二氢香豆素骨架拼接硫代吡咯啉酮螺环氧化吲哚化合物及其制备方法及应用与流程
本发明涉及化学技术领域,尤其是一种二氢香豆素骨架拼接硫代吡咯啉酮螺环氧化吲哚化合物及其制备方法及应用。
背景技术:
把具有生物活性基团拼接到具有活性分子骨架中在有机化学和医药化学中是极其重要的研究领域。(1)多官能团氧化吲哚广泛存在天然产物和合成药物分子中,其中,尤其3-吡咯螺环氧化吲哚因为具有广泛的生物活性,吸引了许多化学工作者及医药化学团队的广泛关注,例如,天然产物3-吡咯螺环氧化吲哚类化合物pteropodine和alstonisine表现明显的生物活性。(2)多取代香豆素也普遍存在天然产物和药物分子中。例如:天然产物分子cinchonain-lb,splitomicin和fiduxosin(abt-980)共享一个香豆素分子单元,这些化合物在解除病痛、经济发展中起着重大作用。鉴于3-吡咯螺环氧化吲哚骨架具有潜在的生物活性,多取代二氢香豆素属于潜在的生物活性骨架。因此,把多取代二氢香豆素拼接到3-吡咯螺环氧化吲哚骨架,合成一系列新的潜在多活性官能团的氧化吲哚衍生物,可以为生物活性筛选提供化合物源,对药物的筛选和制药行业具有重要的应用价值(如附图8所示)。
技术实现要素:
本发明的目的是:提供一种二氢香豆素骨架拼接硫代吡咯啉酮螺环氧化吲哚化合物及其制备方法与应用,它是一类重要的医药中间体类似物和药物分子类似物,对药物筛选和制药行业具有重要的应用价值,且其合成方法非常经济简便。
本发明还发现该类化合物在制备防治肿瘤疾病药物中的应用。
本发明是这样实现的:二氢香豆素骨架拼接硫代吡咯啉酮螺环氧化吲哚化合物,该化合物具有如下通式(ⅰ)的结构:
式中,r1为甲基或乙基或苯基或苄基;r2为氢或氟或甲基;r3为二乙胺基或溴或甲氧基或氢;r4为酯基或氢或腈基。
二氢香豆素骨架拼接硫代吡咯啉酮螺环氧化吲哚化合物的制备方法,将各种取代的3-ncs氧化吲哚、3-吸电子基团取代的香豆素,按摩尔比为1:1的比例在有机溶剂中无催化剂条件下,进行1,3-偶极子3+2环加成反应,获得二氢香豆素骨架拼接硫代吡咯啉酮螺环氧化吲哚化合物。
合成路线如下:
其中合成路线中的化合物,其取代基满足r1为甲基或乙基或苯基或苄基;r2为氢或氟或甲基;r3为二乙胺基或溴或甲氧基或氢;r4为酯基或氢或腈基。
所述的有机溶剂为乙腈、苯、甲苯、二甲苯、三甲苯、氯仿、或二氯甲烷。
各种取代的靛红、(e)-硝基异恶唑烯烃化合物与脯氨酸或硫代脯氨酸或肌氨酸,在有机溶剂中的反应温度为室温,反应时间为5-30分钟。
二氢香豆素骨架拼接硫代吡咯啉酮螺环氧化吲哚化合物在制备防治肿瘤疾病药物中的应用。
通过采用上述技术方案,以各种取代的3-ncs氧化吲哚、3-吸电子基团取代的香豆素,按摩尔比为1:1的比例在有机溶剂中无催化剂条件下,进行1,3-偶极子3+2环加成反应,获得二氢香豆素骨架拼接硫代吡咯啉酮螺环氧化吲哚化合物,该类化合物包含潜在的生物活性香豆素骨架和螺环氧化吲哚骨架,可以为生物活性筛选提供化合物源,对药物的筛选和制药行业具有重要的应用价值。且该化合物对三种肿瘤细胞株如人前列腺(pc-3),人肺癌细胞(a549)以及人白血病细胞(k562)具有抑制活性的作用。本发明操作简单易行,原料合成便宜易得,可以在各种有机溶剂中进行,也具有较好的空气稳定性,适用性广,对于各种取代基都有很好的兼容性。
