本发明涉及有机化学领域,具体涉及一种多羰基官取代手性吡咯烷衍生物及其制备方法。
背景技术
含氮杂环化合物种类繁多,普遍存在于药物分子和天然产物中。其中,吡咯烷是一类重要的五元氮杂环化合物,它具有环张力小、稳定性高等特点。手性吡咯烷单元是许多天然生物碱、药物活性分子的重要组成部分。一些含有吡咯烷结构的药物分子具有抗生素、杀菌剂、添加剂等实用价值,这使得它们在市场上的需求量越来越大,因此,手性吡咯烷化合物的合成引起有机合成工作者的广泛关注。这类化合物最初主要是从生物体内提取分离得到,但随着手性技术的不断发展,越来越多含有吡咯烷结构并且具有药物活性的天然、非天然化合物也被发现。早在1997年shin-ya从正青霉属中分离所得的kaitocephalin是一种用于治疗癫痫和脑缺血的天然桔抗剂,它可以有效地避免kainate毒素损害某些神经元;(+)-conessine是一种止泻木属类抗痢木碱,从抗病木皮中分离得到,这类甾族生物有很重要的生物活性,已经用于痢疾的治疗。这些化合物的共同结构特点是都含有吡咯烷结构单元。
含有手性吡咯烷单元的天然产物和活性分子在医药、农药、食品、化工行业等领域都有着很重要的应用。通常,分子的组成结构决定着其物理、化学及光学等性质,进而影响着它在各种物质中所产生的作用,如生物体中产生的抗病毒性、抗菌性、抑制性等生理活性,日用化学品中产生的润滑剂、防腐剂、添加剂等作用,高分子材料的光敏性、导电性、稳定性、磁性等功能。因此,设计并合成更多高效、实用的手性吡咯烷衍生物具有十分重要的现实意义。
早在二十世纪初期,willststter和robinson吡咯烷就报道了吡咯烷的合成方法。合成吡咯烷的经典方法包括:烷基化反应,缩合反应,琥珀酰亚胺、环状烯胺和吡咯的还原,氮杂cope-mannich串联反应以及其他合成方法等。吡咯烷单元还可以作为手性配体或有机小分子催化剂,立体选择性合成官能化的吡咯烷成为有机合成中的重要部分。大量对映选择性地合成吡咯烷的文献已经被报道,如胺化反应,氢化反应,micheal加成-还原反应,环加成反应等。目前还未见使用查尔酮作为添加剂通过不对称[3+2]环加成反应高效合成手性吡咯烷衍生物及其合成方法的报道。
技术实现要素:
为解决多羰基官取代手性吡咯烷衍生物合成方法不多的问题,本发明提出一种多羰基官取代手性吡咯烷衍生物及其制备方法,合成方法操作简单、反应条件温和,底物普适性强,产物产率高且对映选择性高。
本发明是通过以下技术方案实现的:一种多羰基官取代手性吡咯烷衍生物具有如(i)所示的结构式:
式中,r1、r2分别独立的选自氢、卤代基、烷基、卤代烷基、烷氧基中的一种。
所述的制备方法为在惰性气体保护下,利用金属催化前体与手性膦配体形成的络合物作为催化剂,在添加剂的共同作用下,通过烯酮与甲亚胺叶立德的[3+2]环加成反应来合成,反应式如下所示:
所述的具体反应步骤为:在惰性气体保护下,在容器在内加入甲亚胺叶立德和烯酮类化合物,金属催化前体、手性膦配体和添加剂,在反应介质中反应后,萃取,有机相干燥后蒸除溶剂得到粗产物,粗产物通过分离纯化,得到一种多羰基官取代手性吡咯烷衍生物。
烯酮类化合物与甲亚胺叶立德的摩尔比为1∶1~1∶1.2。作为优选,反应物甲亚胺叶立德和烯酮类化合物的浓度为0.1-1.0mol/l的溶液。甲亚胺叶立德和烯酮类化合物作为原料反应易于合成,反应操作简单,粗产品经过快速柱层析除杂后减压浓缩可得纯品,后处理方便。
本发明通过金属催化前体手性膦配体形成的络合物作为催化剂,金属催化前体选自乙酸银或者醋酸铜与乙酸银任意比的混合物,使用量为烯酮类化合物摩尔量的1~10%。
手性膦配体选用xing-phos、(r)-dtbm-segphos、fei-phos、tao-phos、hznu中一种,使用量为烯酮类化合物摩尔量的1~10%。
作为优选,所述的手性膦配体选自结构式如(ii)所示:
作为优选,所述添加剂选自查尔酮、丙烯酸乙酯、1-苯基-2-硝基丙烯、β-硝基苯乙烯、n-苯基马来酰亚胺、2,6-二氯-ω-硝基苯乙烯,更优选为查尔酮;添加剂可控制产物的立体构型从而得到高对映选择性的目标产物。使用量为烯酮类化合物摩尔量的1~10%。
反应介质选自丙酮、乙酸乙酯、乙腈、氯苯、二噁烷、甲苯、四氢呋喃、二氯甲烷、乙醚中一种,使用量为使溶质充分反应的量。
作为优选,反应温度为-40℃~0℃,反应时间为10~15h后用乙酸乙酯萃取,有机相干燥后蒸除溶剂得到粗产物,粗产物通过硅胶柱层析分离纯化,得到目标产物。
本发明以易于合成的甲亚胺叶立德和烯酮类化合物为原料,经[3+2]环加成反应即可高效合成系列手性吡咯烷衍生物。反应操作简单,粗产品经过快速柱层析除杂后减压浓缩可得纯品,后处理方便。同时通过添加剂来控制产物的立体构型可以得到高对映选择性的目标产物,对传统的[3+2]环加成反应来讲,产生了更多衍生的可能。并且合成的化合物是一类重要的含氮杂环化合物可广泛应用于各种有机合成、医药、材料化学及精细化工领域。
与现有技术相比,本发明的有益效果是:
(1)合成方法操作简单、反应条件温和,底物普适性强,产物产率高且对映选择性高;
(2)合成产物含有多个官能团和多个手性中心,具有潜在的生物活性,可作为精细化工中间体广泛用于各种有机反应及药物合成中,具有可观的应用价值。
附图说明
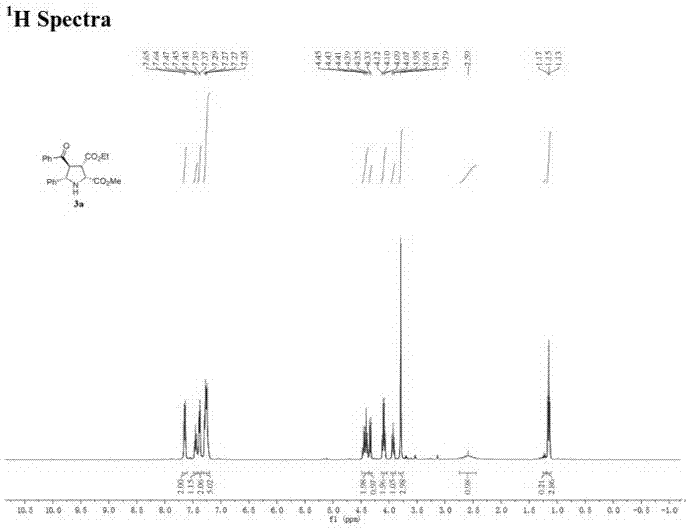
图1为化合物3a结构式的核磁共振氢谱图;
图2为化合物3a结构式的碳谱图;
图3为化合物3b结构式的核磁共振氢谱图;
图4为化合物3b结构式的碳谱图;
具体实施方式
下面结合实施例对本发明作进一步详细说明,实施例中所用原料均可市购或采用常规方法制备。
实施例1:
在氮气气氛下,将(r)-dtbm-segphos(13mg,0.0108mmol,6mol%),agoac(1.6mg,0.0096mmol,5mol%)加入到溶解有查尔酮(3.8mg,0.0182mmol,10mol%)的2ml丙酮溶液中,-40℃下预搅30min后,依次加入亚甲胺叶立德1a(0.2mmol),烯酮类化合物2a(0.182mmol),-40℃下反应12h,tlc监测反应结束后,经硅藻土过滤,萃取,滤液浓缩,经硅胶柱层析纯化,得到无色油状液体3a,产率68%,再经手性高效液相色谱法测得ee值为96%。
该产物的理化指标:1hnmr(400mhz,cdcl3)δ7.65(d,j=7.4hz,2h),7.45(t,j=7.4hz,1h),7.38(d,j=6.7hz,2h),7.27(dd,j=11.3,7.7hz,5h),4.42(dd,j=17.5,8.1hz,2h),4.34(d,j=8.4hz,1h),4.09(q,j=7.1hz,2h),3.93(t,j=7.9hz,1h),3.79(s,3h),2.59(s,1h),1.15(t,j=7.1hz,3h).13cnmr(101mhz,cdcl3)δ199.7,172.3,171.6,140.3,136.6,133.4,128.7(d,j=7.4hz),128.4,128.1,127.1,77.4,77.1,76.8,68.0,62.5,61.4,56.9,52.8,52.2,14.0.hrms(esi)m/z:[m+h]+calculatedforc22h23no5:382.1657,found:382.1652.enantiomericexcesswasdeterminedbyhplcwithachiralpakas-hcolumn(hexanes∶2-propanol=90∶10,1.0ml/min,254nm,96%ee);minorenantiomertr=12.5min,majorenantiomertr=20.0min.
