用于治疗癌症的苯乙炔基取代苯和杂环及其应用的制作方法
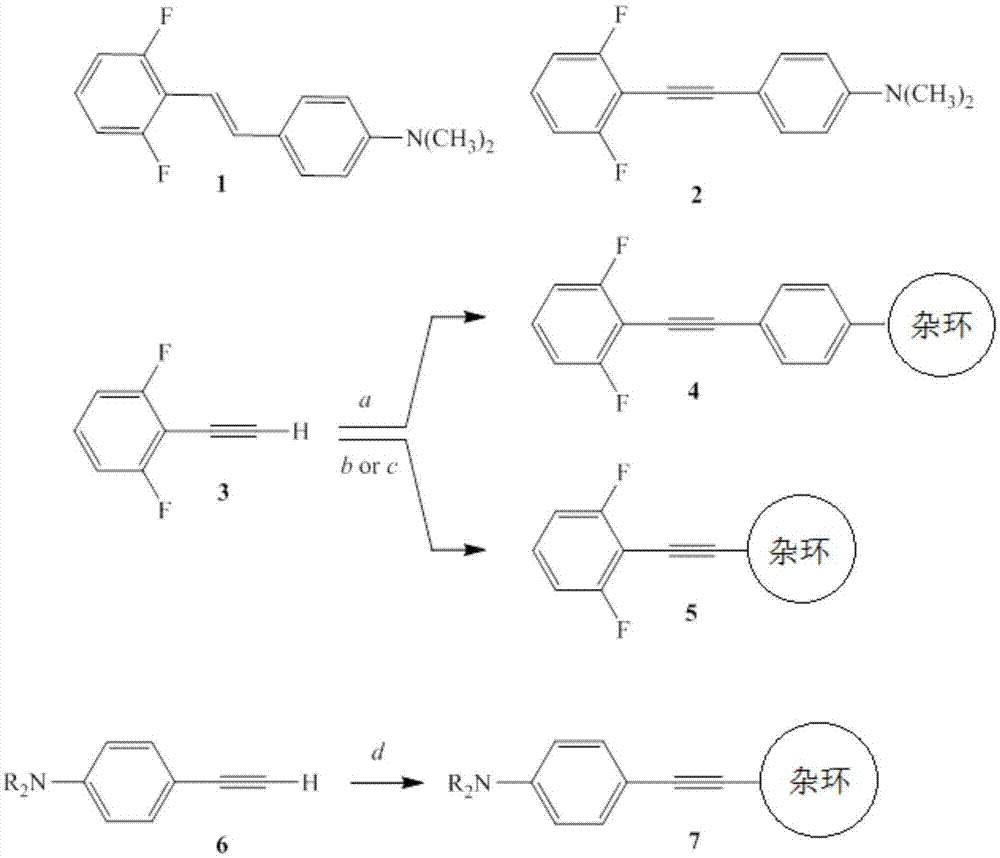
本发明涉及治疗癌症和/或治疗或预防癌症转移的化合物。具体而言,本主题技术涉及带有连接到苯上的含氮杂环的卤代苯乙炔基取代的苯,它们的盐和它们的药物组合物以及其在制备治疗和/或预防癌症药物中的应用。
背景技术:
报道了一系列抑制wnt靶基因如c-myc表达,和抑制在体外和体内抑制结肠癌细胞生长的氟化n,n-二烷基氨基二苯乙烯类似物。参见,例如jmedchem2011,54:1288-1297;acschembiol2013,8(4):796-803;jmedchem2014,57:6083-91;u.s.8,664,276。此外,对于某些医疗用途而言某些二芳基乙炔是已知的。参见,例如wo2012149049;wo2012149048;wo2010092043;wo2009038759;wo2008073350;和wo2001029011。进而,tsai的u.s.8,716,355公开了在治疗癌症中的羟基化二苯乙炔和相关化合物,hadfield等公开了作为抗肿瘤剂的二芳基炔烃的制备和评估。hadfield等,synthcommun1998,28(8):1421-1431。用于治疗癌症的卤代二芳基乙炔和方法也已有报道。参见公开号为2015-0272908的美国专利申请;sviripa等,“halogenateddiarylacetylenesrepressc-mycexpressionincancercells”,bioorgmedchemlett2014,24:3638-40。然而,对能用于治疗癌症和其他疾病的另外的化合物仍存在不断的需要。技术实现要素:本发明的优点包括具有抗肿瘤活性的卤代苯乙炔基取代的苯或杂环化合物及其药物组合物,以及通过施用一种或多种卤代苯乙炔基取代的杂环或其药物组合物在患者中抑制癌症细胞生长和/或治疗癌症的方法。本公开的一个方面涉及卤代苯乙炔基-取代的苯或杂环,其可用于杀死过度增殖细胞例如癌细胞以治疗人恶性和良性癌症,所述癌症包括但不限于结肠直肠癌(crc),白血病,乳腺癌,卵巢癌,肺癌,前列腺癌和肝癌。在本发明的该方面中,提供了某些对癌细胞具有抗肿瘤活性的卤代苯乙炔基取代的杂环。本发明的卤代苯乙炔基取代的苯和杂环包括根据式(i)、(ii)和(iii)的化合物或其药学上可接受的盐:其中x1至x5各自独立地表示h、低级烷基、烷氧基例如低级烷氧基、卤素和/或nr1r2,其中r1和r2各自独立地表示h或低级烷基,n为1或0,并且当n为1时,y1至y4各自独立地表示h、低级烷基或烷氧基、或nr1r2。杂环表示可以未被取代或被一个或多个低级烷基、或烷氧基例如低级烷氧基、和/或一个或多个卤素取代的杂环;其中杂环选自氧代哌啶、吗啉、哌嗪、n-甲基哌嗪、n-吡咯和2,5-二甲基-n-吡咯、喹啉、异喹啉、吲哚、吲唑、萘啶和吡啶基;并且y5表示一个或多个nr1r2,和/或一个或多个可以相同或不同的卤素。式(i)、(ii)或(iii)的卤代苯乙炔基取代的苯或杂环或其药学上可接受的盐可以与药学上可接受的载体一起包含在药物组合物中。本发明的另一方面涉及上述卤代苯乙炔基取代的苯或杂环在制备治疗和/或预防癌症药物中的应用,例如在制备抑制癌细胞生长和/或抑制哺乳动物(例如人)的肿瘤生长药物或治疗与过度增殖细胞相关的疾病药物中的应用。在本发明的该方面的一个实施方式中,将有效量的一种或多种卤代苯乙炔基取代的苯或杂环、其药用盐和/或药物组合物施用于需要治疗癌症的患者,该有效量足以在患者中治疗/抑制癌细胞生长。在本发明的该方面的一个实施方式中,将治疗有效量的一种或多种卤代苯乙炔基取代的苯和杂环、其药用盐和/或药物组合物施用于患有结直肠癌的患者。在另一个实施方式中,将治疗有效量的一种或多种带有连接到苯上的含氮杂环的卤代苯乙炔基取代的苯和一种或多种卤代含氮苯乙炔基取代的杂环、其药用盐和/或其药物组合物施用于患有肝癌或前列腺癌的患者。从以下详细描述,本发明的其他优点对本领域技术人员来说将变得显而易见,其中通过仅说明完成本发明所考虑的最佳模式仅示出和描述了本发明的优选实施方式。如将认识到的,本发明能够具有其他和不同的实施方式,并且其若干细节能够在各种明显的方面进行修改,所有这些都不脱离本发明。因此,附图和说明书本质上被认为是说明性的,而不是限制性的。附图说明图1显示2,6-二氟-4’-(n,n-二甲氨基)二苯乙烯(图中标“1”)和2,6-二氟苯基-4’-(n,n-二甲氨基)苯基乙炔(图中标“2”)的结构式。图1还说明了杂环取代的苯乙炔基苯(图中标“4”)和苯乙炔基取代的杂环(图中标“5”)和(图中标“7”)的合成过程。图中a表示:杂环取代的苯基碘,ipr2etn,pd(pph3)4,cui,h2o,80℃;b表示:杂芳基碘,ipr2etn,pd(pph3)4,cui,h2o,80℃;c表示:氯取代的杂芳基碘,ipr2etn,pd(pph3)4,cui,h2o,75℃,然后是单烷基胺或二烷基胺,thf,130℃,2-3h,压力管;d表示:氟化杂芳基碘,ipr2etn,pd(pph3)4,cui,h2o,80℃。图2a是显示4-((2,6-二氟苯基)乙炔基)-n,n-二甲基苯胺(图中以“2”表示),4-((2,6-二氟苯基)乙炔基)-n,n-二甲基异喹啉-1-胺(图中以“5nn”表示)和4-((2,6-二氟苯基)乙炔基)-n-甲基异喹啉-1-胺(图中以“5oo”表示)对300nm浓度的ls174t细胞中cyclind1和p21wif1/cip1表达的影响的蛋白质印迹。图2b是在ls174t细胞增殖试验中化合物“2”、“5nn”和“5oo”的剂量反应图。对“2”、“5nn”和“5oo”而言ic50分别为25.1±1.3,11.8±1.5和4.2±0.2nm。具体实施方式本发明涉及卤代苯乙炔基取代的苯和杂环化合物、它们的盐和它们的药物组合物,以及一种或多种卤代苯乙炔基取代的苯和杂环、其药用盐或其药物组合物在制备抑制癌细胞生长和/或治疗癌症药物中的应用。已发现在一个芳环中具有至少一个、优选两个卤素取代基,以及在芳基或杂芳基环对位具有胺(例如n-甲氨基或n,n-二甲氨基)的卤代苯乙炔基取代的苯和杂环,通过抑制c-myc和诱导细胞周期蛋白-依赖性激酶抑制剂-1(即p21wif1/cip1)来抑制ls174t结肠癌细胞的增殖。这样的化合物和组合物可用作抗肿瘤剂。本发明的卤代苯乙炔基取代的苯和杂环在卤代苯乙炔基取代的苯和杂环的苯或杂环上包含至少一个胺基,例如伯、仲或叔胺。这样的化合物可用作抗肿瘤剂并可以由下式或其药学上可接受的盐表示:x1至x5的取代基各自独立地表示h、低级烷基或烷氧基例如低级烷氧基、卤素和/或nr1r2,其中r1和r2各自独立地表示h或低级烷基。在本发明的一个实施方式中,x1至x5中的至少一个是卤素并且其余的x1至x5表示h、低级烷基或低级烷氧基。在本发明的另一个实施方式中,x1至x5至少一个是nr1r2。卤素基团是指f、cl、br或i。优选取代基x1至x5包括至少两个卤素基团,例如f和/或cl。在本发明的一个实施方式中,x1至x5是卤素基团,例如两个f基团,两个cl基团或一个f和一个cl基团。变量n可以为1或0。当n为1时,式(i)的卤代苯乙炔基取代的苯可以由以下式(ii)表示。对于式(ii),y1至y4各自独立地表示h、低级烷基或nr1r2,其中r1和r2各自独立地表示h或低级烷基。当n是0时,式(i)可以由以下式(iii)的卤代苯乙炔基取代的杂环表示。式(ii)和(iii)的x1至x5的取代基如式(i)所述,包括其所有组合。对于式(i),(ii)和(iii)中的每一个,杂环表示有杂原子的环。例如,杂环表示可以选自氧代哌啶,吗啉,哌嗪,n-甲基哌嗪,n-吡咯和2,5-二甲基-n-吡咯,喹啉,异喹啉,吲哚,吲唑,萘啶和吡啶基中的杂环。对于式(i),(ii)和(iii)中的每一个,杂环可以未被取代或被一个或多个低级烷基或烷氧基(例如低级烷氧基)和/或一个或多个卤素取代。在式(i),(ii)和(iii)每一个中变量y5代表一个或多个nr1r2,和/或一个或多个可以相同或不同的卤素,即每个nr1r2基团可以具有相同或不同的r1和r2,其中r1和r2各自独立地表示h或低级烷基,和/或具有一个或多个相同或不同卤素的杂环。在本发明的一个实施方式中,式(i),(ii)和(iii)每一个中的y5表示一个或多个nr1r2。在本发明的另一个实施方式中,式(i),(ii)和(iii)每一个中的y5表示一个或多个卤素。例如,对于式(iii),x1至x5中的至少一个为nr1r2并且杂环表示具有至少一个卤素且优选两个卤素基团(例如氟和/或氯基团)的吡啶基环。术语“低级烷基”包括饱和脂肪族基团,包括具有1至约10个碳(c1-c10)的直链烷基、支链烷基、环烷(脂环)基、烷基取代的环烷基和环烷基取代的烷基,例如在其主链结构中具有1至约6个碳原子(c1-c6)。在本发明的一个方面中,低级烷基具体包括甲基、乙基、丙基、异丙基、正丁基等。本发明的卤代苯乙炔基取代的苯和杂环的实施方式包括其中x1至x5中的至少两个是卤素基团,例如其中x1至x5中的至少两个是(i)氟和氯,(ii)两个氟,(iii)两个氯,并且其中r1和r2都是低级烷基(例如甲基、乙基或丁基)。优选地,x1和/或x5是卤素基团,例如苯乙炔基取代的苯和杂环在相对于炔键的邻位具有一个或两个卤素取代基,并且x1至x4是h或低级烷基或烷氧基。在一些实施方式中,卤代苯乙炔基取代的苯和杂环包括其中y3是nr1r2且y1、y2、y4和y5独立地表示h、低级烷基或nr1r2的化合物或其药学上可接受的盐。在其他实施方式中,y3是nr1r2,且y1、y2、y4和y5独立地表示h或低级烷基,例如y1、y2、y4和y5表示h。在又一些实施方式中,r1或r2中的至少一个是低级烷基。本发明的药物组合物包括药学上可接受的载体和一种或多种根据式(i)、(ii)或(iii)的化合物或式(i)、(ii)或(iii)化合物药学上可接受的盐。在本发明的一个方面中,式(i)、(ii)或(iii)的化合物,其药学上可接受的盐或其药学上可接受的组合物用于治疗癌症。治疗时给需要这种治疗的患者施用有效量的一种或多种卤代苯乙炔基取代的杂环、其药用盐或其药物组合物。该方法的实施方式包括其中所治疗的癌症选自结直肠癌、白血病、乳腺癌、卵巢癌、肺癌、前列腺癌和肝癌。在关注结直肠癌机制的研究过程中,我们使用化学生物学来探测调节基因表达的细胞信号传导事件。这一过程中的关键步骤选择性靶向提供了开发治疗剂以治疗这些癌症的潜力。例如,我们报道了2’,6’-二卤代苯乙烯基苯胺(1)(图1)抑制wnt靶基因如c-myc的表达,以及在体外和体内抑制结肠癌ls174t细胞生长。参见例如jmedchem2011,54:1288-1297;acschembiol2013,8(4):796-803;jmedchem2014,57,6083-91。