附图说明
附图1及附图2为本发明的实施例的化合物3a谱图数据;
附图3及附图4为本发明的实施例的化合物3b谱图数据;
附图5及附图6为本发明的实施例的化合物3c谱图数据。
附图7为本发明的实施例1的化合物3b-1单晶图;
附图8为本发明的设计构思图。
具体实施方式
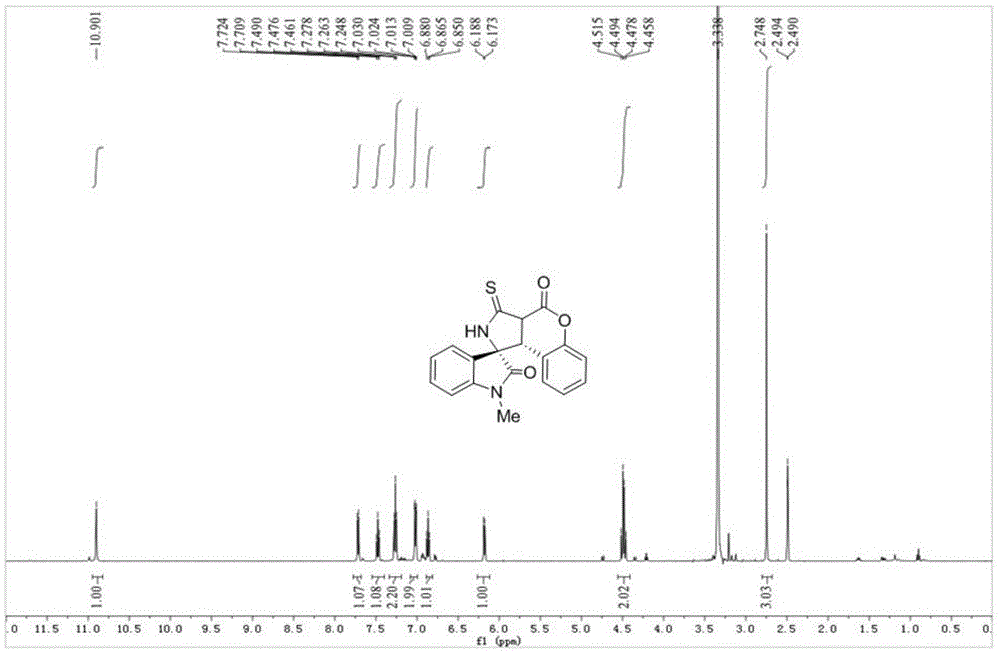
本发明的实施例:在反应管中依次加入40.8mgn-甲基-3-ncs氧化吲哚1a(0.2mmol),38.0mg3-羧酸香豆素2a(0.22mmol)和2.0mlcnch3溶液,室温反应15分钟,tlc检测基本反应完全,直接上样经柱层析(洗脱剂:v(石油醚):v(乙酸乙酯)=4:1)纯化得63.1mg化合物3a,淡黄色固体,熔点:220.3-221.9℃,dr:10:1;产率90.0%。核磁共振和高分辨质谱测试等结果如下:1hnmr(dmso-d6,400mhz)δ:2.75(s,3h),4.46-4.52(m,2h),6.18(d,j=7.0hz,1h),6.85-6.88(m,1h),7.01-7.03(m,2h),7.25-7.28(m,2h),7.46-7.49(m,1h),7.71(d,j=7.5hz,1h),10.90(brs,1h);13cnmr(dmso-d6,100mhz)δ:26.3,47.4,54.0,74.6,109.7,116.2,117.1,124.0,124.3,125.1,125.6,127.5,130.2,131.4,144.3,151.4,160.6,173.6,199.7,218.2;hrms(esi-tof)m/z:calcd.forc19h14n2nao3s[m+na]+:373.0617;found:373.0621.
化合物3b至3p-1的制备方法同化合物3a,投料比与化合物3a相同,可得到化合物化合物3b至3p-1,反应产率和dr值见表1和表2,但需强调的是本发明的化合物不限于表1所表示的内容。
表1为一种二氢香豆素骨架拼接硫代吡咯啉酮螺环氧化吲哚化合物的化学结构
表2为一种二氢香豆素骨架拼接硫代吡咯啉酮螺环氧化吲哚化合物的化学结构
本实施例制备化合物3b:淡黄色固体;熔点:134.2-135.1℃;产率:83%,19:1dr;核磁共振和高分辨质谱测试等结果如下:1hnmr(dmso-d6,400mhz)δ:0.42-0.45(m,3h),2.42-2.50(m,2h),4.39(d,j=10.0hz,1h),4.46(d,j=10.0hz,1h),6.12(d,j=7.6hz,1h),6.80-6.84(m,1h),6.98-7.03(m,2h),7.20-7.26(m,2h),7.41-7.45(m,1h),7.68(d,j=7.2hz,1h);13cnmr(dmso-d6,100mhz)δ:12.1,34.4,48.1,54.0,74.7,109.7,116.2,117.1,123.9,124.3,125.3,125.8,127.7,130.3,131.5,143.3,151.5,160.6,173.3,199.9;hrms(esi-tof)m/z:calcd.forc20h16n2nao3s[m+na]+:387.0774;found:387.0776.
