O16。Liebermann-Burchard反应和Molish反应均呈阳性。FAB-MSm/z 755[M+H]+。1H-NMR(DMSO-d6, 400MHz)δ :0.78(3H, d, J=7.2 Hz, CH3-27), 1.07(3H, d, J=6.0 Hz, Rha-6″-CH3), 1.15(3H, s, CH3-19), 5.66(1H, s, H-6), 5.16(1H, d, J=5.6 Hz, Rha-1″-H), 5.01(1H, d, J=5.6 Hz, Ara-1′-H)。13C-NMR(DMSO-d6, 400MHz)δ :87.7(C-1), 35.8(C-2), 67.9(C-3), 44.7(C-4), 131.6(C-5), 125.3(C-6), 29.7(C-7), 31.4(C-8), 44.9(C-9), 43.5(C-10), 23.2(C-11), 28.0(C-12), 165.7(C-13), 128.6(C-14), 201.1(C-15), 77.7(C-16), 45.2(C-17), 13.9(C-19), 38.0(C-20), 60.9(C-21), 108.7(C-22), 75.1(C-23), 76.0(C-24), 37.3(C-25), 65.8(C-26), 10.8(C-27), 98.3(C-1′), 71.9(C-2′), 88.2(C-3′), 70.2(C-4′), 67.9(C-5′), 99.9(C-1″), 71.9(C-2″), 76.6(C-3″), 75.9(C-4″), 70.5(C-5″), 16.7(C-6″)。化合物16为trillenoside C。
化合物17:无色针状结晶,分子式为C45H62O20。Liebermann-Burchard 反应和Molish反应均呈阳性。FAB-MS m/z 923[M+H]+。1H-NMR(DMSO-d6,400MHz)δ :0.83(3H,d,J=6.4Hz,CH3-27),0.97(3H,s,CH3-19),1.09(3H,d,J=6.0Hz,Rha-6″-CH3),1.92(3H,s,-OCOCH3),2.03(3H,s,-OCOCH3),2.04(3H,s,-OCOCH3),2.11(3H,s,-OCOCH3),5.66(1H,d,J=6.0Hz,H-6),5.29(1H,d,J=1.2Hz,Rha-1″-H),5.01(1H,d,J=4.8Hz,Ara-1′-H)。13C-NMR(DMSO-d6,400MHz)δ :82.6(C-1),36.1(C-2),68.7(C-3),44.2(C-4),138.3(C-5),124.0(C-6),28.5(C-7),31.0(C-8),46.8 (C-9),41.9(C-10),24.5(C-11),26.9(C-12),175.0(C-13),138.0(C-14),202.9(C-15),79.7(C-16),46.8(C-17),12.8(C-19),47.6(C-20),62.7(C-21),112.3(C-22),72.3(C-23),66.7(C-24),37.7(C-25),64.0(C-26),12.8(C-27),96.4(C-1′),74.0(C-2′),73.5(C-3′),68.7(C-4′),66.5(C-5′),98.5(C-1″),69.0(C-2″),70.2(C-3″),70.1(C-4″),73.3(C-5″),17.5(C-6″),170.4(C-OCOCH3),169.6(C-OCOCH3),169.7(C-OCOCH3),169.5(C-OCOCH3),20.8(CH3-OCOCH3),20.6(CH3-OCOCH3),20.5(CH3-OCOCH3),20.4(CH3-OCOCH3)。化合物17为Epitrillenogenin-24-O-
acetate-1-O-[2,3,4-tri-O-acetyl-α-L-rhamnopyranosyl-(1→2)-O-α-L-arabinopyranoside],即TS-d。
化合物18:无色针状结晶,分子式为C43H60O19。Liebermann-Burchard 反应和Molish 反应均呈阳性。FAB-MS m/z 881[M+H]+。1H-NMR(DMSO-d6,400MHz)δ :0.83(3H,d,J=6.4Hz,CH3-27),0.97(3H,s,CH3-19),1.04(3H,d,J=6.0Hz,Rha-6″-CH3),2.03(6H,s,2×OCOCH3),2.07(3H,s,-OCOCH3),4.68(1H,d,J=9.6Hz,H-16),4.96(1H,d,J=9.6Hz,H″-4),5.01(1H,d,J=5.2Hz,H″-2),5.13(1H,d,J=9.2Hz,H″-1),5.19(1H,m,H″-3),5.23(1H,m,H″-6),5.52(1H,s,H-6),5.34(1H,d,J=3.6Hz,Rha-1″-H),4.87(1H,d,J=6.8Hz,Ara-1′-H)。化合物18为Epitrillenogenin-1-O-[2,3,4-tri-O-acetyl-α-L-rhamnopyranosyl-(1→2)-
O-α-L-arabinopyranoside],即TS-e。
化合物19:无色针状结晶,分子式为C43H60O19。Liebermann-Burchard 反应和Molish 反应均呈阳性。FAB-MS m/z 881[M+H]+。1H-NMR(DMSO-d6,400MHz)δ :0.80(3H,d,J=6.4Hz,CH3-27),0.97(3H,s,CH3-19),1.09(3H,d,J=6.0Hz,Rha 6″-CH3),1.92(3H,s,-OCOCH3),2.04(3H,s,-OCOCH3),2.11(3H,s,-OCOCH3),5.53(1H,d,J=6.0Hz,H-6),5.30(1H,d,J=1.2Hz,Rha-1″-H),5.04(1H,d,J=3.6Hz,Ara-1′-H)。13C-NMR(DMSO-d6,400MHz)δ :82.4(C-1),36.0(C-2),68.8(C-3),44.3(C-4),138.3(C-5),124.1(C-6),28.6(C-7),31.0(C-8),46.8(C-9),41.9(C-10),24.4(C-11),27.1(C-12),176.2(C-13),137.3(C-14),203.5(C-15),80.0(C-16),47.9(C-17),12.9(C-19),48.5(C-20),60.3(C-21),112.8(C-22),72.1(C-23),66.7(C-24),37.7(C-25),63.8(C-26),12.8(C-27),96.3(C-1′),74.1(C-2′),73.7(C-3′),68.8(C-4′),66.5(C-5′),98.4(C-1″),69.0(C-2″),70.2(C-3″),70.1(C-4″),73.2(C-5″),17.5(C-6″),169.7(C-OCOCH3),169.6(C-OCOCH3),169.5(C-OCOCH3),20.6(CH3-OCOCH3),20.5(CH3-OCOCH3),20.4(CH3-OCOCH3)。化合物19为Epitrillenogenin-24-O-acetate-
1-O-[2,4-di-O-acetyl-α-L-rhamnopyranosyl-(1→2)-O-α-L-arabinopyranoside],即TS-g。
化合物20:无色针状结晶,分子式为C41H58O18。Liebermann-Burchard 反应和Molish 反应均呈阳性。FAB-MS m/z839 [M+H]+。1H-NMR(DMSO-d6,400MHz)δ :0.83(3H,d,J=6.4Hz,CH3-27),0.97(3H,s,CH3-19),1.02(3H,d,J=6.0Hz,Rha-6″-CH3),2.03(6H,s,2×OCOCH3),4.61(1H,d,J=8.0Hz,H-16),4.92 (1H,d,J=6.0Hz,H″-4),5.03(1H,s,H″-2),5.52(1H,d,J=5.2Hz,H-6), 5.17(1H,d,J=4.0Hz,Rha-1″-H),4.83(1H,d,J=7.6Hz,Ara-1′-H)。化合物20为Epitrillenogenin-1-O-[2,4-di-O-acetyl-α-L-rhamnopyranosyl-(1→2)-O-α-L-arabinopyranoside]。
化合物21:无色针状结晶,分子式为C39H56O17。Liebermann-Burchard 反应和Molish反应均呈阳性。FAB-MS m/z 797[M+H]+。1H-NMR(DMSO-d6,400MHz)δ :0.83(3H,d,J=6.4Hz,CH3-27),0.97(3H,s,CH3-19),1.01(3H,d,J=6.0Hz,Rha-6″-CH3),2.03(3H,s,-OCOCH3),4.66(1H,d,J=9.2Hz,H-16),4.94(1H,d,J=6.0Hz,H″-4),5.52(1H,d,J=4.8Hz,H-6),5.33(1H,d,J=2.0Hz,Rha-1″-H),4.83(1H,d,J=6.8Hz,Ara-1′-H)。化合物21为Epitrillenogenin-1-O-[4-O-acetyl-α-L-
rhamnopyranosyl-(1→2)-O-α-L-arabinopyranoside]。
化合物22:无色针状结晶,分子式为C39H62O12。Liebermann-Burchard 反应和Molish 反应均呈阳性。ESI-MS m/z 723[M+H]+。1H-NMR(DMSO-d6, 400MHz)δ :0.73(3H, d, J=6.0 Hz, CH3-27), 0.73(3H, s, CH3-18), 0.90(3H, d, J=7.6 Hz, CH3-21), 0.96(3H, s, CH3-19), 1.08(3H, d, J=6.0 Hz, Rha-6″-CH3); 5.33(1H, d, J=4.8 Hz, H-6), 4.95(1H, d, J=5.6 Hz, Glc-1′-H), 5.11(1H, d, J=6.0 Hz, Rha-1″-H)。13C-NMR(DMSO-d6, 400MHz)δ :36.4(C-1), 29.8(C-2), 76.0(C-3), 36.8(C-4), 140.3(C-5), 121.2(C-6), 31.5(C-7), 29.8(C-8), 49.6(C-9), 36.3(C-10), 20.3(C-11), 37.6(C-12), 39.5(C-13), 55.7(C-14), 31.0(C-15), 80.2(C-16), 65.9(C-17), 16.0(C-18), 18.9(C-19), 41.1(C-20), 14.6(C-21), 108.4(C-22), 29.0(C-23), 28.4(C-24), 31.0(C-25), 67.9(C-26), 17.0(C-27), 98.1(C-1′), 77.7(C-2′), 76.5(C-3′), 70.2(C-4′), 76.3(C-5′), 61.8(C-6′), 100.0(C-1″), 70.5(C-2″), 70.6(C-3″), 71.9(C-4″), 65.8(C-5″), 17.7(C-6″)。化合物22为薯蓣皂苷元-3-O-α-L-吡喃鼠李糖基(1→2)-O-β-D-吡喃葡萄糖苷,即重楼皂苷V。