我们确定这种试剂专门靶向低聚酶的催化子单元,即甲硫氨酸s-腺苷转移酶-2(mat2),其将s-腺苷甲硫氨酸(sam)提供给调节性甲基转移酶并且在选择的一组癌症中上调。我们进一步确定这些2’,6’-二卤代苯乙烯基苯胺(1)的杂环变体具有最小的总毒性,而没有所期望的人类ether-à-go-go-相关蛋白(herg)活化,合理的生物利用度和药代动力学。根据需要,这些基于1,2-二苯乙烯的试剂不影响甲硫氨酸s-腺苷转移酶-1(mat1),其作为其他细胞需要所必需的sam的主要来源。与其他mat2a抑制剂的最新报道不同,我们还在使用结直肠癌细胞的异种移植模型中证实了体内效力。关于可能潜在地使将来的药代动力学和药效学研究复杂化的二苯乙烯1中的热或光化学e/z异构化的担忧导致合成和评价类似的二芳基乙炔2(图1)。根据二苯乙烯1的sar研究,我们发现在一个苯环上带有n,n-二甲基氨基且在另一个苯环上带有一个或优选两个邻位取代的氟或氯基团的二芳基乙炔2,在结直肠癌ls174t细胞增殖试验中保持了体外的效力。二芳基乙炔2还具有对心脏钾herg通道影响最小的期望性质。分子停靠(docking)研究表明,二芳基乙炔和杂环对应物,如它们的二苯乙烯对应物,靶向mat2的催化子单元(mat2a)。为了获得相对于少量溶于水的4-((2,6-二氟苯基)乙炔基)-n,n-二甲基苯胺(2)改进的效力、溶解度和生物利用度,我们进行了另外的结构-活性研究。最初的工作集中在2中的n,n-二甲基氨基的替换,用杂环代替在杂环取代的二苯基乙炔4中的n,n-二甲基氨基(图1)。2,6-二氟苯基乙炔(3)与4-碘苯基取代的杂环的sonogashira偶联提供了氧代哌啶基、吗啉基、n-甲基哌嗪基,n-吡咯基和2,5-二甲基-n-吡咯基类似物4a-4e(表1)。在细胞增殖测定中n-吡咯基类似物4d在1μm浓度下显示在与4-((2,6-二氟苯基)乙炔基)-n,n-二甲基苯胺(2)相当的活性,但在100nm下显示显著降低的活性。下表1提供了如图1中化合物4(例如式(ii))所示的本发明的某些杂环取代的苯乙炔基苯在各种浓度下对结肠癌ls174t细胞的增殖的抑制(%)。表1还提供了2,6-二氟苯基-4’-(n,n-二甲基氨基)苯基乙炔(2)这样的数据用于比较。表1a,数据来自sviripa等,“halogenateddiarylacetylenesrepressc-mycexpressionincancercells”,bioorgmedchemlett2014,24:3638-40。由于二苯乙烯系列中的杂环变体显示出改善的溶解度和最小的herg活化,所述变体其中1中的n,n-二甲基苯胺环(图1)被n,n-二甲基氨基吡啶或n,n-二甲基氨基嘧啶环取代,接下来我们着重于用如在图1中的化合物5(例如式(iii))所示的苯乙炔基取代的杂环中的杂环代替二芳基乙炔系列中的n,n-二甲基苯胺环。2,6-二氟苯基乙炔与碘取代的杂环的sonogashira偶联提供了大部分这些类似物(表2)。在一些情况下,例如5nn-5oo的合成,优选将2,6-二氟苯基乙炔与1-氯-4-碘异喹啉偶联,随后利用亲核芳族取代反应用仲胺取代氯基。另一种方法涉及2,6-二氟苯基乙炔与1-氨基-4-碘异喹啉的偶联,得到4-((2,6-二氟苯基)乙炔基)异喹啉-1-胺,随后通过中间体1,2,4-三唑使用1,2-双[(二甲基氨基)亚甲基]肼进行胺基的n,n-二甲基化,但后一过程的总收率低于亲核芳族取代反应中的收率。如表2中所概括的,使用这些方法,我们合成了2,6-二氟苯基乙炔基取代的杂环,包括吡啶5a-5c,吡嗪5d,吲哚5e-5f,1h-吲唑5g-5k,1h-吡咯并[2,3-b]吡啶5e,喹啉5m-5ff,异喹啉5gg-5oo,1,6-萘啶5qq和喹唑啉5rr。下表2提供了本发明的由图1中化合物5(例如式(iii))所示的2,6-二氟苯基乙炔基取代的杂环在各种浓度下抑制结肠癌ls174t细胞的细胞增殖(%)。表2a,2,6-二氟苯基乙炔基的连接位置;b,来自sviripa等,“halogenateddiarylacetylenesrepressc-mycexpressionincancercells”,bioorgmedchemlett2014,24:3638-40的数据;c,如盐酸盐测试。在10μm浓度下,表2中的大部分2,6-二氟苯基乙炔基取代的杂环5显示出对ls174t结肠癌细胞增殖试验的有效作用,并且在1μm浓度下,几种吡啶5b和5c、一种吲哚5f、两种1h-吲唑5i和5k、两种喹啉5x和5ff以及四种异喹啉5mm-5oo和5qq显示大于75%的抑制。在2,6-二氟苯乙炔基取代的杂环5中突出的两个侯选物为4-((2,6-二氟苯基)乙炔基)-n,n-二甲基异喹啉-1-胺(5nn)和4-((2,6-二氟苯基)乙炔基)-n-甲基异喹啉-1-胺(5oo)。另外,几种二氟化苯乙炔基取代的杂环如5pp和5qq提供了完全水溶性的盐酸盐。总之,我们鉴定了具有超过母体化合物4-((2,6-二氟苯基)乙炔基)-n,n-二甲基苯胺(2)的效力和/或水溶性的杂环变体5。使用钼酸盐比色法测定磷酸盐的mat2a抑制试验的变异性使得测定细胞周期抑制成为评估二芳基乙炔效力的优选分析工具。我们测试了这些杂环取代的二苯基乙炔4(表1)和氟化苯乙炔基取代的杂环5(表2)对ls174t结肠癌细胞增殖的影响。如蛋白质印迹(图2a)所示,最活跃的化合物2、5nn和5oo在300nm浓度下抑制细胞周期蛋白d1的表达,并且如同对细胞周期抑制剂所预期的那样,同时诱导p21wif1/cip1。与其中n-甲基苯胺基和n,n-二甲基苯胺基类似物2在抑制ls174t细胞增殖中显示相当活性(即,2,ic5025.1nm)的二芳基乙炔系列不同,苯乙炔基取代的杂环中的n-甲基化模式表明n-甲基氨基异喹啉类似物5oo(ic504.2nm)比n,n-二甲基氨基异喹啉类似物5nn(ic5011.8nm)更有效(图2b)。总之,苯乙炔基取代的杂环化合物5通过改变c-myc的表达并由此诱导p21wif1/cip1来抑制ls174t结肠癌细胞的增殖。这些结果与使用卤代二苯乙烯和二芳基乙炔的先前发现一致,并且鉴于在5中相对于二苯乙烯不存在异构化,提高的效力和改善的物理性质(例如水溶性)表明苯乙炔基取代的杂环通过类似机制抑制结肠癌增殖。对这些苯乙炔基取代的杂环的进一步研究是正在进行的研究的主题。我们还关注由图1中的化合物7代表的苯乙炔基取代的杂环,例如式(iii)。4-氨基,4-n-甲基氨基或4-n,n-二甲基氨基乙炔与碘取代的氟代杂环的sonogashira偶联提供了这些类似物7(表3)。表3化合物杂环苯环300nm100nm30nm7a2,6-二氟-4-吡啶c6h512.2±7.124.7±3.75.3±7.97b2,6-二氟-4-吡啶c6h4-4-nh236.2±4.530.5±2.211.4±7.87c2,6-二氟-4-吡啶c6h4-4-nhch362.6±1.349.5±5.520.3±57d2,6-二氟-4-吡啶c6h4-4-n(ch3)260.6±5.220.4±0.516.7±7.7在300nm浓度下,表3中的几种2,6-二氟-4-吡啶基乙炔基取代的苯7显示出对ls174t结肠癌细胞增殖试验的有效作用。这种体外试验的效力并不是促使化合物向临床研究前进的唯一标准,另外的研究、实验和计算表明,这些化合物具有最小的毒性和改善的adme参数。使用体内异种移植研究,几种这些化合物7也显示出非常有希望的活性。实施例以下实施例旨在进一步说明本发明的某些优选实施方式,本质上不是限制性的。本领域技术人员将认识到或能够确定使用不超过常规实验的本文所述的特定物质和程序的许多等同物。材料化学品购自sigmaaldrich或fisherscientific或根据文献程序合成。除非另有说明,否则来自商业供应商的溶剂不经进一步纯化而使用。在varian(1h,400mhz;13c,100mz)和bruker(1h,700mhz;13c,176mz)仪器上测定核磁共振谱。在ltq-orbitrapvelos质谱仪(thermofisherscientific,waltham,ma,usa)上记录高分辨率电喷雾电离(esi)质谱。ft分辨率定为100,000(400m/z)。使用流速为5μl/min的注射泵通过直接输注引入样品。使用dhb(2,5-二羟基苯甲酸)基质,在brukerutraflexstreme飞行时间质谱仪(billerica,ma)上获得maldi质谱。通过atlanticmicrolabs,inc.,norcross,ga的燃烧分析确定了化合物的纯度。除非另有说明,将化合物在制备层merck硅胶f254上层析。用于2,6-二氟苯基乙炔与芳基碘化物或杂芳基碘化物的sonogashira偶联的一般程序。向在7ml水中的2.1mmol芳基碘化物或杂芳基碘化物、3mmol二异丙基乙基胺、0.02mmolpd(pph3)4和0.02mmolcui的混合物中加入2mmol2,6-二氟苯基乙炔。在75℃搅拌混合物1-2小时。将混合物冷却,通过过滤或使用二氯甲烷萃取收集产物。产物4或5如下所述通过重结晶和/或色谱纯化。1-(4-((2,6-二氟苯基)乙炔基)苯基)哌啶-2-酮(4a).收率75%;使用1:10甲醇-二氯甲烷的制备层色谱法(rf=0.52);mp148-150℃。1hnmr(400mhz,dmso-d6)δ7.58(d,j=8.5hz,2h),7.56-7.47(m,1h),7.40(d,j=8.5hz,2h),7.26(t,j=8hz,2h),3.64(t,j=5.6hz,2h),2.42(t,j=6.3hz,2h),1.94-1.77(m,4h)。13cnmr(101mhz,dmso-d6)δ169.01,162.01(dd,j=5.2,251.5hz,2c),144.53,131.78(2c),131.39(t,j=10hz),126.19(2c),118.35,111.94(dd,j=5.3,18.2hz,2c),100.77(t,j=19.8hz),98.63(t,j=3.1hz),75.77,50.29,32.70,22.91,20.79。hrms(esi)理论计算c19h16f2no[mh+]:312.1194。实际测定:312.1195。理论分析计算c19h15f2no:c,73.30;h,4.86;n,4.50。实际测定:c,73.04;h,4.85;n,4.52。4-(4-((2,6-二氟苯基)乙炔基)苯基)吗啉(4b).收率67%;使用1:5乙酸乙酯-己烷的柱色谱法并从己烷重结晶;mp144-145℃。1hnmr(400mhz,dmso-d6)δ7.53-7.44(m,1h),7.42(d,j=8.9hz,2h),7.22(t,j=7.9hz,2h),6.98(d,j=8.8hz,2h),3.73(t,j=4.8hz,4h),3.2(t,j=4.9hz,4h)。