本实施例制备化合物3c:淡黄色固体;熔点:140.5-141.9℃;产率:87%,19:1dr;核磁共振和高分辨质谱测试等结果如下:1hnmr(dmso-d6,500mhz)δ:4.53-4.59(m,2h),6.28(d,j=7.0hz,1h),6.65-6.69(m,3h),6.94-6.97(m,1h),7.08(d,j=8.0hz,1h),7.31-7.44(m,6h),7.81(d,j=7.0hz,1h),10.99(brs,1h);13cnmr(dmso-d6,125mhz)δ:48.1,53.4,74.5,109.5,115.7,116.8,125.1,125.2,125.9,128.6,129.8,132.9,143.4,151.0,159.9,173.1,199.6;hrms(esi-tof)m/z:calcd.forc24h16n2nao3s[m+na]+:435.0774;found:435.0778.
本实施例制备化合物3d:淡黄色固体;熔点:179.6-180.1℃;产率:yield81%,10:1dr;核磁共振和高分辨质谱测试等结果如下:1hnmr(dmso-d6,400mhz)δ:2.80(s,3h),3.70(s,3h),4.41(d,j=6.8hz,1h),4.47(d,j=6.8hz,1h),6.10(d,j=5.6hz,1h),6.47-6.49(m,1h),6.64(s,1h),7.03(d,j=5.2hz,1h),7.25-.28(m,1h),7.47-7.50(m,1h),7.71(d,j=5.2hz,1h),10.88(brs,1h);13cnmr(dmso-d6,100mhz)δ:25.9,46.6,53.4,55.5,74.1,101.7,107.4,109.2,110.4,123.5,124.6,125.3,127.8,130.9,143.8,151.9,160.2,173.2,199.3;hrms(esi-tof)m/z:calcd.forc20h16n2nao4s[m+na]+:403.0723;found:403.0718.
本实施例制备化合物3e:淡黄色固体;熔点:166.5-167.4℃;产率:yield77%,13:1dr;核磁共振和高分辨质谱测试等结果如下:1hnmr(dmso-d6,400mhz)δ:2.79(s,3h),4.48-4.52(m,2h),6.25(s,1h),6.99-7.08(m,2h),7.27-7.29(m,1h),7.44-7.47(m,1h),7.49-7.52(m,1h),7.71(d,j=4.8hz,1h),10.95(brs,1h);13cnmr(dmso-d6,100mhz)δ:26.4,47.0,53.5,74.6,109.7,115.4,118.8,119.4,124.2,125.1,125.2,129.9,131.6,133.0,144.3,150.8,160.1,173.5,199.4;hrms(esi-tof)m/z:calcd.forc19h13brn2nao3s[m+na]+:450.9722;found:450.9729.
本实施例制备化合物3f:淡黄色固体;熔点:168.3-168.7℃;产率:yield80%,12:1dr;核磁共振和高分辨质谱测试等结果如下:1hnmr(dmso-d6,400mhz)δ:3.27-3.31(m,1h),3.42-3.46(m,1h),3.67(s,3h),4.34(d,j=6.8hz,1h),4.45(d,j=6.8hz,1h),6.05(d,j=5.6hz,1h),6.44-6.47(m,1h),6.62(s,1h),7.04(d,j=5.2hz,1h),7.22-7.25(m,1h),7.43-7.46(m,1h),7.67(d,j=4.8hz,1h),10.89(brs,1h);13cnmr(dmso-d6,100mhz)δ:12.1,34.4,47.7,53.9,56.0,74.6,102.1,107.8,109.6,110.9,123.8,125.2,125.8,128.3,131.3,143.2,152.4,160.6,160.8,173.4,199.9;hrms(esi-tof)m/z:calcd.forc21h18n2nao4s[m+na]+:417.0879;found:417.0887.
本实施例制备化合物3g:淡黄色固体;熔点:210.1-211.1℃;产率:yield75%,15:1dr;核磁共振和高分辨质谱测试等结果如下:1hnmr(dmso-d6,400mhz)δ:3.30-3.34(m,1h),3.47-3.53(m,1h),4.47(d,j=6.8hz,1h),4.53(d,j=6.8hz,1h),6.21(s,1h),7.05(d,j=6.0hz,1h),7.13(d,j=5.2hz,1h),7.28-7.30(m,1h),7.46-7.48(m,1h),7.50-7.53(m,1h),7.71(d,j=4.8hz,1h),10.99(brs,1h);13cnmr(dmso-d6,100mhz)δ:11.8,34.4,47.6,53.4,74.6,109.7,115.7,118.8,119.3,124.1,125.3,125.4,130.0,131.6,132.9,143.0,150.8,160.0,173.2,199.5;hrms(esi-tof)m/z:calcd.forc20h15brn2nao3s[m+na]+:464.9879;found:464.9884.