化合物23:无色针状结晶,分子式为C45H72O16。Liebermann-Burchard反应和Molish 反应均呈阳性。ESI-MS m/z 869[M+H]+。1H-NMR(DMSO-d6, 400MHz)δ :0.85(3H, d, J=6.4 Hz, CH3-27), 0.86(3H, s, CH3-18), 0.98(3H, d, J=8.0 Hz, CH3-21), 1.05(3H, s, CH3-19), 1.08(3H, d, J=6.0 Hz, Rha-6″-CH3), 1.10(3H, d, J=6.0 Hz, Rha-6′″-CH3); 5.33(1H, d, J=4.8 Hz, H-6), 5.02(1H, d, J=6.0 Hz, Rha-1″-H), 4.93(1H, d, J=5.2 Hz, Rha-1′″-H), 4.92(1H, d, J=7.2 Hz, Glc-1′-H)。化合物23为薯蓣皂苷元-3-O-α-L-吡喃鼠李糖基(1→4)-[O-α-L-吡喃鼠李糖基(1→2)]-O-β-D-吡喃葡萄糖苷,即重楼皂苷Ⅲ。
化合物24:白色结晶(甲醇),分子式为C51H82O20, FAB-MS m/z: 1037[M+Na]+。1H-NMR(MeOD,400 MHz)δ :0.81(3H, d, J=6.4 Hz, CH3-27), 0.87(3H, s, CH3-18), 0.92(3H, d, J=6.4 Hz, CH3-21), 1.10(3H, s, CH3-19), 1.24(6H, d, J=6.0 Hz, Rha-6″ and 6′″-CH3), 1.27(3H, d, J=6.0 Hz, Rha-6′′′′-CH3), 5.55(1H, d, J=5.6 Hz, H-6), 5.29(1H, d, J=1.2 Hz, Rha-1″-H), 5.18(1H, d, J=2.8 Hz, Rha-1′′′′-H), 4.83(1H, s, Rha-1′″-H), 4.40(1H, d, J=6.8 Hz, Glc-1′-H); 13C-NMR(MeOD,100 MHz)δ :38.6(C-1), 32.8(C-2), 84.6(C-3), 41.5(C-4), 139.5(C-5), 126.1(C-6), 36.6(C-7), 32.4(C-8), 51.4(C-9), 37.3(C-10), 24.8(C-11), 41.6(C-12), 43.3(C-13), 57.9(C-14), 34.0(C-15), 85.2(C-16), 63.4(C-17), 15.4(C-18), 18.6(C-19), 43.4(C-20), 14.5(C-21), 112.0(C-22), 32.8(C-23), 30.8(C-24), 31.3(C-25), 66.7(C-26), 17.0(C-27), 101.0(C-1′), 80.4(C-2′), 80.3(C-3′), 78.1(C-4′), 77.9(C-5′), 65.4(C-6′), 101.4(C-1″), 73.9(C-2″), 72.9(C-3″), 75.0(C-4″), 67.9(C-5″), 17.5(C-6″), 106.4(C-1′″), 69.7(C-2′″), 71.1(C-3′″), 82.6(C-4′″), 66.9(C-5′″), 18.0(C-6′″), 111.9(C-1′′′′), 70.4(C-2′′′′), 71.8(C-3′′′′), 74.8(C-4′′′′), 69.2(C-5′′′′) , 17.2(C-6′′′′)。化合物24为薯蓣皂苷元-3-O-α-L-吡喃鼠李糖基(1→4)-O-α-L-吡喃鼠李糖基(1→4)-[O-α-L-吡喃鼠李糖基(1→2)]-O-β-D-吡喃葡萄糖苷。(diosgenin-3-O-α-L-rhamnopyranosyl-
(1→4)-O-α-L-rhamnopyranosyl-(1→4)-[O-α-L-rhamnopyranosyl(1→2)]-O-β-D-glucopyranoside)。
化合物25:白色结晶(甲醇), 分子式为C48H76O23, FAB-MS m/z:1043[M+Na]+。1H-NMR(MeOD,400 MHz)δ :0.88(3H, d, J=6.8 Hz, CH3-27), 0.92(3H, s, CH3-18), 1.10(3H, s, CH3-19), 1.24(3H, d, J=6.0 Hz, Rha-6″-CH3), 5.55(1H, d, J=5.2 Hz, H-6), 5.30(1H, s, Rha-1″-H), 5.18(1H, d, J=2.8 Hz, Api-1′′′′-H), 4.40(1H, d, J=6.8 Hz, Xyl-1′″-H), 4.32(1H, d, J=6.4 Hz, Ara-1′-H); 13C-NMR(MeOD,100MHz)δ :84.7(C-1), 37.3(C-2), 69.2(C-3), 43.5(C-4), 139.7(C-5), 126.0(C-6), 32.7(C-7), 34.1(C-8), 51.4(C-9), 43.4(C-10), 24.7(C-11), 41.1(C-12), 41.7(C-13), 58.1(C-14), 33.0(C-15), 84.5(C-16), 58.5(C-17), 17.2(C-18),15.4(C-19), 45.9(C-20), 62.8(C-21), 112.0(C-22), 70.7(C-23), 73.9(C-24), 36.5(C-25), 61.3(C-26), 12.9(C-27), 101.0(C-1′), 74.4(C-2′), 85.1(C-3′), 70.4(C-4′), 66.9(C-5′), 101.4(C-1″), 71.9(C-2″), 80.3(C-3″), 72.9(C-4″), 69.7(C-5″), 18.6(C-6″), 106.3(C-1′″), 74.8(C-2′″), 78.1(C-3′″), 71.1(C-4′″), 65.4(C-5′″), 112.6(C-1′′′′), 77.9(C-2′′′′), 80.4(C-3′′′′), 75.0(C-4′′′′), 66.9(C-5′′′′)。化合物25为(23s,24s,25s)-螺甾-5-烯-1β, 3β, 21, 23, 24-五羟基-1β-O-β-D-呋喃芹糖基-(1→3)-O-α-L-吡喃鼠李糖基-(1→2)-[O-β-D-吡喃木糖基(1→3)]-O-α-L-吡喃阿拉伯糖苷(trikamsteroside E)。
化合物26:白色结晶(甲醇),分子式为C48H76O22, FAB-MS m/z:1027[M+Na]+。。1H-NMR(MeOD,400 MHz)δ :0.87(3H, d, J=6.8 Hz, CH3-27), 0.88(3H, s, CH3-18), 0.92(3H, d, J=6.8 Hz, CH3-21), 1.10(3H, s, CH3-19), 1.24(3H, d, J=6.0 Hz, Rha-6″-CH3), 5.55(1H, d, J=5.6 Hz, H-6), 5.29(1H, d, J=1.2 Hz, Rha-1″-H), 5.18(1H, d, J=2.8 Hz, Api-1′′′′-H), 4.40(1H, d, J=6.8 Hz, Xyl-1′″-H), 4.31(1H, d, J=6.4 Hz, Ara-1′-H); 13C-NMR(MeOD,100 MHz)δ :84.6(C-1), 37.6(C-2), 69.0(C-3), 43.5(C-4), 139.6(C-5), 126.0(C-6), 32.7(C-7), 34.0(C-8), 51.4(C-9), 43.4(C-10), 24.8(C-11), 41.5(C-12), 41.6(C-13), 37.3(C-14), 32.8(C-15), 84.0(C-16),58.0(C-17), 17.2(C-18), 15.4(C-19), 30.8(C-20), 14.4(C-21), 112.0(C-22), 70.3(C-23), 74.0(C-24), 36.7(C-25), 61.3(C-26), 12.8(C-27), 101.0(C-1′), 74.5(C-2′), 85.1(C-3′), 69.7(C-4′), 66.9(C-5′), 101.5(C-1″), 71.9(C-2″), 80.3(C-3″), 72.9(C-4″), 69.3(C-5″), 18.6(C-6″), 106.3(C-1′″), 74.8(C-2′″), 78.1(C-3′″), 71.1(C-4′″), 66.8(C-5′″), 113.1(C-1′′′′), 77.9(C-2′′′′), 80.3(C-3′′′′), 75.0(C-4′′′′), 65.4(C-5′′′′)。化合物26为(23s,24s,25s)-螺甾-5-烯-1β, 3β, 23, 24-四羟基-1β-O-β-D-呋喃芹糖基-(1→3)-O-α-L-吡喃鼠李糖基-(1→2)-[O-β-D-吡喃木糖基(1→3)]-O-α-L-吡喃阿拉伯糖苷(deoxytrikamsteroside E)。
化合物27:白色结晶(甲醇),分子式为C43H68O19, FAB-MS m/z:911[M+Na]+。1H-NMR(MeOD,400 MHz)δ :0.87(3H, d, J=6.8 Hz, CH3-27), 0.88(3H, s, CH3-18), 1.10(3H, s, CH3-19), 1.24(3H, d, J=6.0 Hz, Rha-6″-CH3), 5.60(1H, d, J=6.0 Hz, H-6), 5.30(1H, d, J=1.2 Hz, Rha-1″-H), 4.44(1H, d, J=6.0 Hz, Xyl-1′″-H), 4.41(1H, d, J=6.8 Hz, Ara-1′-H); 13C-NMR(MeOD,100 MHz)δ :85.3(C-1), 36.3(C-2), 69.3(C-3), 43.2(C-4), 139.8(C-5), 125.2(C-6), 30.1(C-7), 32.7(C-8), 50.1(C-9), 42.8(C-10), 23.7(C-11), 39.2(C-12), 40.6(C-13), 36.9(C-14), 30.8(C-15), 83.0(C-16), 61.9(C-17), 18.0(C-18), 14.3(C-19), 45.6(C-20), 65.4(C-21), 112.1(C-22), 70.5(C-23), 74.4(C-24), 34.9(C-25), 64.4(C-26), 12.8(C-27), 101.4(C-1′), 74.8(C-2′), 80.3(C-3′), 71.1(C-4′), 69.7(C-5′), 101.5(C-1″), 72.9(C-2″), 78.1(C-3″), 73.3(C-4″), 69.9(C-5″), 18.6(C-6″), 106.4(C-1′″), 75.0(C-2′″), 78.0(C-3′″), 71.9(C-4′″), 66.9(C-5′″)。化合物27为(23s,24s,25s)-螺甾-5-烯-1β, 3β, 21, 23, 24-五羟基-1β-O-α-L-吡喃鼠李糖基-(1→2)-[O-β-D-吡喃木糖基(1→3)]-O-α-L-吡喃阿拉伯糖苷((23s,24s,25s)-spirostan-5-ene-1β, 3β, 21, 23, 24-pentahydroxy-1β-