13cnmr(101mhz,dmso-d6)δ161.85(dd,j=5.3,250.6hz,2c),151.36,132.57(2c),130.51(t,j=10.1hz),114.35(2c),111.89(dd,j=5.4,250.6hz,2c),110.38,101.55(t,j=19.9hz),100.09(t,j=3hz),74.28,65.91(2c),47.21(2c)。hrms(esi)理论计算c18h16f2no[mh+]:300.1194。实际测定:300.1195。理论分析计算c18h15f2no:c,72.23;h,5.05;n,4.68。实际测定:c,71.98;h,5.09;n,4.72。1-(4-((2,6-二氟苯基)乙炔基)苯基)-4-甲基哌嗪(4c).收率60%,用1:10甲醇-二氯甲烷的制备层色谱法(rf=0.43);mp126-128℃。1hnmr(400mhz,dmso-d6)δ7.53-7.42(m,1h),7.39(d,j=8.8hz,2h),7.22(t,j=7.9hz,2h),6.96(d,j=8.9hz,2h),3.24(t,j=4.9hz,4h),2.43(t,j=5.1hz,4h),2.21(s,3h)。13cnmr(101mhz,dmso-d6)δ161.82(dd,j=5.4,250.6hz,2c),151.21,132.56(2c),130.43(t,j=10.1hz),114.49(2c),111.8(dd,j=5.3,18.2hz,2c),109.85,101.59(t,j=19.9hz),100.22(t,j=3hz),74.17,54.35(2c),46.87(2c),45.73。hrms(esi)理论计算c19h19f2n2[mh+]:313.1511。实际测定:313.1489。理论分析计算c19h18f2n2:c,73.06;h,5.81;n,8.97。实际测定:c,72.84;h,5.75;n,8.89。1-(4-((2,6-二氟苯基)乙炔基)苯基)-1h-吡咯(4d).收率79%;用1:5乙酸乙酯-己烷的制备层色谱法(rf=0.68);mp122-124℃。1hnmr(400mhz,dmso-d6)δ7.76-7.61(m,4h),7.60-7.49(m,1h),7.48(t,j=2.2hz,2h),7.32-7.17(m,2h),6.31(t,j=2.2hz,2h)。13cnmr(101mhz,dmso-d6)δ162.01(dd,j=5,251.5hz,两个c),140.3,132.98(2c),131.36(t,j=10.1hz),119.15(2c),118.92(2c),117.61,111.93(dd,j=5.4,18.3hz,2c),111.19(2c),100.91(t,j=19.7hz),98.45(t,j=3hz),76.09。hrms(esi)理论计算c18h12f2n[mh+]:280.0932。实际测定:280.0924。理论分析计算c18h11f2n:c,77.41;h,3.97;n,5.02。实际测定:c,77.20;h,4.21;n,4.89。1-(4-((2,6-二氟苯基)乙炔基)苯基)-2,5-二甲基-1h-吡咯(4e).收率84%;用1:10乙酸乙酯-己烷的柱色谱法;mp124-126℃。1hnmr(400mhz,dmso-d6)δ7.71(d,j=8.4hz,2h),7.62-7.51(m,1h),7.36(d,j=8.5hz,2h),7.30-7.25(m,2h),5.83(s,2h),2.00(s,6h)。13cnmr(101mhz,dmso-d6)δ162.07(dd,j=5.2,251.8hz,2c),139.2,132.43(2c),131.66(t,j=10.2hz),128.49(2c),127.55(2c),120.42,111.96(dd,j=5.3,19hz,2c),106.48(2c),100.69(t,j=19.7hz),98.05(t,j=3hz),76.71,12.86(2c)。hrms(esi)理论计算c20h16f2n[mh+]:308.1245。实际测定:308.1229。理论分析计算c20h15f2n:c,78.16;h,4.92;n,4.56。实际测定:c,77.91;h,5.03;n,4.42。6-((2,6-二氟苯基)乙炔基)-n,n-二甲基吡啶-3-胺(5a).收率68%;用1:2乙酸乙酯-己烷的制备层色谱法(rf=0.53);mp120-122℃。1hnmr(400mhz,dmso-d6)δ8.13(d,j=3hz,1h),7.55-7.46(m,1h),7.44(d,j=8.7hz,1h),7.29-7.2(m,2h),7.06(dd,j=3.1,8.8hz,1h),3(s,6h)。13cnmr(101mhz,dmso-d6)δ162.01(dd,j=5.3,251.2hz,2c),145.52,134.97,130.95(t,j=10.1hz),127.79,127.47,117.6,111.96(dd,j=5.3,18.2hz,2c),101.11(t,j=19.8hz),99.79(t,j=3.1hz),72.88,39.33(2c)。hrms(esi)理论计算c15h13f2n2[mh+]:259.1041。实际测定:259.1023。理论分析计算c15h12f2n2:c,69.76;h,4.68;n,10.85。实际测定:c,69.61;h,4.85;n,10.83。5-((2,6-二氟苯基)乙炔基)-n,n-二甲基吡啶-2-胺(5b).收率79%;用1:5乙酸乙酯-己烷的制备层色谱法(rf=0.41);mp110-112℃。1hnmr(700mhz,dmso-d6)δ8.29(d,j=2.1hz,1h),7.64(dd,j=2.4,8.9hz,1h),7.53-7.43(m,1h),7.22(t,j=7.9hz,2h),6.68(d,j=8.9hz,1h),3.08(s,6h)。13cnmr(176mhz,dmso-d6)δ161.74(dd,j=5.2,250.7hz,2c),158.12,150.93,139.51,130.56(t,j=10.1hz),111.81(dd,j=3.9,20.2hz,2c),105.54,104.57,101.48(t,j=19.8hz),98.02(t,j=3hz),76.11,37.52(2c)。hrms(esi)理论计算c15h13f2n2[mh+]:259.1041。实际测定:259.1023。理论分析计算c15h12f2n2:c,69.76;h,4.68;n,10.85。实际测定:c,69.49;h,4.81;n,10.76。5-((2,6-二氟苯基)乙炔基)-2-(1h-吡咯-1-基)吡啶(5c).收率66%,用1:5乙酸乙酯-己烷的制备层色谱法(rf=0.47)并从abs.乙醇中重结晶;mp121-123℃。1hnmr(700mhz,dmso-d6)δ8.63(d,j=2.2hz,1h),8.12(dd,j=2.3,8.6hz,1h),7.81(d,j=8.6hz,1h),7.73(t,j=2.3hz,2h),7.62-7.51(m,1h),7.28(t,j=8hz,2h),6.34(t,j=2.3hz,2h)。13cnmr(176mhz,dmso-d6)δ162.02(dd,j=5,252hz,2c),151.04,150.19,141.74,131.8(t,j=10.1hz),118.35(2c),114.63,112.07(m,4c),111.27,100.54(t,j=19.7hz),95.5(t,j=2.9hz),78.6。hrms(esi)理论计算c17h11f2n2[mh+]:280.0885。实际测定:280.0886。理论分析计算c17h10f2n2:c,72.85;h,3.60;n,10.00。实际测定:c,72.74;h,3.69;n,9.84。5-((2,6-二氟苯基)乙炔基)-n,n-二甲基吡嗪-2-胺(5d).收率73%,用1:10乙酸乙酯-己烷的柱色谱法;mp137-138℃。1hnmr(400mhz,dmso-d6)δ8.3(d,j=1.4hz,1h),8.19(d,j=1.4hz,1h),7.52(m,1h),7.25(t,j=8hz,2h),3.13(s,6h)。13cnmr(101mhz,dmso-d6)δ161.93(dd,j=251.5,5.2hz,2c),152.96,145.35,131.25(t,j=10.1hz),130.64,123.25,111.93(dd,j=5.2,18.6hz,2c),100.85(t,j=19.8hz),97.31(t,j=3hz),75.74,37.2(2c)。hrms(esi)理论计算c14h12f2n3[mh+]:260.0994。实际测定:260.0994。理论分析计算c14h11f2n3:c,64.86;h,4.28;n,16.21。实际测定:c,64.63;h,4.25;n,16.39。5-((2,6-二氟苯基)乙炔基)-1h-吲哚(5e).收率63%;用1:5乙酸乙酯-己烷的制备层色谱法(rf=0.44);mp131-133℃。1hnmr(400mhz,dmso-d6)δ11.4(s,1h),7.82(s,1h),7.52-7.44(m,3h),7.27(dd,j=1.6,8.5hz,1h),7.23(t,j=8hz,2h),6.51-6.49(m,1h)。13cnmr(101mhz,dmso-d6)δ161.94(dd,j=5.4,250.5hz,2c),136.08,130.44(t,j=10.1hz),127.63,126.95,124.24,124.18,112.06,111.81(dd,j=5.4,18.3hz,2c),111.35,101.69(t,j=19.7hz),101.59,101.49(t,j=3hz),73.26。hrms(esi)理论计算c16h10f2n[mh+]:254.0776。实际测定:254.0760。理论分析计算c16h9f2n:c,75.88;h,3.58;n,5.53。实际测定:c,76.07;h,3.63;n,5.41。5-((2,6-二氟苯基)乙炔基)-1-甲基-1h-吲哚(5f).收率76%;用1:2乙酸乙酯-己烷的柱色谱法;mp100-102℃。1hnmr(400mhz,dmso-d6)δ7.82(d,j=1.6hz,1h),7.55-7.45(m,2h),7.43(d,j=3hz,1h),7.33(dd,j=1.6,8.5hz,1h),7.24(t,j=7.9hz,2h),6.5(d,j=2.6hz,1h),3.82(s,3h)。