本实施例制备化合物3h:淡黄色固体;熔点:157.5-158.3℃;产率:yield76%,12:1dr;核磁共振和高分辨质谱测试等结果如下:1hnmr(dmso-d6,400mhz)δ:3.67(s,3h),4.41(d,j=10.0hz,1h),4.50(d,j=10.0hz,1h),6.13(d,j=8.4hz,1h),6.50-6.53(m,1h),6.63-6.70(m,4h),7.26-7.29(m,1h),7.32-7.42(m,4h),7.73-7.79(m,1h),10.93(brs,1h);13cnmr(dmso-d6,100mhz)δ:48.3,53.9,56.2,74.9,102.4,107.8,109.9,111.0,124.7,125.5,125.7,126.4,129.0,130.2,131.4,143.9,152.4,160.4,161.0,173.6,200.1;hrms(esi-tof)m/z:calcd.forc25h18n2nao4s[m+na]+:465.0879;found:465.0884.
本实施例制备化合物3ee:淡黄色固体;熔点:233.4-233.8℃;产率:yield72%,5:1dr;核磁共振和高分辨质谱测试等结果如下:1hnmr(dmso-d6,400mhz)δ:4.56-4.61(m,2h),6.35(s,1h),6.73-6.74(m,2h),7.06(d,j=6.0hz,1h),7.34-7.37(m,1h),7.39-7.42(m,2h),7.45-7.48(m,3h),7.52-7.55(m,1h),7.80(d,j=4.8hz,1h),11.03(brs,1h);13cnmr(dmso-d6,100mhz)δ:48.0,53.4,75.0,109.9,115.6,118.8,119.6,124.9,125.1,125.8,126.2,126.8,129.2,130.0,130.4,130.6,131.7,133.1,133.3,143.8,150.8,159.9,173.4,199.7;hrms(esi-tof)m/z:calcd.forc24h15brn2nao3s[m+na]+:512.9879;found:512.9886.
本实施例制备化合物3j:淡黄色固体;熔点:185.4-186.3℃;产率:yield77%,15:1dr;核磁共振和高分辨质谱测试等结果如下:1hnmr(dmso-d6,400mhz)δ:3.74(s,3h),4.44(d,j=5.6hz,1h),4.49(s,2h),4.81(d,j=10.4hz,1h),6.14(d,j=5.6hz,1h),6.46-6.48(m,1h),6.61(d,j=5.2hz,2h),6.69(s,1h),6.80(d,j=5.2hz,1h),7.06-7.09(m,2h),7.15-7.18(m,1h),7.20-7.23(m,1h),7.33-7.36(m,1h),7.74(d,j=5.2hz,1h),10.95(brs,1h);13cnmr(dmso-d6,100mhz)δ:43.2,47.1,54.0,56.0,74.4,102.3,107.8,110.2,111.3,124.1,125.4,127.1,127.7,128.7,128.8,131.3,135.6,152.6,160.7,161.0,174.1,199.8;hrms(esi-tof)m/z:calcd.forc26h20n2nao4s[m+na]+:479.1036;found:479.1037.
本实施例制备化合物3k:淡黄色固体;熔点:197.5-198.1℃;产率:yield81%,16:1dr;核磁共振和高分辨质谱测试等结果如下:1hnmr(dmso-d6,400mhz)δ:4.48(d,j=16.0hz,1h),4.56(d,j=10.0hz,1h),4.63(d,j=10.4hz,1h),4.84(d,j=16.0hz,1h),6.35(d,j=2.4hz,1h),6.61-6.63(m,2h),6.86(d,j=8.0hz,1h),7.09(d,j=8.8hz,1h),7.15-7.21(m,3h),7.25-7.29(m,1h),7.40-7.44(m,1h),7.57-7.60(m,1h),7.77-7.79(m,1h),11.04(brs,1h);13cnmr(dmso-d6,100mhz)δ:43.4,46.9,53.7,74.5,110.4,116.1,118.8,119.6,124.3,125.1,125.7,126.8,127.8,129.1,130.5,131.7,133.4,135.6,143.5,151.0,160.3,173.9,199.4;hrms(esi-tof)m/z:calcd.forc25h17brn2nao3s[m+na]+:527.0035;found:527.0031.
本实施例制备化合物3l:淡黄色固体;熔点:150.3-151.9℃;产率:yield78%,17:1;核磁共振和高分辨质谱测试等结果如下:1hnmr(dmso-d6,500mhz)δ:2.38(s,3h),2.72(s,3h),4.42(d,j=8.0hz,1h),4.50(d,j=10.0hz,1h),6.21(d,j=7.5hz,1h),6.86-6.91(m,2h),7.02(d,j=13.0hz,1h),7.24-7.28(m,2h),7.54(s,1h),10.88(brs,1h);13cnmr(dmso-d6,125mhz)δ:21.2,26.3,47.5,54.0,74.7,109.4,116.2,117.0,124.3,125.6,127.6,130.2,131.5,133.2,141.9,151.4,160.6,173.5,199.6;hrms(esi-tof)m/z:calcd.forc20h16n2nao3s[m+na]+:387.0774;found:387.0779.