O-α-L-rhamnopyranosyl-(1→2)-[O-β-D-xylopyranosyl(1→3)]-O-α-L-pyranarabinoside)。
化合物28:白色针晶,可溶于甲醇、乙醇。Liebermann-Burchard 反应阳性,Molish 反应阴性,由此推测化合物28为甾体苷元。1H-NMR(DMSO-d6, 400MHz)δ :0.76(3H, s, CH3-18), 0.81(3H, s, CH3-19), 1.05(3H, s, CH3-27), 1.06(3H, s, CH3-21), 1.08(3H, s, CH3-26), 2.19(1H, dd, J=4.0,13.2 Hz, H-5), 2.25(1H, t, J=8.4 Hz, H-17), 3.00(1H, brt, J=8.4 Hz, H-9), 3.11(1H, d, J=9.6 Hz, H-22), 3.60(1H, brs, H-2), 3.76(1H, brs, H-3), 5.62(1H, d, J=2.0 Hz, H-7); 13C-NMR(DMSO-d6, 400MHz)δ:36.6(C-1), 66.7(C-2), 66.5(C-3), 31.5(C-4), 50.1(C-5), 202.6(C-6), 120.4(C-7), 165.2(C-8), 33.1(C-9), 37.6(C-10), 20.0(C-11), 30.3(C-12), 46.8(C-13), 82.9(C-14), 30.3(C-15), 20.2(C-16), 48.7(C-17), 17.1(C-18), 23.8(C-19), 75.7(C-20), 21.0(C-21), 76.1(C-22), 26.1(C-23), 41.4(C-24), 68.7(C-25), 29.0(C-26), 30.0(C-27)。化合物28为β-脱皮激素。
化合物29:白色针晶,可溶于甲醇、乙醇。Liebermann-Burchard 反应阳性,Molish 反应阴性,由此推测化合物29为甾体苷元。1H-NMR(DMSO-d6, 400MHz)δ :0.77(3H, s, CH3-18), 0.79(3H, s, CH3-19), 1.05(3H, s, CH3-27), 1.06(3H, s,CH3-21), 1.07(3H, s, CH3-26), 2.01(1H, t, J=8.4 Hz, H-17), 2.24(1H, dd, J=4.8,12.8 Hz, H-5), 3.03(1H, m, H-24), 3.08(1H, brt, J=8.4 Hz, H-9), 3.61(1H, brs, H-2), 3.83(1H, brs, H-3), 5.67(1H, d, J=1.8 Hz, H-7); 13C-NMR(DMSO-d6, 400MHz)δ :36.9(C-1), 68.7(C-2), 68.3(C-3), 33.3(C-4), 48.5(C-5), 199.7(C-6), 119.3(C-7), 164.9(C-8), 34.8(C-9), 38.0(C-10), 20.2(C-11), 30.9(C-12), 43.7(C-13), 82.8(C-14), 30.2(C-15), 20.9(C-16), 46.8(C-17), 16.4(C-18), 23.2(C-19), 76.2(C-20), 22.4(C-21), 41.4(C-22), 26.1(C-23), 78.4(C-24), 75.6(C-25), 29.0(C-26), 30.0(C-27)。化合物29为pinnatasterone。
化合物30:白色针晶,可溶于甲醇、乙醇。Liebermann-Burchard 反应呈阳性,Molish 反应呈阴性,由此可知化合物30为甾体皂苷元。1H-NMR(DMSO-d6,400MHz)δ :0.82(3H,d,J=6.4Hz,CH3-27),0.92(3H,s,CH3-19),4.22(1H,d,J=6.4Hz,H-3),4.66(2H,d,J=5.2Hz,H-21),5.48(1H,d,J=5.6Hz,H-6)。化合物30为∆5,13-20βF,22αF,25αF螺甾烯-3β,21α-二醇(20βF,22αF,25αF-spirostan-
5,13-ene-3β,21α-diol)。
化合物31:白色针晶,可溶于甲醇、乙醇。Liebermann-Burchard 反应呈阳性,Molish 反应呈阴性,由此可知化合物31为甾体苷元。1H-NMR(DMSO-d6, 400MHz)δ :0.76(3H, s, CH3-18), 0.81(3H, s, CH3-19), 0.84(6H, s, CH3-21,CH3-27), 1.04(3H, s, CH3-26), 2.02(1H, t, J=7.6 Hz, H-17), 2.98(1H, brt, J=8.0 Hz, H-9), 3.01(1H, d, J=7.2 Hz, H-22), 4.23(1H, brt, J=5.2,6.0 Hz, H-2), 4.42(1H, d, J=5.2 Hz, H-3), 5.62(1H, s, H-7)。化合物31为polypodineB。
化合物32:白色针状结晶(甲醇)。Liebermann-Burchard 反应阳性,Molish 反应阴性,由此推测化合物32为甾体苷元。1H-NMR(MeOD,400 MHz)δ :0.90(3H, s, CH3-18), 1.13(3H, s, CH3-19), 1.18(6H, s, CH3-26,27), 1.19(3H, s, CH3-21), 3.07(1H, brt, J=8.0 Hz, H-9), 3.48(1H, brs, H-1), 3.60(1H, brs, H-2), 3.74(1H, brs, H-3), 5.86(1H, d, J=2.0 Hz, H-7); 13C-NMR(MeOD,100MHz)δ :76.0(C-1), 69.0(C-2), 70.0(C-3), 37.6(C-4), 80.3(C-5), 202.3(C-6), 120.3(C-7), 166.6(C-8), 39.7(C-9), 47.8(C-10), 22.7(C-11), 32.6(C-12), 48.8(C-13), 84.9(C-14), 31.8(C-15), 21.4(C-16), 50.5(C-17), 18.0(C-18), 14.0(C-19), 77.8(C-20), 21.0(C-21), 78.4(C-22), 27.3(C-23), 42.4(C-24), 71.3(C-25), 29.0(C-26), 29.7(C-27)。化合物32为intergristerone B。
化合物33:白色针状结晶(甲醇),分子式为C39H64O17, FAB-MS m/z: 827[M+Na]+。1H-NMR(MeOD,400 MHz)δ :0.90(3H, s, CH3-19), 1.13(3H, s, CH3-18), 1.18(6H, s, CH3-26,27), 1.19(3H, s, CH3-21), 3.07 (1H,t, J=8.4 Hz, H-5), 3.20 (1H, t, J=10.4 Hz, H-17), 3.51(1H, t, J=8.4 Hz, H-9), 4.06(1H, m, H-3), 4.08(1H, m, H-2), 5.28(1H, d, J=6.4 Hz, Glc-1′′-H), 5.58(1H, d, J=8.4 Hz, Gal-1′-H), 5.87(1H, d, J=2.0 Hz, H-7); 13C-NMR(MeOD,100 MHz)δ :39.7(C-1), 69.0(C-2), 80.3(C-3), 29.7(C-4), 50.5(C-5), 202.2(C-6), 120.3(C-7), 166.7(C-8), 32.6(C-9), 37.6(C-10), 21.0(C-11), 31.8(C-12), 47.8(C-13), 84.9(C-14), 30.8(C-15), 21.4(C-16), 49.0(C-17), 14.0(C-18), 18.0(C-19), 77.8(C-20), 22.7(C-21), 91.0(C-22), 27.3(C-23), 42.4(C-24), 70.0(C-25), 28.9(C-26), 28.9(C-27), 101.5(Gal-C-1′), 69.7(C-2′), 71.8(C-3′), 71.1(C-4′), 74.8(C-5′), 65.4(C-6′), 106.3(Glc-C-1″), 76.0(C-2″), 78.4(C-3″), 71.3(C-4″), 78.1(C-5″), 66.9(C-6″)。化合物33为sileneoside G。