13cnmr(101mhz,dmso-d6)δ161.94(dd,j=5.4,250.6hz,2c),136.45,131.28,130.51(t,j=10.1hz),127.96,124.41,124.24,111.83(dd,j=5.3,19.6hz,2c),111.45,110.42,101.63(t,j=20.0hz),101.28(t,j=3hz),100.92,73.52,32.63。hrms(esi)理论计算c17h12f2n[mh+]:268.0932。实际测定:268.0917。理论分析计算c17h11f2n:c,76.39;h,4.15;n,5.24。实际测定:c,76.17;h,4.31;n,5.04。4-((2,6-二氟苯基)乙炔基)-1h-吲唑(5g).收率71%,用1:2乙酸乙酯-己烷的柱色谱法;mp178-179℃。1hnmr(400mhz,dmso-d6)δ13.45(s,1h),8.11(s,1h),7.69(d,j=7.8hz,1h),7.63-7.52(m,1h),7.47-7.37(m,2h),7.3(t,j=8hz,2h)。13cnmr(101mhz,dmso-d6)δ162.05(dd,j=5.2,251.8hz,2c),139.64,132.2,131.67(t,j=10.2hz),126.16,124.5,123.35,112.98,112.22,112.05(dd,j=5.3,18.2hz,2c),100.9(t,j=19.7hz),96.74(t,j=3hz),79.12。hrms(esi)理论计算c15h9f2n2[mh+]:255.0728。实际测定:255.0712。理论分析计算c15h8f2n2:c,70.86;h,3.17;n,11.02。实际测定:c,70.60;h,3.25;n,10.92。5-((2,6-二氟苯基)乙炔基)-1h-吲唑(5h).收率25%,用1:2乙酸乙酯-己烷的制备层色谱法(rf=0.63);mp193-195℃。1hnmr(700mhz,dmso-d6)δ13.34(s,1h),8.15(s,1h),8.08(s,1h),7.62(d,j=8.6hz,1h),7.54-7.45(m,2h),7.25(t,j=7.9hz,2h)。13cnmr(176mhz,dmso-d6)δ162.01(dd,j=5.2,251hz,2c),139.52,134.15,130.94(t,j=10.1hz),128.85,124.98,122.82,112.96,111.88(dd,j=3.8,20.2hz,2c),110.94,101.24(t,j=19.8hz),100.03(t,j=3hz),74.15。hrms(esi)理论计算c15h9f2n2[mh+]:255.0728。实际测定:255.0727。理论分析计算c15h8f2n2:c,70.86;h,3.17;n,11.02。实际测定:c,70.64;h,3.22;n,11.09。6-((2,6-二氟苯基)乙炔基)-1h-吲唑(5i).收率87%,用1:1乙酸乙酯-己烷的柱色谱法;mp192-194℃。1hnmr(400mhz,dmso-d6)δ13.31(s,1h),8.15(s,1h),7.85(d,j=8.3hz,1h),7.77(s,j=1.2hz,1h),7.6-7.48(m,1h),7.33-7.21(m,3h)。13cnmr(101mhz,dmso-d6)δ162.09(dd,j=5.2,251.5hz,2c),139.31,133.87,131.44(t,j=10.2hz),123.20,123.04,121.37,118.43,113.63,111.99(dd,j=5.3,19hz,2c),100.94(t,j=19.7hz),99.67(t,j=3.1hz),75.66。hrms(esi)理论计算c15h9f2n2[mh+]:255.0728。实际测定:255.0712。理论分析计算c15h8f2n2:c,70.86;h,3.17;n,11.02。实际测定:c,70.61;h,3.06;n,10.88。5-((2,6-二氟苯基)乙炔基)-1-甲基-1h-吲唑(5j).收率47%;用1:5乙酸乙酯-己烷的制备层色谱法(rf=0.31);mp107-109℃。1hnmr(700mhz,dmso-d6)δ8.12(d,j=0.9hz,1h),8.06(s,1h),7.73(d,j=8.7hz,1h),7.55(dd,j=1.5,8.7hz,1h),7.53-7.48(m,1h),7.25(t,j=7.9hz,2h),4.08(s,3h)。13cnmr(176mhz,dmso-d6)δ162.01(dd,j=5.2,251.0hz,2c),139.19,133.06,130.98(t,j=10hz),128.78,125.17,123.39,113.04,111.89(dd,j=3.8,20.2hz,2c),110.54,101.2(t,j=19.8hz),99.87(t,j=3hz),74.38,35.53。hrms(esi)理论计算c16h11f2n2[mh+]:269.0885。实际测定:269.0885。理论分析计算c16h10f2n2:c,71.64;h,3.76;n,10.44。实际测定:c,71.41;h,3.75;n,10.53。6-((2,6-二氟苯基)乙炔基)-1-甲基-1h-吲唑(5k).收率75%;用1:5乙酸乙酯-己烷的制备层色谱法(rf=0.35);mp117-119℃。1hnmr(400mhz,dmso-d6)δ8.12(s,1h),7.99(s,1h),7.83(d,j=8.4hz,1h),7.63-7.47(m,1h),7.32-7.2(m,3h),4.09(s,3h)。13cnmr(101mhz,dmso-d6)δ162.09(dd,j=5.2,251.6hz,2c),139.09,132.68,131.44(t,j=10.2hz),123.56,123.20,121.49,118.42,113.46,111.94(dd,j=5.2,18.6hz,2c),100.88(t,j=19.7hz),99.65(t,j=3hz),75.81,35.58。hrms(esi)理论计算c16h11f2n2[mh+]:269.0885。实际测定:269.0886。理论分析计算c16h10f2n2:c,71.64;h,3.76;n,10.44。实际测定:c,71.48;h,3.83;n,10.39。5-((2,6-二氟苯基)乙炔基)-1h-吡咯并[2,3-b]吡啶(5e).收率45%;用1:2乙酸乙酯-己烷的柱色谱法;mp192-194℃。1hnmr(400mhz,dmso-d6)δ11.99(s,1h),8.41(d,j=2hz,1h),8.22(d,j=2hz,1h),7.59(t,j=3hz,1h),7.57-7.47(m,1h),7.26(t,j=8hz,2h),6.52(dd,j=1.8,3.5hz,1h)。13cnmr(101mhz,dmso-d6)δ161.95(dd,j=5.2,251.2hz,2c),147.80,145.04,131.21,131.02(t,j=10.3hz),127.83,119.20,111.76(dd,j=5,19hz,2c),109.53,101.29(t,j=19.9hz),100.42,98.22(t,j=3hz),75.88。hrms(esi)理论计算c15h9f2n2[mh+]:255.0728。实际测定:255.0710。理论分析计算c15h8f2n2:c,70.86;h,3.17;n,11.02。实际测定:c,70.57;h,3.17;n,10.88。2-((2,6-二氟苯基)乙炔基)喹啉(5m).收率83%;用1:5乙酸乙酯-己烷的柱色谱法;mp127-129℃。1hnmr(400mhz,dmso-d6)δ8.48(d,j=8.5hz,1h),8.09-8.02(m,2h),7.89-7.82(m,1h),7.76(d,j=8.4hz,1h),7.72-7.66(m,1h),7.66-7.58(m,1h),7.32(t,j=8.1hz,2h)。13cnmr(101mhz,dmso-d6)δ162.44(dd,j=5,252.8hz,2c),147.66,141.73,137.07,132.57(t,j=10.2hz),130.62,128.75,128.05,127.82,127.15,124.25,112.16(dd,j=4.6,19hz,2c),99.97(t,j=19.7hz),98.51(t,j=3hz),75.62。hrms(esi)理论计算c17h10f2n[mh+]:266.0776。实际测定:266.0756。理论分析计算c17h9f2n:c,76.98;h,3.42;n,5.28。实际测定:c,76.73;h,3.38;n,5.17。3-((2,6-二氟苯基)乙炔基)喹啉(5n).收率64%;用1:10乙酸乙酯-己烷的制备层色谱法(rf=0.42);mp115-117℃。1hnmr(400mhz,dmso-d6)δ9(d,j=2.1hz,1h),8.72(d,j=2.2hz,1h),8.06(d,j=8.4hz,2h),7.85(t,j=7.6hz,1h),7.69(t,j=7.6hz,1h),7.64-7.52(m,1h),7.30(t,j=8.1hz,2h)。13cnmr(101mhz,dmso-d6)δ162.12(dd,j=5,252.2hz,2c),151.13,146.64,139.13,132.02(t,j=10.2hz),131.04,128.87,128.29,127.7,126.73,115.41,112.06(dd,j=5,18.7hz,2c),100.46(t,j=19.7hz),96.21(t,j=3.1hz),78.9。hrms(esi)理论计算c17h10f2n[mh+]:266.0776。实际测定:266.0757。理论分析计算c17h9f2n:c,76.98;h,3.42;n,5.28。实际测定:c,76.68;h,3.50;n,5.11。4-((2,6-二氟苯基)乙炔基)喹啉(5o).收率68%;用1:5乙酸乙酯-己烷的柱色谱法;mp101-102℃。1hnmr(400mhz,dmso-d6)δ8.97(d,j=4.4hz,1h),8.29(d,j=8.1hz,1h),8.12(d,j=8.3hz,1h),7.95-7.84(m,1h),7.83-7.75(m,2h),7.70-7.58(m,1h),7.34(t,j=8.1hz,2h)。