本实施例制备化合物3a-1:黄色固体;熔点:228.5-230.7℃;产率:yield90%,6:1dr;核磁共振和高分辨质谱测试等结果如下:1hnmr(dmso-d6,400mhz)δ:1.10-1.14(m,3h),2.75(s,3h),4.15-4.18(m,2h),4.60(s,1h),6.27-6.29(m,1h),6.87-6.93(m,1h),7.03(d,j=7.6hz,1h),7.09(d,j=7.6hz,1h),7.26-7.32(m,2h),7.47-7.51(m,1h),7.64(d,j=7.2hz,1h),11.32(brs,1h);13cnmr(dmso-d6,100mhz)δ:14.3,26.6,52.6,63.9,67.3,73.8,110.0,114.3,117.4,124.5,124.7,124.9,128.0,131.2,132.0,144.3,150.9,158.1,166.4,172.9,196.9;hrms(esi-tof)m/z:calcd.forc22h18n2nao5s[m+na]+:445.0829;found:445.0834.
本实施例制备化合物3b-1:黄色固体;熔点:190.2-191.7℃;产率:yield89%,16:1dr;核磁共振和高分辨质谱测试等结果如下:1hnmr(dmso-d6,500mhz)δ:0.46-0.49(m,3h),1.13-1.19(m,4h),3.27-3.30(m,1h),3.41-3.46(m,1h),4.16-4.24(m,2h),4.58(s,1h),6.27(d,j=7.0hz,1h),6.89-6.94(m,1h),7.09-7.13(m,2h),7.28-7.34(m,2h),7.49-7.52(m,1h),7.65(d,j=7.0hz,1h),11.35(brs,1h);13cnmr(dmso-d6,125mhz)δ:12.0,14.2,34.5,53.1,63.8,67.2,73.7,109.9,114.1,117.2,124.2,124.8,125.1,128.0,131.1,131.9,143.1,150.9,157.9,166.3,172.5,197.0;hrms(esi-tof)m/z:calcd.forc23h20n2nao5s[m+na]+:459.0985;found:459.0987.
本实施例制备化合物3c-1:黄色固体;熔点:150.2-152.1℃;产率:yield80%,20:1dr;核磁共振和高分辨质谱测试等结果如下:1hnmr(dmso-d6,500mhz)δ:1.15-1.19(m,3h),4.20-4.24(m,2h),4.70(s,1h),6.39-6.41(m,1h),6.67-6.72(m,3h),6.99-7.03(m,1h),7.17(d,j=8.0hz,1h),7.36-7.49(m,7h),7.75(d,j=6.0hz,1h),11.43(brs,1h);13cnmr(dmso-d6,125mhz)δ:14.2,53.5,63.9,67.2,74.0,110.2,114.1,117.4,124.6,126.3,128.1,129.2,130.3,132.0,133.1,143.8,150.9,157.8,166.2,172.8,197.1;hrms(esi-tof)m/z:calcd.forc27h20n2nao5s[m+na]+:507.0985;found:507.0987.
本实施例制备化合物3d-1:黄色固体;熔点:234.5-236.1℃;产率:yield90%,20:1dr;核磁共振和高分辨质谱测试等结果如下:1hnmr(dmso-d6,500mhz)δ:1.14-1.17(m,3h),4.19-4.22(m,2h),4.46(d,j=16.0hz,1h),4.71(s,1h),4.79(d,j=16.0hz,1h),6.38(d,j=7.5hz,1h),6.56(d,j=7.5hz,2h),6.83(d,j=8.0hz,1h),6.94-6.97(m,1h),7.08-7.11(m,2h),7.14-7.19(m,2h),7.25-7.29(m,1h),7.38-7.41(m,1h),7.44-7.47(m,1h),7.70(d,j=7.5hz,1h),11.41(brs,1h);13cnmr(dmso-d6,125mhz)δ:13.7,42.8,52.0,63.4,66.9,73.1,110.1,113.7,117.0,124.0,124.1,124.8,126.5,127.2,128.1,128.5,130.9,131.4,134.9,142.9,150.5,157.6,165.8,172.7,196.3;hrms(esi-tof)m/z:calcd.forc28h22n2nao5s[m+na]+:521.1142;found:521.1146.
本实施例制备化合物3e-1:黄色固体;熔点:260.4-261.3℃;产率:yield85%,12:1dr;核磁共振和高分辨质谱测试等结果如下:1hnmr(dmso-d6,400mhz)δ:1.15-1.18(m,3h),2.81(s,3h),4.16-4.23(m,2h),4.70(s,1h),6.43(s,1h),7.09-7.13(m,2h),7.30-7.33(m,1h),7.50-7.56(m,2h),7.65(d,j=7.5hz,1h),11.36(brs,1h);13cnmr(dmso-d6,100mhz)δ:13.7,26.1,51.2,63.4,66.4,73.3,109.5,115.6,116.3,119.1,123.7,124.0,124.8,130.1,131.6,133.4,143.8,149.7,157.1,165.6,172.4,196.0;hrms(esi-tof)m/z:calcd.forc22h17brn2nao5s[m+na]+:522.9934;found:522.9939.