化合物34:白色结晶(甲醇)。1H-NMR(MeOD,400 MHz)δ :1.02(3H, d, J=6.0 Hz, CH3-27), 1.02(3H, s, CH3-19),1.11(3H, s, CH3-18), 1.24(6H, d, J=6.0 Hz, Rha-6″ and 6′″-CH3), 2.34(3H, s, CH3-21), 5.19(1H, d, J=1.2 Hz, Rha-1″-H), 5.17(1H, d, J=2.8 Hz, Rha-1′′′-H) , 4.48(1H, d, J=7.6 Hz, Glc-1′′′′-H), 4.24(1H, d, J=7.6 Hz, Glc-1′-H); 13C-NMR(MeOD,100 MHz)δ :30.8(C-1), 30.2(C-2),75.2(C-3), 30.8(C-4), 34.8(C-5), 30.1(C-6), 30.8(C-7), 37.0(C-8), 38.5(C-9), 34.8(C-10), 22.3(C-11), 38.0(C-12), 39.5(C-13), 51.8(C-14), 33.1(C-15), 88.4(C-16), 58.4(C-17), 15.3(C-18), 19.8(C-19), 122.7(C-20), 14.5(C-21), 142.0(C-22), 23.8(C-23), 30.8(C-24), 32.8(C-25), 73.9(C-26), 16.7(C-27), 100.5(C-1′), 79.4(C-2′), 79.1(C-3′), 79.0(C-4′), 78.2(C-5′), 62.7(C-6′), 102.2(C-1″), 72.2(C-2″), 73.9(C-3″), 77.9(C-4″),69.8(C-5″), 17.5(C-6″), 101.0(C-1′″), 72.4(C-2′″), 77.9(C-3′″), 78.1(C-4′″), 71.7(C-5′″), 18.0(C-6′″), 104.5(C-1′′′′), 77.7(C-2′′′′), 79.4(C-3′′′′),78.1(C-4′′′′), 79.1(C-5′′′′) , 62.8(C-6′′′′)。化合物34为26-O-β-D-吡喃葡萄糖基-(25s)-5α-呋甾烷-△20(22)-烯-3β,26-二醇-3-O-α-L-吡喃鼠李糖基(1→4)-[O-α-L-吡喃鼠李糖基(1→2)]-O-β-D-吡喃葡萄糖苷。(26-O-β-D-glucopyranosyl-(25s)-5α-furostan-△20(22)-ene-3β,26-glycol-3-O-α-L-rhamnopyranosyl-(1→4)-[O-α-L-
rhamnopyranosyl(1→2)]-O-β-D-glucopyranoside)。
化合物35:白色结晶(甲醇)。1H-NMR(MeOD,400 MHz)δ :0.93(3H, d, J=6.0 Hz, CH3-27), 1.09(3H, s, CH3-19),1.13(3H, s, CH3-18), 1.23(3H, d, J=6.0 Hz, Rha-6″), 1.95(3H, s, CH3-21), 5.18(1H, s, Rha-1″-H), 4.47(1H, d, J=8.0 Hz, Glc-1′′′-H), 4.23(1H, d, J=7.6 Hz, Glc-1′-H); 13C-NMR(MeOD,100 MHz)δ :30.7(C-1), 28.3(C-2),75.2(C-3), 30.7(C-4),34.0(C-5), 28.3(C-6), 30.7(C-7), 38.1(C-8), 39.5(C-9), 34.0(C-10), 21.9(C-11), 39.5(C-12), 44.6(C-13), 51.3(C-14),34.0(C-15), 85.3(C-16), 61.6(C-17), 17.1(C-18), 19.8(C-19), 122.2(C-20), 15.9(C-21), 142.1(C-22), 23.7(C-23), 30.7(C-24), 32.1(C-25), 74.0(C-26), 17.4(C-27), 100.6(C-1′), 79.4(C-2′), 79.2(C-3′), 79.1(C-4′), 78.2(C-5′), 62.8(C-6′), 102.2(C-1″), 72.2(C-2″), 72.4(C-3″), 77.9(C-4″),69.7(C-5″), 18.0(C-6″), 104.6(C-1′″), 77.7(C-2′″), 79.4(C-3′″), 71.9(C-4′″), 79.2(C-5′″), 62.8(C-6′″)。化合物35为26-O-β-D-吡喃葡萄糖基-(25s)-5α-呋甾烷-△20(22)-烯-3β,26-二醇-3-O-α-L-吡喃鼠李糖基(1→4)-O-β-D-吡喃葡萄糖苷。(26-O-β-D-glucopyranosyl-(25s)-5α-furostan-△20(22)-ene-3β,26-glycol-3-O-α-L-rhamnopyranosyl-(1→4)-O-β-D-
glucopyranoside)。
化合物36:白色结晶(甲醇)。1H-NMR(MeOD,400 MHz)δ :0.92(3H, d, J=6.0 Hz, CH3-27), 1.05(3H, s, CH3-19), 1.23(3H, d, J=6.0 Hz, Rha-6″), 1.40(3H, s, CH3-18),2.12(3H, s, CH3-21), 5.40(1H, s, H-6), 5.18(1H, d, J=2.0 Hz, Rha-1″-H), 4.48(1H, d, J=7.6 Hz, Glc-1′′′-H), 4.20(1H, d, J=7.6 Hz, Glc-1′-H); 13C-NMR(MeOD,100 MHz)δ :30.7(C-1), 28.0(C-2),75.2(C-3), 30.7(C-4),107.4(C-5), 159.9(C-6), 30.7(C-7), 38.1(C-8), 39.3(C-9), 34.4(C-10), 20.3(C-11), 38.3(C-12), 41.7(C-13), 50.6(C-14),34.0(C-15), 82.5(C-16), 63.4(C-17), 16.9(C-18), 19.7(C-19), 122.9(C-20), 13.2(C-21), 141.1(C-22), 20.8(C-23), 30.7(C-24), 33.3(C-25), 74.0(C-26), 18.0(C-27), 100.6(C-1′), 79.4(C-2′), 79.2(C-3′), 79.1(C-4′), 78.2(C-5′), 62.8(C-6′), 102.2(C-1″), 72.2(C-2″), 72.4(C-3″), 77.9(C-4″),69.7(C-5″), 18.0(C-6″), 104.6(C-1′″), 77.7(C-2′″), 79.4(C-3′″), 71.9(C-4′″), 79.2(C-5′″), 62.8(C-6′″)。化合物36为26-O-β-D-吡喃葡萄糖基-(25s)-5α-呋甾烷-△5(6),20(22)-二烯-3β,26-二醇-3-O-α-L-吡喃鼠李糖基(1→4)-O-
β-D-吡喃葡萄糖苷。(26-O-β-D-glucopyranosyl-(25s)-5α-furostan-△5(6),20(22)-diene-
3β,26-glycol-3-O-α-L-rhamnopyranosyl-(1→4)-O-β-D-glucopyranoside)。
化合物37(新化合物):白色结晶(甲醇)。1H-NMR(MeOD,400 MHz)δ :0.93(3H, d, J=6.4 Hz, CH3-27), 1.09(3H, s, CH3-18), 1.13(3H, s, CH3-19),1.23(3H, d, J=6.0 Hz, Rha-6″), 1.24(3H, d, J=6.0 Hz, Rha-6′′′), 1.28(3H, d, J=6.0 Hz, Rha-6″″), 1.95(3H, s, CH3-21), 5.40(1H, d, J=4.4 Hz,H-6), 5.18(1H, d, J=1.6 Hz, Rha-1″-H), 5.17(1H, d, J=1.6 Hz, Rha-1″″-H), 4.82(1H, d, J=1.6 Hz, Rha-1′′′-H), 4.49(1H, d, J=7.6 Hz, Glc-1′-H), 4.23(1H, d, J=7.6 Hz, Glc-1′′′″-H); 13C-NMR(MeOD,100 MHz)δ :38.0(C-1), 37.2(C-2),71.7(C-3), 39.4(C-4),142.1(C-5), 122.3(C-6), 30.7(C-7), 38.2(C-8), 51.3(C-9), 32.0(C-10), 21.9(C-11), 32.6(C-12), 44.6(C-13), 52.1(C-14),38.6(C-15),213.9(C-16), 143.8(C-17), 17.1(C-18), 19.8(C-19), 147.0(C-20), 15.9(C-21), 207.9(C-22), 28.2(C-23), 39.3(C-24), 34.0(C-25), 75.8(C-26), 17.4(C-27), 100.4(C-1′), 79.4(C-2′), 78.1(C-3′), 80.8(C-4′), 77.9(C-5′), 61.9(C-6′), 102.4(C-1″), 72.1(C-2″), 73.9(C-3″), 76.7(C-4″),69.8(C-5″), 18.6(C-6″), 103.2(C-1′″), 73.0(C-2′″), 72.4(C-3′″), 73.8(C-4′″), 70.4(C-5′″), 17.9(C-6′″), 102.6(C-1″″), 72.9(C-2″″), 72.4(C-3″″), 79.5(C-4″″), 69.1(C-5″″), 18.0(C-6″″), 104.5(C-1′′′″), 75.2(C-2′′′″), 78.1(C-3′′′″), 79.1(C-4′′′″), 78.0(C-5′′′″), 62.8(C-6′′′″)。化合物37为26-O-β-D-吡喃葡萄糖基-(25R)-Δ5(6)、17(20)-二烯-16,22-二酮-呋甾烷-3β,26-二醇-3-O-α-L-吡喃鼠李糖基(1→4)-O-α-L-吡喃鼠李糖基(1→4)[O-α-L-吡喃鼠李糖基(1→2)-]-O-β-D-吡喃葡萄糖苷。(26-O-β-D-glucopyranosyl-(25R)-Δ5(6)、17(20)-diene-16,22-dione-furostan-3β,26-diol-3-O-α-L-rhamnopyranosyl-(1→4)-O-α-L-rhamnopyranosyl-(1→4)[O-α-L-rhamnopyranosyl-(1→2)]-O-β-D-glucopyranoside)。