13cnmr(101mhz,dmso-d6)δ162.22(dd,j=4.9,252.9hz,2c),150.26,147.50,132.8(t,j=10.2hz),130.44,129.78,128.15,127.1,126.35,124.97,123.86,112.21(dd,j=5.3,19hz,2c),100.06(t,j=19.8hz),94.17(t,j=3hz),84.92。hrms(esi)理论计算c17h10f2n[mh+]:266.0776。实际测定:266.0757。理论分析计算c17h9f2n:c,76.98;h,3.42;n,5.28。实际测定:c,77.21;h,3.43;n,5.24。2-氯-3-((2,6-二氟苯基)乙炔基)喹啉(5p).收率53%;用1:5乙酸乙酯-己烷的柱色谱法;mp137-138℃。1hnmr(400mhz,dmso-d6)δ8.89(s,1h),8.1(d,j=8.2hz,1h),8(d,j=8.5hz,1h),7.93-7.84(m,1h),7.77-7.69(m,1h),7.68-7.56(m,1h),7.31(t,j=8.1hz,2h)。13cnmr(101mhz,dmso-d6)δ162.2(dd,j=5,252.8hz,2c),148.86,146.09,143,132.47(t,j=10.1hz),132.3,128.28,128.16,127.84,126.14,115.72,112.17(dd,j=4.5,19hz,2c),100.22(t,j=19.7hz),94.07(t,j=2.9hz),82.23。hrms(esi)理论计算c17h9clf2n[mh+]:300.0386。实际测定:300.0368。理论分析计算c17h8clf2n:c,68.13;h,2.69;n,4.67。实际测定:c,68.16;h,2.73;n,4.38。4-氯-3-((2,6-二氟苯基)乙炔基)喹啉(5q).收率40%;从甲醇重结晶;mp140-142℃。1hnmr(400mhz,dmso-d6)δ9.03(s,1h),8.27(d,j=8.4hz,1h),8.14(d,j=8.4hz,1h),7.94(t,j=7.7hz,1h),7.84(t,j=7.7hz,1h),7.7-7.56(m,1h),7.32(t,j=8.2hz,2h)。13cnmr(101mhz,dmso-d6)δ162.11(dd,j=4.9,253.1hz,2c),151.25,147.27,143,132.62(t,j=10.2hz),131.84,129.65,129.22,124.83,124.17,115.77,112.16(dd,j=4.6,19hz,2c),100.15(t,j=19.7hz),93.43(t,j=3hz),84.18。hrms(esi)理论计算c17h9clf2n[mh+]:300.0386。实际测定:300.0366。理论分析计算c17h8clf2n:c,68.13;h,2.69;n,4.67。实际测定:c,67.84;h,2.82;n,4.53。3-((2,6-二氟苯基)乙炔基)喹啉-6-胺(5r).收率67%;用2:1乙酸乙酯-己烷的柱色谱法;mp184-185℃。1hnmr(400mhz,dmso-d6)δ8.54(d,j=2.1hz,1h),8.26(d,j=2.1hz,1h),7.73(d,j=9hz,1h),7.63-7.51(m,1h),7.29(t,j=8hz,2h),7.22(dd,j=2.4,9hz,1h),6.83(d,j=2.5hz,1h),5.82(s,2h)。13cnmr(101mhz,dmso-d6)δ162.10(dd,j=5.1,251.9hz,2c),148.05,145.57,140.98,135.88,131.76(t,j=10.2hz),129.62,128.76,123,115.13,112.07(dd,j=5,18.7hz,2c),104.41,100.8(t,j=19.7hz),97.1(t,j=3.1hz),78.02。hrms(esi)理论计算c17h11f2n2[mh+]:281.0885。实际测定:281.0864。理论分析计算c17h10f2n2:c,72.85;h,3.60;n,10.00。实际测定:c,72.57;h,3.64;n,9.84。3-((2,6-二氟苯基)乙炔基)-7-氟喹啉(5s).收率87%;用1:5乙酸乙酯-己烷的柱色谱法;mp142-143℃。1hnmr(400mhz,dmso-d6)δ9.03(d,j=2hz,1h),8.77(d,j=2.1hz,1h),8.17(dd,j=6.3,9.1hz,1h),7.83(dd,j=2.5,10.3hz,1h),7.66(td,j=2.6,8.9hz,1h),7.61-7.54(m,1h),7.3(t,j=8.1hz,2h)。13cnmr(101mhz,dmso-d6)δ163.16(d,j=250.1hz),162.12(dd,j=5.1,252.3hz,2c),152.29,147.61(d,j=13hz),139.24(d,j=1.3hz),132.07(t,j=10.2hz),131.14(d,j=10.3hz),124.09,118.13(d,j=25.5hz),114.95(d,j=2.7hz),112.58(d,j=20.6hz),112.10(dd,j=5.3,18.9hz,2c),100.4(t,j=19.7hz),95.94(t,j=2.9hz),78.94。hrms(esi)理论计算c17h9f3n[mh+]:284.0682。实际测定:284.0661。理论分析计算c17h8f3n:c,72.09;h,2.85;n,4.95。实际测定:c,71.83;h,2.94;n,4.70。3-((2,6-二氟苯基)乙炔基)-8-氟喹啉(5t).收率79%;从甲醇重结晶;mp136-137℃。1hnmr(400mhz,dmso-d6)δ9.05(d,j=2hz,1h),8.8(s,1h),7.97-7.84(m,1h),7.75-7.65(m,2h),7.64-7.56(m,1h),7.31(t,j=8.1hz,2h)。13cnmr(101mhz,dmso-d6)δ162.15(dd,j=5,252.4hz,2c),157.08(d,j=255.6hz),151.38(d,j=1.5hz),139.04(d,j=2.9hz),136.37(d,j=12hz),132.25(t,j=10.2hz),128.44(d,j=2hz),127.91(d,j=8hz),124.26(d,j=4.6hz),116.55,115.22(d,j=18.4hz),112.11(dd,j=5,18.6hz,2c),100.27(t,j=19.7hz),95.71(t,j=3hz),79.59。hrms(esi)理论计算c17h9f3n[mh+]:284.0682。实际测定:284.0661。理论分析计算c17h8f3n:c,72.09;h,2.85;n,4.95。实际测定:c,71.79;h,2.92;n,4.95。7-氯-4-((2,6-二氟苯基)乙炔基)喹啉(5u).收率80%;在1:150甲醇-二氯甲烷中的柱色谱法;mp130-132℃。1hnmr(400mhz,dmso-d6)δ8.99(d,j=4.4hz,1h),8.25(d,j=8.9hz,1h),8.17(d,j=2.1hz,1h),7.83(dd,j=2.1,8.9hz,1h),7.80(d,j=4.5hz,1h),7.7-7.59(m,1h),7.34(t,j=8.2hz,2h)。13cnmr(101mhz,dmso-d6)δ162.24(dd,j=4.9,253.1hz,2c),151.62,147.83,135.06,133.04(t,j=10.3hz),128.78,128.42,127.32,127.01,125.05,124.22,112.24(dd,j=4.5,19hz,2c),99.89(t,j=19.6hz),93.66(t,j=3hz),85.43。hrms(esi)理论计算c17h9clf2n[mh+]:300.0386。实际测定:300.0366。理论分析计算c17h8clf2n:c,68.13;h,2.69;n,4.67。实际测定:c,67.96;h,2.68;n,4.68。5-((2,6-二氟苯基)乙炔基)喹啉(5v).收率68%;用1:10乙酸乙酯-己烷的制备层色谱法(rf=0.45);mp98-100℃。1hnmr(400mhz,dmso-d6)δ9.04-9(m,1h),8.64(d,j=8.2hz,1h),8.15(dd,j=2.5,8.5hz,1h),7.94(dd,j=2.8,7.2hz,1h),7.9-7.78(m,1h),7.78-7.69(m,1h),7.66-7.52(m,1h),7.40-7.23(m,2h)。13cnmr(101mhz,dmso-d6)δ162.06(dd,j=5.1,251.9hz,2c),151.47,147.34,133.17,131.9(t,j=10.2hz),131.13,131.06,129.35,127.62,122.86,119.2,112.06(dd,j=5.3,18.3hz,2c),100.56(t),95.63(t,j=3.1hz),81.48。hrms(esi)理论计算c17h10f2n[mh+]:266.0776。实际测定:266.0758。理论分析计算c17h9f2n:c,76.98;h,3.42;n,5.28。实际测定:c,76.80;h,3.47;n,5.15。6-((2,6-二氟苯基)乙炔基)喹啉(5w).收率80%;用1:2乙酸乙酯-己烷的柱色谱法;mp91-93℃。1hnmr(400mhz,dmso-d6)δ8.96(dd,j=1.8,4.2hz,1h),8.44(d,j=8.1hz,1h),8.33(d,j=1.9hz,1h),8.07(d,j=8.7hz,1h),7.86(dd,j=1.9,8.7hz,1h),7.61(dd,j=4.2,8.3hz,1h),7.59-7.52(m,1h),7.29(t,j=8.1hz,2h)。13cnmr(101mhz,dmso-d6)δ162.12(dd,j=5.1,251.9hz,2c),151.86,147.35,136.13,132.05,131.74(t,j=10.2hz),131.38,129.7,127.75,122.44,119.23,112.01(dd,j=4.9,18.7hz,2c),100.67(t,j=19.7hz),98.47(t,j=3hz),76.91。hrms(esi)理论计算c17h10f2n[mh+]:266.0776。实际测定:266.0758。理论分析计算c17h9f2n:c,76.98;h,3.42;n,5.28。实际测定:c,76.97;h,3.33;n,5.29。7-((2,6-二氟苯基)乙炔基)喹啉(5x).