本实施例制备化合物3f-1:黄色固体;熔点:168.2-170.1℃;产率:yield86%,11:1dr;核磁共振和高分辨质谱测试等结果如下:1hnmr(dmso-d6,500mhz)δ:1.13-1.16(m,3h),2.41(s,3h),2.75(s,3h),4.16-4.24(m,2h),4.61(s,1h),6.34(d,j=7.5hz,1h),6.91-6.95(m,2h),7.11(d,j=8.0hz,1h),7.31-7.34(m,2h),7.50(s,1h),11.31(brs,1h);13cnmr(dmso-d6,125mhz)δ:13.7,20.6,26.0,52.1,63.3,66.8,73.3,109.2,113.8,116.8,124.2,127.6,130.6,131.6,133.3,141.4,150.4,157.6,165.9,172.3,196.3;hrms(esi-tof)m/z:calcd.forc23h20n2nao5s[m+na]+:459.0991;found:459.0994.
本实施例制备化合物3g-1:黄色固体;熔点:132.4-135.0℃;产率:yield87%,9:1dr;核磁共振和高分辨质谱测试等结果如下:1hnmr(dmso-d6,500mhz)δ:1.15-1.18(m,3h),2.41(s,3h),2.79(s,3h),3.33(s,3h),4.18-4.21(m,2h),4.68(s,1h),6.47(s,1h),6.98(d,j=8.0hz,1h),7.11(d,j=8.5hz,1h),7.34(d,j=8.0hz,1h),7.48(s,1h),7.50-7.52(m,1h),11.34(brs,1h);13cnmr(dmso-d6,125mhz)δ:13.8,20.7,26.1,51.2,63.4,66.4,73.4,109.2,115.6,116.4,119.1,123.7,125.3,130.1,131.7,133.3,141.4,149.7,157.2,165.6,172.2,196.0;hrms(esi-tof)m/z:calcd.forc23h19brn2nao5s[m+na]+:537.0090;found:537.0093.
本实施例制备化合物3h-1:黄色固体;熔点:127.5-128.4℃;产率:yield64%,12:1dr;核磁共振和高分辨质谱测试等结果如下:1hnmr(dmso-d6,500mhz)δ:1.19-1.21(m,3h),2.85(s,3h),4.19-4.26(m,2h),4.81(s,1h),6.63(s,1h),7.16(d,j=8.5hz,2h),7.43-7.47(m,1h),7.56(d,j=8.5hz,1h),7.63(d,j=8.0hz,1h),11.40(brs,1h);13cnmr(dmso-d6,125mhz)δ:14.1,26.7,51.2,63.8,66.7,73.8,109.9,110.9,113.4(d,jcf=24.8hz),116.1,116.6,117.2(d,jcf=25.4hz),119.5,125.8,130.7,133.8,140.3,150.1,157.5,159.5(d,jcf=236.8hz),165.9,172.7,196.3;hrms(esi-tof)m/z:calcd.forc22h16brfn2nao5s[m+na]+:540.9840;found:540.9844.
本实施例制备化合物3i-1:黄色固体;熔点:145.5-146.1℃;产率:yield79%,12:1dr;核磁共振和高分辨质谱测试等结果如下:1hnmr(dmso-d6,500mhz)δ:0.77-0.80(m,3h),0.95-1.01(m,6h),1.13-1.18(m,6h),1.49-1.51(m,2h),2.83(s,3h),3.21-3.26(m,4h),4.08-4.12(m,1h),4.16-4.21(m,1h),4.48(s,1h),6.15(d,j=8.5hz,1h),6.20-6.25(m,2h),7.06-7.08(m,1h),7.33-7.37(m,1h),7.53-7.56(m,1h),11.20(brs,1h);13cnmr(dmso-d6,125mhz)δ:12.7,14.3,22.4,25.1,26.8,28.2,31.1,44.0,52.8,67.2,67.8,73.7,98.6,98.9,108.1,110.9,111.0(d,jcf=8.8hz),112.9(d,jcf=25.0hz),117.9(d,jcf=21.3hz),126.8,126.9,128.6,140.5,149.6,152.2,158.6,158.7,161.8(d,jcf=203.8hz),165.5,166.5,173.0,197.3;hrms(esi-tof)m/z:calcd.forc30h34fn3nao5s[m+na]+:590.2095;found:590.2097.