化合物38(新化合物):白色结晶(甲醇)。1H-NMR(MeOD,400 MHz)δ :0.93(3H, d, J=6.4 Hz, CH3-27), 1.09(3H, s, CH3-18), 1.13(3H, s, CH3-19),1.23(3H, d, J=6.0 Hz, Rha-6″), 1.24(3H, d, J=6.0 Hz, Rha-6′″), 1.95(3H, s, CH3-21), 5.40(1H, d, J=4.4 Hz, H-6), 5.19(1H, d, J=1.2 Hz, Rha-1″-H), 4.83(1H, d, J=1.2 Hz, Rha-1′″-H),4.50(1H, d, J=7.6 Hz, Glc-1′-H), 4.23(1H, d, J=7.6 Hz, Glc-1″″-H); 13C-NMR(MeOD,100 MHz)δ :38.0(C-1), 37.3(C-2),71.7(C-3), 39.5(C-4),142.1(C-5), 122.3(C-6),30.8(C-7),38.3(C-8), 51.3(C-9), 32.0(C-10), 21.9(C-11), 32.6(C-12),44.6(C-13), 52.1(C-14),38.6(C-15), 77.0(C-16), 143.9(C-17), 17.1(C-18), 19.8(C-19), 146.9(C-20), 15.9(C-21), 207.9(C-22), 28.3(C-23), 39.3(C-24), 34.0(C-25), 75.2(C-26), 17.4(C-27), 100.4(C-1′), 79.3(C-2′), 78.1(C-3′), 80.0(C-4′), 77.9(C-5′), 62.0(C-6′), 102.3(C-1″), 72.2(C-2″), 73.9(C-3″), 76.6(C-4″),69.8
(C-5″), 18.0(C-6″), 103.0(C-1′″), 72.5(C-2′″), 72.4(C-3′″), 73.7(C-4′″), 70.7(C-5′″), 17.9(C-6′″), 104.5(C-1″″), 75.2(C-2″″), 78.1(C-3″″), 79.2(C-4″″), 78.0(C-5″″), 62.8(C-6″″)。化合物38为26-O-β-D-吡喃葡萄糖基-(25R)-Δ5(6)、17(20)-二烯-16-羟基-22-酮-呋甾烷-3β,26-二醇-3-O-α-L-吡喃鼠李糖基(1→2)[O-α-L-吡喃鼠李糖基(1→4)-]-O-β-D-吡喃葡萄糖苷。(26-O-β-D-glucopyranosyl-(25R)-Δ5(6)、17(20)-diene
-16-hydroxyl-22-one-furostan-3β,26-diol-3-O-α-L-rhamnopyranosyl-(1→2)[O-α-L-
rhamnopyranosyl-(1→4)]-O-β-D-glucopyranoside)。
化合物39:白色结晶(甲醇)。1H-NMR(MeOD,400 MHz)δ :0.93(3H, d, J=6.4 Hz, CH3-27), 1.09(3H, s, CH3-18), 1.13(3H, s, CH3-19), 1.23(3H, d, J=6.0 Hz, Rha-6″), 1.25(3H, d, J=6.0 Hz, Rha-6′″), 1.95(3H, s, CH3-21), 5.40(1H, d, J=4.8 Hz, H-6), 5.19(1H, d, J=1.6 Hz, Rha-1″-H), 4.83(1H, d, J=1.6 Hz, Rha-1′′′-H), 4.50(1H, d, J=7.6 Hz, Glc-1′-H) , 4.23(1H, d, J=8.0 Hz, Glc-1′″′-H); 13C-NMR(MeOD,100 MHz)δ :38.1(C-1), 37.3(C-2),71.7(C-3), 39.5(C-4),142.1(C-5), 122.3(C-6), 30.7(C-7), 38.3(C-8), 51.3(C-9), 32.0(C-10), 21.9(C-11), 32.6(C-12), 44.6(C-13), 52.1(C-14),38.6(C-15), 214.0(C-16), 143.9(C-17), 17.1(C-18), 19.8(C-19), 147.0(C-20), 16.0(C-21), 207.9(C-22), 28.3(C-23), 39.3(C-24), 34.0(C-25), 75.8(C-26), 17.4(C-27), 100.4(C-1′), 79.3(C-2′), 78.1(C-3′), 80.0(C-4′), 77.9(C-5′), 62.0(C-6′), 102.3(C-1″), 72.2(C-2″), 73.9(C-3″), 76.6(C-4″),69.8(C-5″), 18.0(C-6″), 103.0(C-1′″), 72.5(C-2′″), 72.4(C-3′″), 73.7(C-4′″), 70.7(C-5′″), 17.9(C-6′″), 104.6(C-1″″), 75.2(C-2″″), 78.1(C-3″″), 79.2(C-4″″), 78.0(C-5″″), 62.8(C-6″″)。化合物39为26-O-β-D-吡喃葡萄糖基-(25R)-Δ5(6)、17(20)-二烯-16,22-二酮-呋甾烷-3β,26-二醇-3-O-α-L-吡喃鼠李糖基(1→2)[O-α-L-吡喃鼠李糖基(1→4)-]-O-β-D-吡喃葡萄糖苷。(26-O-β-D-glucopyranosyl-(25R)-Δ5(6)、17(20)
-diene-16,22-dione-furostan-3β,26-diol-3-O-α-L-rhamnopyranosyl-(1→2)[O-α-L-
rhamnopyranosyl-(1→4)]-O-β-D-glucopyranoside)。
化合物40:白色结晶(甲醇)。1H-NMR(MeOD,400 MHz)δ :0.93(3H, d, J=6.4 Hz, CH3-27), 1.09(3H, s, CH3-18), 1.13(3H, s, CH3-19), 1.23(3H, d, J=6.0 Hz, Rha-6″), 1.95(3H, s, CH3-21) , 5.39(1H, brs, H-6), 5.16(1H, d, J=2.0 Hz, Rha-1″-H), 4.47(1H, d, J=7.6 Hz, Glc-1′-H), 4.23(1H, d, J=7.6 Hz, Glc-1′′′-H); 13C-NMR(MeOD,100 MHz)δ :38.1(C-1), 37.3(C-2),71.7(C-3), 39.5(C-4),142.1(C-5), 122.3(C-6), 30.7(C-7), 38.3(C-8), 51.3(C-9),32.0(C-10), 21.9(C-11), 32.6(C-12), 44.6(C-13), 52.1(C-14),38.6(C-15), 214.0(C-16), 143.9(C-17), 17.1(C-18), 19.8(C-19), 147.0(C-20), 16.0(C-21), 207.9(C-22), 28.3(C-23), 39.3(C-24), 34.0(C-25), 75.8(C-26), 17.4(C-27), 100.5(C-1′), 79.0(C-2′), 79.1(C-3′),77.9(C-4′), 77.7(C-5′), 62.7(C-6′), 102.2(C-1″), 72.2(C-2″), 73.9(C-3″), 72.4(C-4″),71.8(C-5″), 18.0(C-6″), 104.5(C-1′″), 75.2(C-2′″), 78.1(C-3′″), 79.4(C-4′″), 69.8(C-5′″), 62.8(C-6′″)。化合物40为26-O-β-D-吡喃葡萄糖基-(25R)-Δ5(6)、17(20) -二烯-16,22-二酮-呋甾烷-3β,26-二醇-3-O-α-L-吡喃鼠李糖基(1→2)-O-β-D-吡喃葡萄糖苷。(26-O-β-D-glucopyranosyl-(25R)-Δ5(6)、17(20)-diene-16,22-dione-furostan-
3β-26-diol-3-O-α-L-rhamnopyranosyl-(1→2)-O-β-D-glucopyranoside)。
化合物41: 白色结晶(甲醇)。1H-NMR(MeOD,400 MHz)δ :1.03(3H, d, J=6.0 Hz, CH3-27), 1.00(3H, s, CH3-18), 1.10(3H, s, CH3-19), 1.24(3H, d, J=6.0 Hz, Rha-6″), 1.25(3H, d, J=6.0 Hz, Rha-6′″), 2.28(3H, s, CH3-21), 6.89(1H, d, J=7.6 Hz,H-16′), 6.86(1H, d, J=7.6 Hz,H-22′), 5.42(1H, d, J=4.4 Hz,H-6), 5.20(1H, d, J=1.6 Hz, Rha-1″-H), 4.83(1H, d, J=1.6 Hz, Rha-1′′′-H), 4.50(1H, d, J=7.6 Hz, Glc-1′-H) , 4.23(1H, d, J=8.0 Hz, Glc-1′″′-H); 13C-NMR(MeOD,100 MHz)δ :38.3(C-1), 38.1(C-2),71.6(C-3), 39.6(C-4),142.1(C-5), 122.7(C-6), 30.8(C-7), 38.5(C-8), 51.9(C-9), 32.1(C-10), 22.3(C-11), 32.8(C-12), 48.2(C-13), 59.0(C-14),32.2(C-15), 141.5(C-16), 123.4(C-16′),
152.6(C-17), 16.8(C-18), 19.8(C-19), 131.9(C-20), 15.0(C-21), 140.5(C-22), 128.2(C-22′), 34.8(C-23), 36.2(C-24), 33.2(C-25), 75.9(C-26), 17.5(C-27), 100.5(C-1′), 79.3(C-2′), 78.2(C-3′), 80.0(C-4′), 77.9(C-5′), 62.0(C-6′), 102.3(C-1″), 71.7(C-2″), 73.9(C-3″), 76.6(C-4″), 69.8(C-5″), 18.0(C-6″), 103.0(C-1′″), 72.5(C-2′″), 72.4(C-3′″), 73.7(C-4′″), 70.7(C-5′″), 17.9(C-6′″), 104.5(C-1″″), 75.2(C-2″″), 78.2(C-3″″), 79.3(C-4″″), 78.0(C-5″″), 62.8(C-6″″)。化合物41为3β-O-α-L-rhamnopyranosyl-(1→4)-[O-α-L-
rhamnopyranosyl-(1→2)]-O-β-D-glucopyranosylhomo-aro-cholest-5-ene-26-O-β-D-gluc-opyranoside(aethiosidecA)。