收率74%;用1:10乙酸乙酯-己烷的制备层色谱法(rf=0.49);mp123-125℃.1hnmr(400mhz,dmso-d6)δ8.98(dd,j=1.7,4.2hz,1h),8.43(d,j=8.2hz,1h),8.21(d,j=1.1hz,1h),8.07(d,j=8.4hz,1h),7.73(dd,j=1.6,8.4hz,1h),7.66-7.5(m,2h),7.29(t,j=8.1hz,2h)。13cnmr(101mhz,dmso-d6)δ162.13(dd,j=5,252hz,2c),151.81,147.12,136.01,132.15,131.86(t,j=10.2hz),129.08,128.46,128.24,122.56,122.21,112.7-111.37(dd,j=5,18.6hz,2c),100.62(t,j=19.8hz),98.35(t,j=3.1hz),77.65。hrms(esi)理论计算c17h10f2n[mh+]:266.0776。实际测定:266.0757。理论分析计算c17h9f2n:c,76.98;h,3.42;n,5.28。实际测定:c,77.15;h,3.28;n,5.31。8-((2,6-二氟苯基)乙炔基)喹啉(5y).收率62%;用1:5乙酸乙酯-己烷的柱色谱法;mp108-110℃。1hnmr(400mhz,dmso-d6)δ9.04(dd,j=1.8,4.2hz,1h),8.47(dd,j=1.8,8.3hz,1h),8.11(d,j=8.2hz,1h),8.08(dd,j=1.4,7.2hz,1h),7.7-7.62(m,2h),7.6-7.5(m,1h),7.28(t,j=8hz,2h)。13cnmr(101mhz,dmso-d6)δ162.07(dd,j=5.2,251.6hz,2c),151.47,147.17,136.71,134.39,131.37(t,j=10.2hz),130,128.02,126.3,122.36,121.25,111.94(dd,j=5.3,19hz,2c),101.33(t,j=19.6hz),97.3(t,j=3hz),80.76。hrms(esi)理论计算c17h10f2n[mh+]:266.0776。实际测定:266.0757。理论分析计算c17h9f2n:c,76.98;h,3.42;n,5.28。实际测定:c,76.68;h,3.30;n,5.29。2-氯-6-((2,6-二氟苯基)乙炔基)喹啉(5z).收率67%;用1:5乙酸乙酯-己烷的柱色谱法;mp137-138℃。1hnmr(400mhz,dmso-d6)δ8.51(d,j=8.7hz,1h),8.39(s,1h),8(d,j=8.7hz,1h),7.92(dd,j=8.7,1.6hz,1h),7.69(d,j=8.6hz,1h),7.63-7.53(m,1h),7.29(t,j=8.1hz,2h)。13cnmr(100mhz,dmso-d6)δ162.14(dd,j=252,5.1hz,2c),151.17,146.89,140.01,132.81,131.97,131.93(t,j=10.2hz),128.64,126.69,123.53,119.89,112.06,(dd,j=5.3,19hz,2c),100.54(t,j=19.8hz),98.05(t,j=3hz).77.38。hrms(esi)理论计算c17h9clf2n[mh+]:300.0386。实际测定:300.0366。理论分析计算c17h8clf2n:c,68.13;h,2.69;n,4.67。实际测定:c,67.93;h,2.70;n,4.49。4-氯-6-((2,6-二氟苯基)乙炔基)喹啉(5aa).收率64%;用1:5乙酸乙酯-己烷的制备层色谱法(rf=0.33)并从己烷中重结晶;mp120-122℃。1hnmr(700mhz,dmso-d6)δ8.89(d,j=4.7hz,1h),8.33(d,j=1.8hz,1h),8.14(d,j=8.6hz,1h),7.96(dd,j=1.8,8.6hz,1h),7.84(d,j=4.7hz,1h),7.67-7.5(m,1h),7.29(t,j=8hz,2h)。13cnmr(176mhz,dmso-d6)δ162.14(dd,j=4.9,252.3hz,2c),151.75,148.13,140.97,132.55,132(t,j=10.1hz),130.46,127.06,125.49,122.61,120.88,112.00(dd,j=3.8,20.1hz,2c),100.41(t,j=19.7hz),97.85(t,j=3hz),77.94。hrms(esi)理论计算c17h9clf2n[mh+]:300.0386。实际测定:300.0389。理论分析计算c17h8clf2n:c,68.13;h,2.69;n,4.67。实际测定:c,67.85;h,2.79;n,4.53。4-氯-7-((2,6-二氟苯基)乙炔基)喹啉(5bb).收率63%;用1:5乙酸乙酯-己烷的柱色谱法并从乙腈中重结晶;mp148-150℃。1hnmr(400mhz,dmso-d6)δ8.92(d,j=4.7hz,1h),8.28(d,j=1.7hz,1h),8.25(d,j=8.7hz,1h),7.88(dd,j=1.7,8.6hz,1h),7.84(d,j=4.7hz,1h),7.64-7.52(m,1h),7.30(t,j=8.1hz,2h)。13cnmr(101mhz,dmso-d6)δ162.16(dd,j=5,252.3hz,2c),151.83,148.07,141.2,132.55,132.16(t,j=10.1hz),130.06,125.83,124.76,123.5,122.66,112.08(dd,j=5.3,19hz,2c),100.4(t,j=19.7hz),97.62(t,j=3hz),78.63。hrms(esi)理论计算c17h9clf2n[mh+]:300.0386。实际测定:300.0365。理论分析计算c17h8clf2n:c,68.13;h,2.69;n,4.67。实际测定:c,68.41;h,2.97;n,4.42。6-((2,6-二氟苯基)乙炔基)喹啉-4-胺(5cc).收率93%;用1:10甲醇-二氯甲烷的柱色谱法;mp220-222℃。1hnmr(700mhz,dmso-d6)δ8.54(s,1h),8.35(brs,1h),7.79(d,j=8.6hz,1h),7.73(dd,j=1.7,8.6hz,1h),7.61-7.51(m,1h),7.29(t,j=8hz,2h),7.27(s,2h),6.62(d,j=5.3hz,1h)。13cnmr(176mhz,dmso-d6)δ162.07(dd,j=5,251.6hz,2c),152.28,150.46,147.61,131.44,131.41(t,j=10.1hz),128.67,126.82,118.06,116.27,111.98(dd,j=5,18.6hz,2c),103.1,100.91(t,j=19.8hz),99.23(t,j=3hz),76.01。hrms(esi)理论计算c17h11f2n2[mh+]:281.0885。实际测定:281.0887。理论分析计算c17h10f2n2:c,72.85;h,3.60;n,10.00。实际测定:c,72.84;h,3.52;n,10.06。7-((2,6-二氟苯基)乙炔基)喹啉-4-胺(5dd).收率61%;用乙酸乙酯的柱色谱法;mp219-221℃。1hnmr(700mhz,dmso-d6)δ8.37(brs,1h),8.23(d,j=8.6hz,1h),7.94(s,1h),7.6-7.54(m,1h),7.51(dd,j=1.5,8.6hz,1h),7.29(t,j=8hz,2h),6.93(s,2h),6.6(d,j=5.1hz,1h)。13cnmr(176mhz,dmso-d6)δ162.1(dd,j=5.0,251.8hz,2c),151.45,151.4,148.33,132.32,131.63(t,j=10.1hz),125.22,123.49,121.57,118.88,111.98(dd,j=3.7,20.3hz,2c),103.21,100.78(t,j=19.7hz),98.75(t,j=2.9hz),76.85。hrms(esi)理论计算c17h11f2n2[mh+]:281.0885。实际测定:281.0885。理论分析计算c17h10f2n2:c,72.85;h,3.60;n,10.00。实际测定:c,72.57;h,3.73;n,9.96。通过亲核芳族取代合成n,n-二甲基氨基取代的喹啉的一般程序。130mg(0.43mmol)合适的氯取代的喹啉和2.2ml(4.3mmol,10当量)的含2m甲胺或二甲胺的thf溶液混合后在130℃下在压力管中搅拌2-3小时。将该混合物倒入水中,用二氯甲烷萃取,用无水硫酸镁干燥,浓缩并通过色谱法纯化,如针对单个化合物所述。6-((2,6-二氟苯基)乙炔基)-n,n-二甲基喹啉-2-胺(5ee).收率67%;用1:5乙酸乙酯-己烷的制备层色谱法(rf=0.53)并从abs.乙醇中重结晶;mp142-144℃。1hnmr(700mhz,dmso-d6)δ8.05(d,j=9.1hz,1h),7.98(d,j=1.9hz,1h),7.6(dd,j=2.0,8.6hz,1h),7.54(d,j=8.6hz,1h),7.53-7.48(m,1h),7.29-7.23(m,2h),7.14(d,j=9.2hz,1h),3.19(s,6h)。13cnmr(176mhz,dmso-d6)δ161.97(dd,j=5.2,251.1hz,2c),157.82,147.85,136.93,131.54,131.42,130.93(t,j=10hz),126.25,121.85,113.31,111.88(dd,j=3.9,20.2hz,2c),110.48,101.25(t,j=19.8hz),99.78(t,j=2.9hz),75.07,37.61(2c)。hrms(esi)理论计算c19h15f2n2[mh+]:309.1198。实际测定:309.1198。理论分析计算c19h14f2n2:c,74.01;h,4.58;n,9.09。实际测定:c,74.04;h,4.59;n,9.09。6-((2,6-二氟苯基)乙炔基)-n,n-二甲基喹啉-4-胺(5ff).收率64%;用1:1乙酸乙酯-己烷的制备层色谱法(rf=0.41);mp118-120℃.1hnmr(700mhz,dmso-d6)δ8.62(d,j=5.2hz,1h),8.22(d,j=1.9hz,1h),7.94(d,j=8.6hz,1h),7.76(dd,j=1.9,8.6hz,1h),7.55(m,1h),7.27(t,j=8hz,2h),6.91(d,j=5.2hz,1h),3.02(s,6h)。