本实施例制备化合物3j-1:黄色固体;熔点:171.5-173.4℃;产率:yield81%,15:1dr;核磁共振和高分辨质谱测试等结果如下:1hnmr(dmso-d6,500mhz)δ:1.19-1.24(m,3h),4.23-4.29(m,2h),4.82(s,1h),6.56(s,1h),6.77-6.81(m,3h),7.19(d,j=8.5hz,1h),7.43-7.47(m,2h),7.50-7.55(m,3h),7.62-7.64(m,1h),7.77(d,j=7.5hz,1h),11.48(brs,1h);13cnmr(dmso-d6,125mhz)δ:14.2,52.6,64.0,66.7,74.1,110.1,116.2,116.8,119.7,124.1,125.2,125.7,126.2,129.3,130.4,130.6,132.1,133.1,133.9,143.8,150.1,157.4,166.0,172.7,196.8;hrms(esi-tof)m/z:calcd.forc27h19brn2nao5s[m+na]+:585.0090;found:585.0097.
本实施例制备化合物3k-1:黄色固体;熔点:193.2-194.5℃;产率:yield72%,5:1dr;核磁共振和高分辨质谱测试等结果如下:1hnmr(dmso-d6,500mhz)δ:0.48-0.51(m,3h),1.14-1.21(m,3h),4.16-4.24(m,4h),4.65(s,1h),6.35(s,1h),7.11-7.15(m,2h),7.29-7.33(m,1h),7.51-7.55(m,2h),7.63(d,j=7.5hz,1h),11.39(brs,1h);13cnmr(dmso-d6,125mhz)δ:11.3,13.7,34.1,51.8,63.5,66.3,73.3,109.5,115.8,116.3,119.1,123.9,124.9,130.1,131.6,133.2,142.5,149.7,157.0,165.7,172.0,196.3;hrms(esi-tof)m/z:calcd.forc23h19brn2nao5s[m+na]+:537.0090;found:537.0086.
本实施例制备化合物3l-1:黄色固体;熔点:240.1-241.5℃;产率:yield82%,17:1dr;核磁共振和高分辨质谱测试等结果如下:1hnmr(dmso-d6,500mhz)δ:0.51-0.54(m,3h),0.76-0.79(m,3h),0.95-0.98(m,6h),1.13-1.18(m,6h),1.49-1.52(m,2h),3.19-3.27(m,4h),3.29-3.34(m,1h),3.45-3.51(m,1h),4.08-4.13(m,1h),4.16-4.21(m,1h),5.97(d,j=9.0hz,1h),6.16-6.19(m,1h),6.25(s,1h),7.07(d,j=7.5hz,1h),7.24-7.27(m,1h),7.46-7.49(m,1h),7.55(d,j=7.5hz,1h),11.24(brs,1h);13cnmr(dmso-d6,125mhz)δ:11.9,12.6,14.3,22.4,25.1,28.2,31.1,34.5,44.1,53.9,67.2,67.7,73.7,98.6,98.8,108.2,109.8,124.0,124.6,125.4,128.3,131.6,143.1,149.4,152.2,158.6,166.7,172.7,197.6,217.9;hrms(esi-tof)m/z:calcd.forc31h37n3nao5s[m+na]+:586.2346;found:586.2351.
本实施例制备化合物3m-1:黄色固体;熔点:138.2-140.1℃;产率:yield76%,>20:1dr;核磁共振和高分辨质谱测试等结果如下:1hnmr(dmso-d6,500mhz)δ:0.79(s,3h),1.00-1.03(m,3h),1.12-1.20(m,6h),1.52-1.55(m,2h),3.26-3.31(m,4h),4.11-4.23(m,2h),4.46(s,1h),6.09(d,j=9.0hz,1h),6.28-6.32(m,2h),6.68-6.71(m,3h),7.32-7.45(m,5h),7.65(d,j=7.5hz,1h),11.32(brs,1h);13cnmr(dmso-d6,125mhz)δ:12.6,14.3,22.4,25.2,28.2,31.1,44.3,54.5,67.3,67.6,74.2,110.1,124.9,125.0,125.2,126.7,129.2,130.1,131.7,133.4,143.8,152.3,158.4,166.6,173.1,197.8;hrms(esi-tof)m/z:calcd.forc35h37n3nao5s[m+na]+:634.2346;found:634.2342.
本实施例制备化合物3n-1:黄色固体;熔点:187.7-189.2℃;产率:yield81%,20:1dr;核磁共振和高分辨质谱测试等结果如下:1hnmr(dmso-d6,500mhz)δ:1.16-1.18(m,3h),4.19-4.22(m,2h),4.49(d,j=16.0hz,1h),4.79-4.85(m,2h),6.50(s,1h),6.60(d,j=7.0hz,2h),6.89(d,j=8.0hz,1h),7.15-7.20(m,4h),7.28-7.31(m,1h),7.42-7.45(m,1h),7.62(d,j=6.5hz,1h),7.68(d,j=7.5hz,1h),11.43(brs,1h);13cnmr(dmso-d6,125mhz)δ:13.7,42.9,51.1,63.5,66.5,73.1,110.1,116.2,119.2,123.6,124.0,125.1,126.3,127.3,128.6,130.5,131.5,133.7,134.9,142.9,149.8,157.2,165.6,172.7,196.0,217.6;hrms(esi-tof)m/z:calcd.forc28h21brn2nao5s[m+na]+:599.0247;found:599.0249.