化合物42: 白色结晶(甲醇)。1H-NMR(MeOD,400 MHz)δ :1.03(3H, d, J=6.0 Hz, CH3-27), 1.00(3H, s, CH3-18), 1.10(3H, s, CH3-19), 1.24(6H, d, J=6.0 Hz, Rha-6″,6′″), 1.28(3H, d, J=6.0 Hz, Rha-6′″′), 2.28(3H, s, CH3-21), 6.89(1H, d, J=7.6 Hz,H-16′), 6.86(1H, d, J=7.6 Hz,H-22′), 5.43(1H, s,H-6), 5.18(1H, s, Rha-1″-H), 5.17(1H, s, Rha-1″″-H), 4.83(1H, d, J=1.6 Hz, Rha-1′′′-H), 4.50(1H, d, J=7.6 Hz, Glc-1′-H) , 4.23(1H, d, J=8.0 Hz, Glc-1′′′″-H); 13C-NMR(MeOD,100 MHz)δ :38.3(C-1), 38.1(C-2),71.6(C-3), 39.5(C-4),142.1(C-5), 122.7(C-6), 30.8(C-7), 38.5(C-8), 51.9(C-9), 32.0(C-10), 22.3(C-11), 32.8(C-12), 48.2(C-13), 59.0(C-14),32.2(C-15), 141.5(C-16), 123.4(C-16′),
152.6(C-17), 16.8(C-18), 19.8(C-19), 131.9(C-20), 15.0(C-21), 140.5(C-22), 128.2(C-22′), 34.8(C-23), 36.2(C-24), 33.2(C-25), 75.9(C-26), 17.5(C-27), 100.5(C-1′), 79.5(C-2′), 78.2(C-3′), 80.9(C-4′), 77.9(C-5′), 61.9(C-6′), 102.4(C-1″), 71.7(C-2″), 74.0(C-3″), 76.7(C-4″), 69.8(C-5″), 18.6(C-6″), 103.2(C-1′″), 73.0(C-2′″), 72.2(C-3′″), 73.9(C-4′″), 70.5(C-5′″), 18.0(C-6′″), 102.6(C-1″″), 73.0(C-2″″), 72.4(C-3″″), 79.5(C-4″″), 69.1(C-5″″), 18.0(C-6″″), 104.5(C-1′′′″), 75.2(C-2′′′″), 78.2(C-3′′′″), 79.3(C-4′′′″), 77.9(C-5′′′″), 62.8(C-6′′′″)。化合物42为3β-O-α-L-rhamnopyranosyl-(1→4)-O-α-L-
rhamnopyranosyl-(1→4)-[O-α-L-rhamnopyranosyl-(1→2)]-O-β-D-glucopyranosylhomo-aro-cholest-5-ene-26-O-β-D-glucopyranoside(parispseudosideA)。
化合物43: 白色结晶(甲醇)。1H-NMR(MeOD,400 MHz)δ :1.02(3H, d, J=6.0 Hz, CH3-27), 1.02(3H, s, CH3-18), 1.10(3H, s, CH3-19), 1.24(3H, d, J=6.0 Hz, Rha-6″), 2.34(3H, s, CH3-21), 7.52(1H, d, J=7.6 Hz,H-22′), 5.42(1H, s,H-6), 5.19(1H, d, J=1.2 Hz, Rha-1″-H), 4.48(1H, d, J=7.6 Hz, Glc-1′-H) , 4.24(1H, d, J=7.6 Hz, Glc-1′″-H); 13C-NMR(MeOD,100 MHz)δ :38.2(C-1), 38.1(C-2), 71.8(C-3), 39.6(C-4),142.1(C-5), 122.7(C-6), 30.7(C-7), 38.5(C-8), 51.8(C-9), 32.9(C-10), 22.3(C-11), 32.8(C-12), 47.9(C-13), 58.4(C-14), 32.2(C-15), 136.8(C-16), 160.3(C-16′), 154.0(C-17), 16.7(C-18), 19.8(C-19), 129.8(C-20), 15.3(C-21), 140.9(C-22), 125.3(C-22′), 34.8(C-23), 35.9(C-24), 33.7(C-25), 75.2(C-26), 17.5(C-27), 100.6(C-1′), 79.4(C-2′), 78.2(C-3′), 80.3(C-4′), 77.7(C-5′), 62.8(C-6′), 102.1(C-1″), 71.9(C-2″), 72.3(C-3″), 75.9(C-4″), 69.8(C-5″), 18.0(C-6″), 104.6(C-1′″), 74.0(C-2′″), 78.2(C-3′″), 79.3(C-4′″), 77.9(C-5′″), 62.9(C-6′″)。化合物43为3β-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranosylhomo-16′-hydro-
xyl-aro-cholest-5-ene-26-O-β-D-glucopyranoside(16′-hydroxyl-parispseudoside B)。
化合物44: 白色粉末,溶于乙醇、丙酮。1H-NMR(CDCl3,400MHz):δ5.63(1H,dd,J=11.2Hz, H-2),5.59(1H,dd,J=11.2、17.6Hz)和5.26(1H,dd,J=9.0、17.5Hz)为1位烯键上2个H的信号,5.49(2H,d,J=19.6Hz,H-15),5.34(1H,m, H-6),1.25(3H,s,CH3-12)、1.30(3H,s,CH3-13)和1.61(3H,s,CH3-14),4.64(1H,d,J=8.0Hz)和4.63(1H,d,J=8.0Hz)处可见2个葡萄糖的端基氢信号。化合物44为7,11-dimethyl-3-methylene-1,6-dodecadien-10,11-dihydroxyl -10- O-β-D-glucopyranosyl-(1→4)- O-β-D-glucopyranoside。
化合物45: 白色粉末(甲醇)。1H-NMR (MeOD,400MHz):δ 6.37 (1H,dd,J=10.8 Hz, H-2),5.68 (1H,m,H-6),5.27 (1H,dd,J=6.4,17.6 Hz, H-1a),5.24 (1H,d,J=17.6 Hz, H-15a),5.03 (1H,d,J=10.8,17.6 Hz, H-1b),5.00 (1H,d,J=17.6 Hz, H-15b),4.33 (1H,d,J=8.0 Hz,Glc-1′-H), 1.61 (3H,s,CH3-14)、1.15 (3H,s,CH3-12), 1.13 (3H,s,CH3-13); 13C-NMR(MeOD,100 MHz)δ :113.5(C-1), 140.2(C-2), 147.6(C-3), 32.5(C-4), 27.8(C-5), 126.9(C-6), 136.4(C-7), 37.0(C-8), 31.3(C-9), 89.3(C-10), 71.5(C-11), 24.5(C-12), 26.6(C-13), 16.0(C-14), 116.4(C-15), 105.3(C-1′), 75.4(C-2′), 78.1(C-3′), 73.5(C-4′), 78.0(C-5′), 62.5(C-6′)。化合物45为延龄草倍半萜苷C,即7,11-二甲基-3-亚甲基-1,6-十二碳二烯-10,11-二羟基-10-O-β-D-吡喃葡萄糖苷(7,11-dimethyl-3-methylene-1,6-dodecadien-10,11-dihydroxyl -10-O-β-D-glucopy-
ranoside)。
化合物46:白色结晶(甲醇)。1H-NMR(DMSO-d6,400MHz)δ :9.02(1H,d,J=2.0Hz,H-6),8.77(1H,m,H-1a),8.17(1H,m,H-1b),8.17(1H,m,H-2b),5.81(1H,m,H-15a),5.37(1H,m,H-15b),5.22(1H,t,J=6.8Hz,H-10),1.64(3H,s,CH3-14),1.20(3H,s,CH3-13),1.16(3H,s,CH3-12)。化合物46为7,11-二甲基-3-亚甲基-10,11-二羟基-1,6-十二碳二烯二醇(7,11-dimethyl-3-methylene-10,11-dihydroxyl-1,6-dodecadien-diol)。
化合物47:白色结晶(氯仿),分子式为 C15H30O2,ESI-MS [M+H]+ 峰m/z:243。1H-NMR(CDCl3,400MHz)δ :5.34(1H,ddd,J=3.2,6.4,8.8Hz,H-7),2.01(2H,dd,J=6.0,6.4Hz,H-8),2.35(2H,t,J=7.6,7.2Hz,H-3)和1.63(2H,dd,J=7.2,7.6Hz,H-5),0.88(3H,s)、1.12(3H,s)、1.18(3H,s)、和1.58(3H,s)处出现的4个甲基信号。化合物47为2,6,10-三甲基-2,10-二羟基-6-十二碳烯二醇(2,6,10-trimethyl-2,10-dihydroxyl-6-dodecene-diol)。
化合物48:白色结晶(氯仿),分子式为 C15H28O3,ESI-MS [M+H]+ 峰m/z:257。1H-NMR(CDCl3,400MHz)δ :5.89(1H,dd,J=10.8Hz,H-2),5.37(1H,dd,J=10.8,17.5Hz,H-1a),5.29(1H,dd,J=10.8,17.5Hz,H-1b),5.22(1H,t,J=7.6Hz,H-6),1.16(3H,s)、1.20(3H,s)、1.25(3H,s)和1.62(3H,s)处出现的4个甲基信号。化合物48为即3,7,11-三甲基-3,9,11-三羟基-1,6-十二碳二烯三醇(3,7,11-trimethyl-3,9,11-trihydroxyl-1,6-dodecadiene-glycerol)。
化合物49(新化合物):淡黄色固体(甲醇),分子式为 C21H31O4。1H-NMR(CDCl3,400MHz)δ :9.02(1H,d,J=2.0 Hz,H-2′),8.77(1H,dd,J=1.2,4.8 Hz,H-4′),8.17(1H,dd,J=1.6,2.0 Hz,H-6′),7.42(1H,ddd,J=1.2,2.0,4.8 Hz,H-5′),5.80(1H,dd,J=10.8 Hz,H-2),5.33(1H,dd,J=10.8,17.5Hz,H-1a),5.32(1H,dd,J=10.8,17.5Hz,H-1b),3.49(1H,t,J=7.6Hz,H-15a,15b),3.35(1H,m,H-10),2.22(2H,m,H-4a,5a),2.06(2H,m,H-4b,5b),1.76(1H,m,H-8a),1.64(3H,s,CH3-14),1.20(3H,s,CH3-12),1.16(3H,s,CH3-13)。化合物49为7,11-二甲基-3-羟甲基-1,6-十二碳二烯-10,11,15-三醇-10-O-间苯酚(7,11-