13cnmr(176mhz,dmso-d6)δ162.08(dd,j=5.1,251.7hz,2c),156.56,151.52,149.18,131.51(t,j=10.1hz),130.73,130.23,128.42,121.95,117.04,111.95(dd,j=3.8,20.2hz,2c),108.06,100.84(t,j=19.7hz),99.05(t,j=3hz),76.28,43.54(2c)。hrms(esi)理论计算c19h15f2n2[mh+]:309.1198。实际测定:309.1201。理论分析计算c19h14f2n2:c,74.01;h,4.58;n,9.09。实际测定:c,73.75;h,4.66;n,9.11。1-((2,6-二氟苯基)乙炔基)异喹啉(5gg).收率72%;用1:5乙酸乙酯-己烷的柱色谱法;mp120-122℃.1hnmr(400mhz,dmso-d6)δ8.6(d,j=5.6hz,1h),8.45-8.37(m,1h),8.13-8.05(m,1h),7.97(d,j=5.6hz,1h),7.92-7.81(m,2h),7.74-7.57(m,1h),7.35(t,j=8.1hz,2h)。13cnmr(101mhz,dmso-d6)δ162.44(dd,j=5,252.7hz,2c),143.08,142.14,135.38,132.71(t,j=10.2hz),131.22,129.13,128.6,127.47,125.45,121.74,112.21(dd,j=5.4,18.3hz,2c),100.04(t,j=19.7hz),96.00(t,j=3.1hz),80。hrms(esi)理论计算c17h10f2n[mh+]:266.0776。实际测定:266.0758。理论分析计算c17h9f2n:c,76.98;h,3.42;n,5.28。实际测定:c,76.92;h,3.50;n,5.20。4-((2,6-二氟苯基)乙炔基)异喹啉(5hh).收率73%;用1:5乙酸乙酯-己烷的柱色谱法;mp102-104℃。1hnmr(400mhz,dmso-d6)δ9.41(s,1h),8.79(s,1h),8.25(t,j=9.1hz,2h),7.99(t,j=7.6hz,1h),7.82(t,j=7.6hz,1h),7.68-7.53(m,1h),7.32(t,j=8.1hz,2h)。13cnmr(101mhz,dmso-d6)δ162.04(dd,j=5,252.1hz,2c),153.34,146.2,134.33,132.32,132.06(t,j=10.2hz),128.63,128.57,127.34,123.75,113.84,112.09(dd,j=4.9,18.6hz,2c),100.58(t,j=19.6hz),94.01(t,j=3hz),83.24。hrms(esi)理论计算c17h10f2n[mh+]:266.0776。实际测定:266.0758。理论分析计算c17h9f2n:c,76.98;h,3.42;n,5.28。实际测定:c,76.68;h,3.49;n,5.10。5-((2,6-二氟苯基)乙炔基)异喹啉(5ii).收率85%;用1:1乙酸乙酯-己烷的柱色谱法;mp115-117℃。1hnmr(400mhz,dmso-d6)δ9.44(s,1h),8.7(d,j=5.8hz,1h),8.27(d,j=8.3hz,1h),8.11(dd,j=1.1,7.2hz,1h),8.08(d,j=5.9hz,1h),7.81-7.72(m,1h),7.66-7.55(m,1h),7.33(t,j=8.1hz,2h)。13cnmr(101mhz,dmso-d6)δ162.06(dd,j=5.1,252hz,2c),153.14,144.54,134.88,134.71,131.97(t,j=10.1hz),129.62,127.91,127.36,117.86,117.53,112.1(dd,j=5.3,19hz,2c),100.63(t,j=19.6hz),95.41(t,j=3hz),81.86。hrms(esi)理论计算c17h10f2n[mh+]:266.0776。实际测定:266.0756。理论分析计算c17h9f2n:c,76.98;h,3.42;n,5.28。实际测定:c,76.75;h,3.47;n,5.23。6-((2,6-二氟苯基)乙炔基)异喹啉(5jj).收率75%,用1:1乙酸乙酯-己烷的柱色谱法;mp97-99℃。1hnmr(400mhz,dmso-d6)δ9.38(s,1h),8.58(d,j=6.1hz,1h),8.3(s,1h),8.2(d,j=8.5hz,1h),7.9(d,j=5.7hz,1h),7.79(dd,j=1.6,8.5hz,1h),7.68-7.5(m,1h),7.3(t,j=8.1hz,2h)。13cnmr(101mhz,dmso-d6)δ162.18(dd,j=5,252.1hz,2c),152.35,143.91,134.84,132.1(t,j=10.2hz),130.25,129.29,128.44,123.12,120.19,112.11(dd,j=5.0,19hz,2c),100.37(t,j=19.7hz),98.29(t,j=3.2hz),78.06。hrms(esi)理论计算c17h10f2n[mh+]:266.0776。实际测定:266.0757。理论分析计算c17h9f2n:c,76.98;h,3.42;n,5.28。实际测定:c,77.16;h,3.39;n,5.21。1-氯-4-((2,6-二氟苯基)乙炔基)异喹啉(5kk).收率48%;用1:5乙酸乙酯-己烷的制备层色谱法(rf=0.62)并从abs.乙醇重结晶;mp145-146℃。1hnmr(700mhz,dmso-d6)δ8.63(s,1h),8.39(d,j=8.2hz,1h),8.3(d,j=8.3hz,1h),8.1(ddd,j=1.2,6.9,8.2hz,1h),7.95(ddd,j=1.1,6.9,8.3hz,1h),7.66-7.58(m,1h),7.33(t,j=8.1hz,2h)。13cnmr(176mhz,dmso-d6)δ162.06(dd,j=4.9,252.5hz,2c),151.11,144.73,136.29,133.31,132.36(t,j=10.1hz),130.35,126.5,125.37,124.82,114.66,112.13(dd,j=3.7,20.1hz,2c),100.31(t,j=19.7hz),93(t,j=3.0hz),84.26。hrms(esi)理论计算c17h9clf2n[mh+]:300.0386。实际测定:300.0388。理论分析计算c17h8clf2n:c,68.13;h,2.69;n,4.67。实际测定:c,68.06;h,2.78;n,4.61。1-((2,6-二氟苯基)乙炔基)-7-氟异喹啉(5ee).收率56%;用1:200甲醇-二氯甲烷的柱色谱法;mp177-178℃。1hnmr(400mhz,dmso-d6)δ8.61(d,j=5.5hz,1h),8.23(dd,j=5.5,9.1hz,1h),8.07-7.96(m,2h),7.83(td,j=2.6,8.9hz,1h),7.71-7.57(m,1h),7.35(t,j=8.2hz,2h)。13cnmr(101mhz,dmso-d6)δ162.43(dd,j=5,252.8hz,2c),161.17(d,j=249.3hz),142.77(d,j=2.4hz),141.58(d,j=6hz),132.93,132.79(d,j=8.6hz),131.15(d,j=8.9hz),129.49(d,j=8.8hz),121.79(d,j=25.6hz),121.56(d,j=1.6hz),112.23(dd,j=5,18.7hz,2c),108.72(d,j=22.2hz),99.91(t,j=19.7hz),95.49(t,j=2.7hz),80.54。hrms(esi)理论计算c17h9f3n[mh+]:284.0682。实际测定:284.0662。理论分析计算c17h8f3n:c,72.09;h,2.85;n,4.95。实际测定:c,71.98;h,2.69;n,4.91。4-((2,6-二氟苯基)乙炔基)异喹啉-1-胺(5mm).收率71%;用1:10甲醇-二氯甲烷的柱色谱法并从甲醇重结晶;mp200-201℃。1hnmr(400mhz,dmso-d6)δ8.31(d,j=8.3hz,1h),8.17(s,1h),8.04(d,j=8.2hz,1h),7.82(t,j=7.6hz,1h),7.59(t,j=7.6hz,1h),7.55-7.43(m,3h),7.26(t,j=7.9hz,2h)。13cnmr(101mhz,dmso-d6)δ162.04(dd,j=5.4,250.5hz,2c),158.49,147.85,136.25,131.71,130.77(t,j=10hz),126.86,125,124.33,116.64,111.85(dd,j=5.4,18.3hz,2c),102.27,102.23(t,j=19.9hz),97.26(t,j=2.9hz),80.27。hrms(esi)理论计算c17h11f2n2[mh+]:281.0885。实际测定:281.0866。理论分析计算c17h10f2n2:c,72.85;h,3.60;n,10.00。实际测定:c,73.13;h,3.69;n,9.93。4-((2,6-二氟苯基)乙炔基)-n,n-二甲基异喹啉-1-胺(5nn).收率75%;用1:5乙酸乙酯-己烷的制备层色谱法(rf=0.47);mp122-124℃。1hnmr(700mhz,dmso-d6)δ8.31(s,1h),8.23-8.16(m,1h),8.13(dd,j=1.2,8.3hz,1h),7.84(ddd,j=1.2,6.9,8.2hz,1h),7.62(ddd,j=1.3,6.8,8.3hz,1h),7.56-7.48(m,1h),7.28(t,j=7.9hz,2h),3.19(s,6h)。13cnmr(176mhz,dmso-d6)δ161.72(dd,j=5.3,250.9hz,2c),160.63,145.03,137.01,130.96,130.82(t,j=10.1hz),126.80,126.2,124.21,118.3,111.91(dd,j=3.8,20.1hz,2c),105.07,101.45(t,j=19.8hz),95.96(t,j=2.9hz),81.00,42.45(2c)。