本实施例制备化合物3o-1:黄色固体;熔点:128.5-130.4℃;产率:yield92%,10:1dr;核磁共振和高分辨质谱测试等结果如下:1hnmr(dmso-d6,400mhz)δ:2.78(s,3h),5.28(s,1h),6.38(d,j=7.0hz,1h),7.02-7.05(m,2h),7.24(d,j=6.8hz,1h),7.29-7.32(m,1h),7.40-7.43(m,1h),7.50-7.53(m,1h),7.98(d,j=7.0hz,1h),11.65(brs,1h);13cnmr(dmso-d6,100mhz)δ:26.6,50.9,56.1,74.1,110.0,113.5,114.9,117.7,123.8,124.4,125.8,126.2,128.3,131.6,132.1,144.2,150.4,154.8,172.9,191.6;hrms(esi-tof)m/z:calcd.forc20h13n3nao3s[m+na]+:398.0575;found:398.0579.
本实施例制备化合物3p-1:黄色固体;熔点:149.2-151.2℃;产率:yield82%,15:1dr;核磁共振和高分辨质谱测试等结果如下:1hnmr(dmso-d6,500mhz)δ:2.43(s,3h),2.79(s,3h),5.29(s,1h),6.44-6.46(m,1h),6.97(d,j=8.0hz,1h),7.07-7.10(m,1h),7.26-7.28(m,1h),7.34-7.36(m,1h),7.44-7.47(m,1h),7.87(s,1h),11.65(brs,1h);13cnmr(dmso-d6,125mhz)δ:21.2,26.6,50.9,56.1,74.1,109.7,113.6,114.9,117.3,117.6,123.8,125.8,125.9,126.8,128.4,130.4,131.5,132.2,133.6,135.9,141.9,150.4,153.9,154.8,172.7,191.6,218.0;hrms(esi-tof)m/z:calcd.forc21h15n3nao3s[m+na]+:412.0726;found:412.0727.
本发明的式(1)化合物具有重要的生物活性,体外对人白血病细胞(k562)的细胞毒性试验表明:此类式(1)所示的结构的二氢香豆素骨架拼接硫代吡咯啉酮螺环氧化吲哚化合物对肿瘤细胞生长具有抑制作用,有可能发展成为新的防治肿瘤药物。但需强调的是本发明的化合物不限于人白血病细胞(k562)表示的细胞毒性。
药理实施例:化合物3i-1,3e-1,3l-1,3m-1和3o-1对k562细胞的细胞毒性
k562(人慢性髓系白血病细胞)用rpmi-1640培养基培养,培养基中含10%的胎牛血清,100u/ml的青霉素和100u/ml链霉素。细胞以每孔5000个细胞的浓度加入到96孔中,在37℃含5%co2潮湿空气的培养箱中培养24小时。
细胞存活率的测定用改良mtt法。细胞经过24小时的孵育后,分别将新配的化合物3i-1,3e-1,3l-1,3m-1和3o-1的二甲基亚砜溶液以浓度梯度加入到各孔中,使孔中化合物最终浓度分别为5μmol/l,10μmol/l,20μmol/l,40μmol/l和80μmol/l。48小时后,每孔加入10μlmtt(5mg/ml)的磷酸盐缓冲液,再继续在37℃培养4小时后,离心5分钟除去未转化的mtt,每孔中加入150μl二甲基亚砜。以溶解还原的mtt晶体甲臜(formazan),用酶标仪在490nm波长测定od值。其中化合物3i-1,3e-1,3l-1,3m-1和3o-1对k562细胞半抑制浓度ic50由spss软件(19版本)分析得到。化合物3i-1对k562肿瘤细胞的ic50为35.57μmol/l;化合物3e-1对k562肿瘤细胞的ic50为23.42μmol/l;化合物3l-1对k562肿瘤细胞的ic50为27.73μmol/l;化合物3m-1对k562肿瘤细胞的ic50为41.87μmol/l;化合物3o-1对k562肿瘤细胞的ic50为39.54μmol/l;而阳性对照顺铂对k562肿瘤细胞的ic50为21.01μmol/l。
实验结论:k562细胞是测试化合物对肿瘤细胞的细胞毒性的有效工具和评价指标。本实验表明此类式(1)所示的二氢香豆素骨架拼接硫代吡咯啉酮螺环氧化吲哚化合物对k562细胞具有较强的细胞毒性,和肿瘤治疗一线用药顺铂同一数量级,有可能发展成新的具有抗肿瘤作用的药物。
从以上药理实施例中我们可以看出这些化合物对人白血病细胞(k562)都显示有一定的细胞毒性。可见这些化合物具有开发成为抗肿瘤药物的潜力,值得继续深入研究下去。
- 还没有人留言评论。精彩留言会获得点赞!