dimethyl-3-hydroxymethyl-1,6-dodecadiene-10,11,15-triol-10-O-meta phenol)。
化合物50(新化合物):淡黄色固体(甲醇),溴甲酚兰显黄色斑点,示有羧基存在。1H-NMR(DMSO-d6,400 MHz)δ :8.02(1H,s,COOH-7),6.33(1H,s, H-3),3.33(1H,m,H-5),3.00(1H,m,H-4),2.28(1H,m,H-6a),2.02(1H,m,H-6b),1.23(6H,s,CH3-8, 9);13C-NMR(DMSO-d6,100 MHz)δ :39.0(C-1),109.7(C-2),139.1(C-3),69.7(C-4),59.3(C-5),28.9(C-6),173.8(C-7),28.5(C-8),28.3(C-9)。化合物50为延龄草酸A(trillium acid A)。
化合物51(新化合物):淡黄色固体(甲醇),溴甲酚兰显黄色斑点,示有羧基存在。1H-NMR(DMSO-d6,400 MHz)δ :11.2(1H,s,COOH-1),7.69(1H,d,J=0.8 Hz, H-3),6.16(1H,m,H-2),4.23(1H,brs,H-7a),3.75(1H,brs,H-8a),3.55(4H,m,H-7a,8a,10),2.06(2H,m,H-9),1.77(3H,d,J=0.8 Hz,CH3-11);13C-NMR(DMSO-d6,100 MHz)δ :163.7(C-1),83.7(C-2),150.4(C-3),109.3(C-4),136.1(C-6),70.4(C-7),87.2(C-8),39.4(C-9),61.3(C-10),12.2(C-11)。化合物51为延龄草酸B(trillium acid B)。
化合物52(新化合物):棕黄色固体(甲醇)。1H-NMR(MeOD,400 MHz)δ :6.77(1H,s),6.76(1H,dd,J=2.0,8.0 Hz),6.70(1H,d,J=1.6 Hz),6.69(1H,s),6.67(1H,dd,J=1.6,8.0 Hz),6.66(1H,s),6.64(1H,dd,J=1.6,8.0 Hz),6.62(1H,dd,J=2.0,8.0 Hz),6.56(1H,dd,J=2.0,8.0 Hz),6.47(2H,dd,J=2.0,7.2 Hz),6.22(1H,dd,J=2.0,7.2 Hz),6.15(1H,d,J=1.6 Hz),6.10(1H,s),5.87(1H,s),5.85(1H,d,J=7.2 Hz),4.70(1H,s),4.68(1Hs),4.49(1H,d,J=7.2 Hz),3.96(1H,s),3.75(3H,s),3.63(6H,s),3.34(2H,s);13C-NMR(MeOD,100 MHz)δ :162.3,161.8,161.2,161.0,159.7,159.6,149.1,148.9,148.8,147.9,147.7,147.1,141.3,140.0,139.4,133.7,133.3,132.8,121.5,120.6,120.3,120.2,119.5,119.0,116.0,115.9,115.7,110.7,110.1,100.0,109.1,106.4,106.0,98.4,97.1,96.9,96.5,90.9,87.1,56.6,56.4,56.3,56.2,53.2,48.0。化合物52为延龄草酸C(trillium acid C)。
化合物53:白色结晶(甲醇)。1H-NMR(DMSO-d6, 400MHz)δ :7.53(1H, d, J=16.0 Hz, H-7), 7.26(1H, d, J=2.0 Hz, H-2), 7.08(1H, dd, J=2.0,8.0 Hz, H-6), 6.72(1H, d, J=8.0 Hz, H-5), 6.40(1H, d, J=16.0 Hz, H-8), 3.79(3H, s, 3-OMe), 3.69(3H, s, 9-OMe)。化合物53为methyl ferulorate。
化合物54:白色结晶(甲醇)。1H-NMR(DMSO-d6, 400MHz)δ :7.59(1H, d, J=16.0 Hz, H-7), 7.28(1H, d, J=2.0 Hz, H-2), 7.15(1H, dd, J=2.0,8.0 Hz, H-6), 6.79(1H, d, J=8.0 Hz, H-5), 6.41(1H, d, J=16.0 Hz, H-8), 5.35(1H, d, J=8.0 Hz, H-1′), 5.23(1H, d, J=3.6Hz, H-1′′), 3.82(3H, s, 3-OMe), 2.04(3H, s, 6′′-COOMe)。化合物54为regaloside A。
化合物55:白色结晶(甲醇)。1H-NMR(DMSO-d6,400MHz)δ :7.59(1H,d,J=16.0Hz,H-7),7.29(1H,d,J=1.6Hz,H-2),7.15(1H,dd,J=1.6,8.0Hz,H-6),6.80(1H,d,J=8.0Hz,H-5),6.41(1H,d,J=16.0Hz,H-8),5.34(1H,d,J=7.6Hz,H-1′),5.20(1H,d,J=3.2Hz,H-1′′),3.82(3H,s,MeO-3)。化合物55为3-O-feruloylsucrose。
化合物56:白色结晶(甲醇)。1H-NMR(DMSO-d6,400MHz)δ :7.78(2H,d,J=16.0Hz,H-7,7′″),7.43(2H,d,J=1.6Hz,H-2,2′″),7.30(2H,dd,J=1.6,8.0Hz,H-6,6′″),7.15(1H,d,J=8.0Hz,H-5,5′″),6.66(1H,d,J=16.0Hz,H-8,8′″),6.58(1H,d,J=8.0Hz,H-3′),6.43(1H,d,J=3.2Hz,H-1′′),6.20(1H,d,J=4.4Hz,H-1′),3.82(6H,s,MeO-3,3′″)。以上数据与文献[9]报道一致,故鉴定化合物56为heronioside A。
实施例8
阿尔茨海默病(AD)的病理特征为大脑皮质和海马出现大量的老年斑(SP)、神经纤维缠结(NFT)、神经元大量丢失和痴呆表现,其中Aβ聚集是中心环节,也是AD发病的关键性因素和关键性治疗靶点,干扰Aβ的产生和阻止其聚集是防治AD的有效途径,开发抑制Aβ纤丝形成和聚集的新药对预防和对症治疗AD具有良好的学术与临床应用价值。本课题组利用Aβ25-35致PC12细胞损伤模型,采用MTT法检测了各实验组细胞的活力,从而筛选中国延龄草属或重楼属药材的根及根茎或果实或全草中甾体皂苷类、倍半萜类、苯丙素苷类和酚酸类中各单体化合物治疗AD的有效成分,为中国延龄草属或重楼属药材治疗AD的新药研究奠定理论基础。
1.1药物制备
(1)1 mg 的Aβ25-35加超纯水4.72 ml配成200 μmol/L,37℃孵育24 h后,置于2-8℃冰箱中贮存,备用。
(2)中国延龄草属或重楼属药材的根及根茎或果实或全草中偏诺皂苷类化合物1-7:偏诺皂苷元-3-O-α-L-吡喃鼠李糖基(1→2)-O-β-D-吡喃葡萄糖苷(Y1)、偏诺皂苷元-3-O-α-L-吡喃鼠李糖基(1→4)-[-O-α-L-吡喃鼠李糖基(1→2)]-O-β-D-吡喃葡萄糖苷(Y2)、偏诺皂苷元-3-O-α-L-吡喃鼠李糖基(1→4)-O-α-L-吡喃鼠李糖基(1→4)-[O-α-L-吡喃鼠李糖基(1→2)]-O-β-D-吡喃葡萄糖苷(Y3)、偏诺皂苷元-3β-O-β-D-吡喃葡萄糖基(1→6)-[O-α-L-吡喃鼠李糖基(1→2)]-O-β-D-吡喃葡萄糖苷(Y4)、27-羟基-偏诺皂苷元-3β-O-β-D-吡喃葡萄糖基(1→6)-[O-α-L-吡喃鼠李糖基(1→2)]-O-β-D-吡喃葡萄糖苷(Y5)、3-乙酰基-偏诺皂苷元(Y6)、dioscoreanoside I(Y7)。将上述各单体化合物用蒸馏水配成5 mmol/L的贮备液,实验时用无血清的DMEM培养液依次稀释成50 nmol/L、100 nmol/L、200 nmol/L、500 nmol/L、1 μmol/L、10 μmol/L、250 nmol/L的工作液。
(3)中国延龄草属或重楼属药材的根及根茎或果实或全草中延龄草皂苷类化合物8-21:trillenoside A(Y8)、deoxytrillenoside A(Y9)、24-acetyl-deoxytrillenoside A(Y10)、24-epiacetyl-deoxytrillenoside A(Y11)、24-methoxy-trillenoside A(Y12)、trillenoside B(Y13)、24-acetyl-trillenoside B(Y14)、24-acetyl-deoxytrillenoside B(Y15)、trillenoside C(Y16)、Epitrillenogenin-24-O-acetate-1-O-[2,3,4-tri-O-acetyl-
α-L-rhamnopyranosyl-(1→2)-O-α-L-arabinopyranoside] (Y17)、Epitrillenogenin-1-
O-[2,3,4-tri-O-acetyl-α-L-rhamnopyranosyl-(1→2)-O-α-L-arabinopyranoside] (Y18)、Epitrillenogenin-24-O-acetate-1-O-[2,4-di-O-acetyl-α-L-rhamnopyranosyl-(1→2)-O-α-L-arabinopyranoside] (Y19)、Epitrillenogenin-1-O-[2,4-di-O-acetyl-α-L-rhamno-
pyranosyl-(1→2)-O-α-L-arabinopyranoside] (Y20)、Epitrillenogenin-1-O-[4-O-acetyl-
α-L-rhamnopyranosyl-(1→2)-O-α-L-arabinopyran-oside] (Y21)。将上述各单体化合物用蒸馏水配成5 mmol/L的贮备液,实验时用无血清的DMEM培养液依次稀释成250 nmol/L、500 nmol/L、1 μmol/L、5 μmol/L、10 μmol/L、100 μmol/L的工作液。