hrms(esi)理论计算c19h15f2n2[mh+]:309.1198。实际测定:309.1200。理论分析计算c19h14f2n2:c,74.01;h,4.58;n,9.09。实际测定:c,73.75;h,4.60;n,9.00。4-((2,6-二氟苯基)乙炔基)-n-甲基异喹啉-1-胺(5oo).收率60%,rf=0.48(1:2乙酸乙酯-己烷),mp118-120℃。1hnmr(400mhz,dmso-d6)δ8.28(d,j=8.6hz,1h),8.25(s,1h),8.08(t,j=4.5hz,1h),8.05(d,j=9.1hz,1h),7.81(t,j=7.5hz,1h),7.61(t,j=7.5hz,1h),7.55-7.44(m,1h),7.26(t,j=7.9hz,2h),3.03(d,j=4.5hz,3h)。13cnmr(101mhz,dmso-d6)δ161.61(dd,j=5.4,250.4hz,2c),156.29,147.12,135.26,130.99,130.33(t,j=10.1hz),126.6,124.05,123.44,117.01,112.01(dd,j=3.8,20.1hz,2c),101.78(t,j=19.9hz),101.45,96.88(t,j=2.9hz),79.90,28.29。hrms(esi)理论计算c18h13f2n2[mh+]:295.1041。实际测定:295.1035。理论分析计算c18h12f2n2:c,73.46;h,4.11;n,9.52。实际测定:c,73.49;h,4.23,n,9.61。4-((2,6-二氟苯基)乙炔基)-1-(4-甲基哌嗪-1-基)异喹啉(5pp).收率79%,rf=0.42(1:10甲醇-二氯甲烷),mp70-72℃。1hnmr(400mhz,dmso-d6)δ8.38(s,1h),8.16(d,j=8.1hz,1h),8.11(d,j=8.4hz,1h),7.88(ddd,j=1.2,6.9,8.2hz,1h),7.68(ddd,j=1.3,6.9,8.3hz,1h),7.61-7.5(m,1h),7.29(t,j=8hz,2h),3.47(t,j=5hz,4h),2.57(t,j=4.7hz,4h),2.27(s,3h)。13cnmr(101mhz,dmso-d6)δ161.82(dd,j=5.3,251.1hz,2c),160.77,144.84,136.70,131.31,131.15(t,j=9.2hz),127.19,126.14,124.54,119.27,112.12(dd,j=3.7,20.1hz,2c),107.32,101.20(t,j=19.7hz),95.34(t,j=3hz),81.52,54.56(2c),50.65(2c),45.78。hrms(esi)理论计算c22h20f2n3[mh+]:364.1620。实际测定:364.1614。理论分析计算c22h19f2n3:c,72.71;h,5.27;n,11.56。实际测定:c,72.91;h,5.12,n,11.67。8-((2,6-二氟苯基)乙炔基)-n,n-二甲基-1,6-萘啶-5-胺(5qq).收率76%,rf=0.29(1:2乙酸乙酯-己烷),mp149-150℃。1hnmr(400mhz,dmso-d6)δ9.04(dd,j=1.7,4.2hz,1h),8.59(dd,j=1.7,8.5hz,1h),8.45(s,1h),7.57(dd,j=4.2,8.5hz,1h),7.53-7.46(m,1h),7.25(t,j=8hz,2h),3.25(s,6h)。13cnmr(101mhz,dmso-d6)δ161.9(dd,j=5.3,250.9hz,2c),160.35,153.88,152.18,148.92,135.3,130.67(t,j=10.1hz),120.82,113.48,111.84(dd,j=3.8,20.1hz,2c),106.58,101.86(t,j=19.8hz),96.37(t,j=3.0hz),79.73,42.31(2c)。hrms(esi)理论计算c18h14f2n3[mh+]:310.1150。实际测定:310.1147。理论分析计算c18h13f2n3:c,69.89;h,4.24;n,13.58。实际测定:c,70.01;h,4.44,n,13.53。7-((2,6-二氟苯基)乙炔基)喹唑啉(5rr).收率68%;从乙腈重结晶;mp166-168℃。1hnmr(400mhz,dmso-d6)δ9.68(s,1h),9.37(s,1h),8.25(d,j=8.4hz,1h),8.2(s,1h),7.89(dd,j=1.6,8.4hz,1h),7.69-7.53(m,1h),7.31(t,j=8.1hz,2h)。13cnmr(101mhz,dmso-d6)δ162.19(dd,j=4.9,252.6hz,2c),160.85,156.02,148.92,132.46(t,j=10.2hz),130.75,130.11,128.79,127.1,124.50,112.14(dd,j=5,18.6hz,2c),100.21(t,j=19.7hz),97.51(t,j=3hz),79.73。hrms(esi)理论计算c16h9f2n2[mh+]:267.0728。实际测定:267.0710。理论分析计算c16h8f2n2:c,72.18;h,3.03;n,10.52。实际测定:c,71.97;h,3.22;n,10.29。3,5-二氟-4-(苯乙炔基)吡啶(7a).收率88%;用1:20乙酸乙酯-己烷(rf=0.48)的制备层色谱法;mp52-54℃。1hnmr(400mhz,dmso-d6)δ8.67(s,2h),7.69-7.62(m,2h),7.58-7.47(m,3h)。13cnmr(101mhz,dmso-d6)δ157.60(d,j=264.1hz,2c),134.52(dd,j=21.8,4.6hz,2c),131.87(2c),130.58,129.09(2c),120.20,108.59(t,j=16.5hz),103.10(t,j=2.9hz),73.82。hrms(esi)理论计算c13h8f2n[mh+]:216.0619。实际测定:216.0619。理论分析计算c13h7f2n:c,72.56;h,3.28;n,6.51。实际测定:c,72.78;h,3.38,n,6.58。4-((3,5-二氟吡啶-4-基)乙炔基)苯胺(7b).收率69%;用1:5乙酸乙酯-己烷的制备层色谱法(rf=0.3);mp156-158℃。1hnmr(400mhz,dmso-d6)δ8.58(s,2h),7.28(d,j=8.4hz,2h),6.59(d,j=8.3hz,2h),5.91(s,2h,nh2)。13cnmr(101mhz,dmso-d6)δ157.31(d,j=262.2hz,2c),151.19,134.17(dd,j=21.5,4.8hz,2c),133.48(2c),113.62(2c),109.81(t,j=16.7hz),106.67(t,j=2.9hz),105.47,71.86。hrms(esi)理论计算c13h9f2n2[mh+]:231.0728。实际测定:231.0730。理论分析计算c13h8f2n2:c,67.82;h,3.50;n,12.17。实际测定:c,67.64;h,3.69,n,12.10。4-((3,5-二氟吡啶-4-基)乙炔基)-n-甲基苯胺(7c).收率54%;用1:5乙酸乙酯-己烷的制备层色谱法(rf=0.26);mp149-151℃。1hnmr(400mhz,dmso-d6)δ8.60(s,2h),7.35(d,j=8.6hz,2h),6.58(d,j=8.6hz,2h),6.49(q,j=5.0hz,1h),2.72(d,j=5.0hz,3h)。13cnmr(101mhz,dmso-d6)δ157.30(d,j=262.2hz,2c),151.50,134.17(dd,j=21.6,4.9hz,2c),133.41(2c),111.54(2c),109.80(t,j=16.6hz),106.66(t,j=2.8hz),105.34,72.10,29.16。hrms(esi)理论计算c14h11f2n2[mh+]:245.0885。实际测定:245.0885。理论分析计算c14h10f2n2:c,68.85;h,4.13;n,11.47。实际测定:c,68.64;h,4.40,n,11.27。4-((3,5-二氟吡啶-4-基)乙炔基)-n,n-二甲基苯胺(7d).收率78%;用1:5乙酸乙酯-己烷的制备层色谱法(rf=0.47);mp139-140℃。1hnmr(400mhz,dmso-d6)δ8.60(s,2h),7.43(d,j=8.5hz,2h),6.74(d,j=8.6hz,2h),2.99(s,6h)。13cnmr(101mhz,dmso-d6)δ157.32(d,j=262.4hz,2c),151.14,134.21(dd,j=21.6,4.8hz,2c),133.21(2c),111.80(2c),109.67(t,j=16.7hz),106.17(t,j=2.9hz),105.72,72.48,39.59(2c)。hrms(esi)理论计算c15h13f2n2[mh+]:259.1041。实际测定:259.1041。理论分析计算c15h12f2n2:c,69.76;h,4.68;n,10.85。实际测定:c,69.50;h,4.78,n,10.78。细胞增殖试验.ls174t细胞在补充有5%胎牛血清和1%青霉素/链霉素的rpmi培养基(mediatech)中生长。对于细胞增殖试验,用dmso或抑制剂处理在12孔板中生长的3x104个细胞/孔。4天后通过vi-cellcellviabilityanalyzer分析细胞数目和存活力。用graphpadprim5计算ic50值。蛋白质印迹.如前所述进行蛋白质印迹。使用以下抗体:抗c-myc(epitomics,1472-1),抗-p21wif1/cip1(cellsignaling,2947),抗-β-微管蛋白(dshb,e7)。在本发明中仅示出和描述了本发明的优选实施方式及其多功能性的实施例。应该理解的是,本发明能够用于各种其他组合和环境,并且能够在本文所表达的本发明构思的范围内进行改变或修改。因此,例如本领域技术人员将认识到或能够确认,使用不超过常规实验,本文所述的特定物质、程序和布置的许多等同方式。这样的等同方式被认为是在本发明的范围内,并且由以下权利要求覆盖。当前第1页12
相关技术
网友询问留言
已有0条留言
- 还没有人留言评论。精彩留言会获得点赞!
1