(4)中国延龄草属或重楼属药材的根及根茎或果实或全草中薯蓣皂苷类化合物22-27:薯蓣皂苷元-3-O-α-L-吡喃鼠李糖基(1→2)-O-β-D-吡喃葡萄糖苷(Y22)、薯蓣皂苷元-3-O-α-L-吡喃鼠李糖基(1→4)-[O-α-L-吡喃鼠李糖基(1→2)]-O-β-D-吡喃葡萄糖苷(Y23)、薯蓣皂苷元-3-O-α-L-吡喃鼠李糖基(1→4)-O-α-L-吡喃鼠李糖基(1→4)-[O-α-L-吡喃鼠李糖基(1→2)]-O-β-D-吡喃葡萄糖苷(Y24)、(23s,24s,25s)-螺甾-5-烯-1β, 3β, 21, 23, 24-五羟基-1β-O-β-D-呋喃芹糖基-(1→3)-O-α-L-吡喃鼠李糖基-(1→2)-[O-β-D-吡喃木糖基(1→3)]-O-α-L-吡喃阿拉伯糖苷(Y25)、(23s,24s,25s)-螺甾-5-烯-1β, 3β, 23, 24-四羟基-1β-O-β-D-呋喃芹糖基-(1→3)-O-α-L-吡喃鼠李糖基-(1→2)-[O-β-D-吡喃木糖基(1→3)]-O-α-L-吡喃阿拉伯糖苷(Y26)、(23s,24s,25s)-螺甾-5-烯-1β, 3β, 21, 23, 24-五羟基-1β-O-α-L-吡喃鼠李糖基-(1→2)-[O-β-D-吡喃木糖基(1→3)]-O-α-L-吡喃阿拉伯糖苷(Y27)。将上述各单体化合物用蒸馏水配成5 mmol/L的贮备液,实验时用无血清的DMEM培养液依次稀释成4 μmol/L、20 μmol/L、40 μmol/L、100 μmol/L、200 μmol/L的工作液。
(5)中国延龄草属或重楼属药材的根及根茎或果实或全草中甾醇皂苷类化合物28-33:β-脱皮激素(Y28)、pinnatasterone(Y29)、∆5,13-20βF,22αF,25αF螺甾烯-3β,21α-二醇(Y30)、polypodineB(Y31)、intergristerone B(Y32)、sileneoside G(Y33)。将上述各单体化合物用蒸馏水配成5 mmol/L的贮备液,用无血清的DMEM培养液稀释成4 μmol/L、40 μmol/L、200 μmol/L的工作液。
(6)中国延龄草属或重楼属药材的根及根茎或果实或全草中呋甾烷皂苷类化合物34-43:26-O-β-D-吡喃葡萄糖基-(25s)-5α-呋甾烷-△20(22)-烯-3β,26-二醇-3-O-α-L-吡喃鼠李糖基(1→4)-[O-α-L-吡喃鼠李糖基(1→2)]-O-β-D-吡喃葡萄糖苷(Y34)、26-O-β-D-吡喃葡萄糖基-(25s)-5α-呋甾烷-△20(22)-烯-3β, 26-二醇-3-O-α-L-吡喃鼠李糖基(1→4)-O-β-D-吡喃葡萄糖苷(Y35)、26-O-β-D-吡喃葡萄糖基-(25s)-5α-呋甾烷-△5(6),20(22)-二烯-3β, 26-二醇-3-O-α-L-吡喃鼠李糖基(1→4)-O-β-D-吡喃葡萄糖苷(Y36)、26-O-β-D-吡喃葡萄糖基-(25R)-Δ5(6)、17(20)-二烯-16,22-二酮-呋甾烷-3β,26-二醇-3-O-α-L-吡喃鼠李糖基(1→4)-O-α-L-吡喃鼠李糖基(1→4)[O-α-L-吡喃鼠李糖基(1→2)-]-O-β-D-吡喃葡萄糖苷(Y37)、26-O-β-D-吡喃葡萄糖基-(25R)-Δ5(6)、17(20)-二烯-16-羟基-22-酮-呋甾烷-3β,26-二醇-3-O-α-L-吡喃鼠李糖基(1→2) [O-α-L-吡喃鼠李糖基(1→4)-]-O-β-D-吡喃葡萄糖苷(Y38)、26-O-β-D-吡喃葡萄糖基-(25R)-Δ5(6)、17(20)-二烯-16,22-二酮-呋甾烷-3β,26-二醇-3-O-α-L-吡喃鼠李糖基(1→2)[O-α-L-吡喃鼠李糖基(1→4)-]-O-β-D-吡喃葡萄糖苷(Y39)、26-O-β-D-吡喃葡萄糖基-(25R)-Δ5(6)、17(20) -二烯-16,22-二酮-呋甾烷-3β,26-二醇-3-O-α-L-吡喃鼠李糖基(1→2)-O-β-D-吡喃葡萄糖苷(Y40)、3β-O-α-L-rhamnopyranosyl-(1→4)-
[O-α-L-rhamnopyranosyl-(1→2)]-O-β-D-glucopyranosylhomo-aro-cholest-5-ene-26-O-β-D-glucopyranoside(aethiosidecA)(Y41)、3β-O-α-L-rhamnopyranosyl-(1→4)-O-α-L-
rhamnopyranosyl-(1→4)-[O-α-L-rhamnopyranosyl-(1→2)]-O-β-D-glucopyranosylhomo-aro-cholest-5-ene-26-O-β-D-glucopyranoside(parispseudosideA)(Y42)、3β-O-α-L-
rhamnopyranosyl-(1→2)-O-β-D-glucopyranosylhomo-16′-hydroxyl-aro-cholest-5-ene-26-O-β-D-glucopyranoside(16′-hydroxylparispseudosideB)(Y43)。将上述各单体化合物用蒸馏水配成5 mmol/L的贮备液,用无血清的DMEM培养液稀释成4 μmol/L、8 μmol/L、40 μmol/L、200 μmol/L的工作液。
(7)中国延龄草属3种药材的根及根茎或果实或全草中新倍半萜类化合物44-49:延龄草倍半萜苷C或7,11-二甲基-3-亚甲基-1,6-十二碳二烯-10,11-二羟基-10-O-β-D-吡喃葡萄糖苷(Y44)、延龄草倍半萜苷A或7,11-二甲基-3-亚甲基-1,6-十二碳二烯-10,11-二羟基-10-O-β-D-吡喃葡萄糖基-(1→4)-O-β-D-吡喃葡萄糖苷(Y45)、7,11-二甲基-3-亚甲基-10,11-二羟基-1,6-十二碳二烯二醇(Y46)、2,6,10-三甲基-2,10-二羟基-6-十二碳烯二醇(Y47)、3,7,11-三甲基-3,9,11-三羟基-1,6-十二碳二烯三醇(Y48)、7,11-二甲基-3-羟甲基-1,6-十二碳二烯-10,11,15-三醇-10-O-间苯酚(Y49)。将上述各单体化合物分别用蒸馏水配成5 mmol/L的贮备液,用无血清的DMEM培养液稀释成8 μmol/L、40 μmol/L、200 μmol/L的工作液。
(8)中国延龄草属3种药材的根及根茎或果实或全草中酚酸类新化合物50-52:trillium acid A(Y50)、trillium acid B(51)、trillium acid C(52)。将上述各单体化合物分别用蒸馏水配成5 mmol/L的贮备液,用无血清的DMEM培养液稀释成8 μmol/L、40 μmol/L、200 μmol/L的工作液。
(9)中国延龄草属3种药材的根及根茎或果实或全草中苯丙素类化合物53-56: methyl ferulorate(53)、regaloside A(54)、3-O-feruloylsucrose(55)、heronioside A(56)。将上述各单体化合物分别用蒸馏水配成5 mmol/L的贮备液,用无血清的DMEM培养液稀释成4 μmol/L、40 μmol/L、200 μmol/L的工作液。
1.2 不同浓度的Aβ25-35对PC12细胞活力的测定,确定造模条件
实验用无血清DMEM低糖培养基配制的终浓度为10 μM、20 μM、40 μM的Aβ25-35。37°C培养24 h或48 h后,每孔加入终浓度为0.5 mg/ml MTT(10 μl),继续培养4 h后吸去DMEM培养基,加入DMSO100 μl,在酶标仪570 nm处测定吸光度(A570nm)值。
1.3不同浓度的中国延龄草属或重楼属药材的根及根茎或果实或全草中甾体皂苷类、倍半萜类、苯丙素苷类和酚酸类中各类单体化合物对Aβ25-35所致PC12细胞损伤的保护作用
空白对照组:每孔加入100 μl无血清DMEM低糖培养基;Aβ25-35模型组:每孔加入用无血清DMEM低糖培养基配制的终浓度为20 μM的Aβ25-35;各给药组每孔加入终浓度为20 μM的Aβ25-35及各药物溶液37°C培养24 h后,加入终浓度为0.5 mg/ml MTT(10 μl),继续培养4 h后吸去DMEM培养基,每孔加入DMSO100 μl,振摇混匀10 min,待孔内颗粒完全溶解后,在酶标仪570 nm处测定吸光度(A570nm)值。
1.4 数据统计
所有数据以± s表示,组间比较用t检验。细胞存活率的计算公式为:实验组 A 值/空白对照组 A 值×100%。
1.5实验结果
(1)确定不同浓度的Aβ25-35对PC12细胞造模浓度和作用时间,取10 μM、20 μM、40 μM不同浓度的Aβ25-35与PC12细胞共孵24 h后或48 h后,细胞活力显著下降,表现为A570nm值明显下降;且随着Aβ25-35剂量和时间的增加PC12细胞活力下降更加明显。结果见表1和图1。
表1 24h和48h后不同浓度的Aβ25-35对PC12细胞活力的影响 (n=10)
注:**与空白对照组比较p < 0.01
结果显示:空白对照组PC12细胞个体饱满、长势好、细胞的折光性正常。模型组PC12细胞可见细胞膜外围的纤毛消失,细胞浆内出现空泡,细胞聚集成团,大多数细胞变形,细胞的折光性变差。20 µM的Aβ25-35处理PC12细胞24 h后,细胞形态发生了很大的改变,能较好的复制了AD的病理变化,是研究AD理想的模型,故最终确定造模浓度和作用时间为20 µM的Aβ25-35处理PC12细胞24 h。
(3)不同浓度的中国延龄草属或重楼属药材的根及根茎或果实或全草中甾体皂苷类、倍半萜类、苯丙素苷类和酚酸类中各类单体化合物对Aβ25-35所致PC12细胞损伤的保护作用:不同浓度的偏诺皂苷类、延龄草皂苷类、薯蓣皂苷类、甾醇类、呋甾烷皂苷类、倍半萜类、苯丙素类、酚酸类单体化合物对Aβ25-35所致PC12细胞损伤均有不同程度的保护作用,可使细胞活力增强,表现为A570nm值明显上升。结果见表2 ~9和图2~11。
表2 不同浓度的偏诺皂苷类单体化合物Y1-Y7对受损的PC12细胞的保护作用(n=5)
注:*与Aβ模型组比较p < 0.05; **与Aβ模型组比较p < 0.01
表3 不同浓度的延龄草皂苷类单体化合物Y8-Y21对受损的PC12细胞的保护作用(n=5)
注:*与Aβ模型组比较p < 0.05; **与Aβ模型组比较p < 0.01
表4 不同浓度的薯蓣皂苷类单体化合物Y22-Y27对受损的PC12细胞的保护作用(n=5)
注:*与Aβ模型组比较p < 0.05; **与Aβ模型组比较p < 0.01
表5 不同浓度的甾醇类单体化合物Y28-Y33对受损的PC12细胞的保护作用(n=5)
注:*与Aβ模型组比较p < 0.05; **与Aβ模型组比较p < 0.01
表6 不同浓度的呋甾烷皂苷类单体化合物Y34-Y40对受损的PC12细胞的保护作用(n=5)
注:*与Aβ模型组比较p < 0.05; **与Aβ模型组比较p < 0.01
表7 不同浓度的倍半萜类单体化合物Y44-Y49对受损的PC12细胞的保护作用(n=5)
注:*与Aβ模型组比较p < 0.05; **与Aβ模型组比较p < 0.01
表8 不同浓度的呋甾烷皂苷化合物Y41-Y43或酚酸类化合物Y50-Y52对受损的PC12细胞的保护作用(n=5)
注:*与Aβ模型组比较p < 0.05; **与Aβ模型组比较p < 0.01
表9 不同浓度的苯丙素类化合物Y53-Y56对受损的PC12细胞的保护作用(n=5)
注:*与Aβ模型组比较p < 0.05; **与Aβ模型组比较p < 0.01。
- 还没有人留言评论。精彩留言会获得点赞!