实施例2:
制备方法与实施例1相同,溶解的查尔酮改为8mol%(3.04mg,0.0146mmol)得到无色油状液体3a,产率58%,经手性高效液相色谱法测得ee值为95%。
实施例3:
制备方法与实施例1相同,溶解的查尔酮改为6mol%(2.28mg,0.0109mmol)得到无色油状液体3a,产率63%,经手性高效液相色谱法测得ee值为94%。
实施例4:
制备方法与实施例1相同,溶解的查尔酮改为4mol%(1.52mg,0.0073mmol)得到无色油状液体3a,产率57%,经手性高效液相色谱法测得ee值为94%。
实施例5:
制备方法与实施例1相同,溶解的查尔酮改为2mol%(0.76mg,0.0036mmol)得到无色油状液体3a,产率64%,经手性高效液相色谱法测得ee值为94%。
实施例6:
制备方法与实施例1相同,反应介质改为2ml乙酸乙酯溶液,得到无色油状液体3a,产率51%,经手性高效液相色谱法测得ee值为89%。
实施例7:
制备方法与实施例1相同,反应介质改为2ml二噁烷溶液,得到无色油状液体3a,产率32%,经手性高效液相色谱法测得ee值为55%。
实施例8:
制备方法与实施例1相同,反应介质改为2ml乙醚溶液,得到无色油状液体3a,产率26%,经手性高效液相色谱法测得ee值为90%。
实施例9:
制备方法与实施例1相同,反应介质改为2ml乙醚溶液,反应温度改为0℃,得到无色油状液体3a,产率32%,经手性高效液相色谱法测得ee值为85%。
实施例10:
制备方法与实施例1相同,反应介质改为2ml乙醚溶液,反应温度改为-20℃,得到无色油状液体3a,产率54%,经手性高效液相色谱法测得ee值为90%。
实施例11:
制备方法与实施例1相同,反应介质改为2ml四氢呋喃溶液,反应温度改为-20℃,得到无色油状液体3a,产率50%,经手性高效液相色谱法测得ee值为89%。
实施例12:
制备方法与实施例1相同,反应介质改为2ml甲苯溶液,反应温度改为-20℃,得到无色油状液体3a,产率43%,经手性高效液相色谱法测得ee值为85%。
实施例13:
制备方法与实施例1相同,反应介质改为2ml乙腈溶液,反应温度改为-20℃,得到无色油状液体3a,产率60%,经手性高效液相色谱法测得ee值为94%。
实施例14:
制备方法与实施例1相同,反应介质改为2ml氯苯溶液,反应温度改为-20℃,得到无色油状液体3a,产率58%,经手性高效液相色谱法测得ee值为95%。
实施例15:
制备方法与实施例1相同,反应介质改为2ml二氯甲烷溶液,反应温度改为-20℃,得到无色油状液体3a,产率61%,经手性高效液相色谱法测得ee值为93%。
实施例16:
在氮气气氛下,将(r)-dtbm-segphos(13mg,0.0108mmol,6mol%),agoac(1.6mg,0.0096mmol,5mol%)加入到溶解有查尔酮(3.8mg,0.0182mmol,10mol%)的2ml丙酮溶液中,-40℃下预搅30min后,依次加入亚甲胺叶立德1b(0.2mmol),烯酮类化合物2a(0.182mmol),-40℃下反应12h,tlc监测反应结束后,经硅藻土过滤,萃取,滤液浓缩,经硅胶柱层析纯化,得到无色油状液体3b,产率78%,再经手性高效液相色谱法测得ee值为92%。
该产物的理化指标:1hnmr(400mhz,cdcl3)δ7.58(d,j=7.3hz,2h),7.36(t,j=7.4hz,1h),7.30(s,1h),7.20(t,j=7.8hz,2h),7.11(d,j=7.1hz,1h),7.09-6.99(m,2h),4.37-4.28(m,2h),4.22(d,j=8.3hz,1h),3.97(q,j=7.1hz,2h),3.78(dd,j=11.4,4.3hz,1h),3.67(s,3h),2.64(s,1h),1.02(t,j=7.1hz,3h).13cnmr(101mhz,cdcl3)δ199.4,172.2,171.1,143.0,136.5,134.5,133.5,129.8,128.5(d,j=15.3hz),128.1,127.2,125.3,77.5,77.2,76.9,66.8,62.0,61.3,56.4,52.5,52.2,13.9.hrms(esi)m/z:[m+h]+calculatedforc22h22clno5:416.1268,found:416.1264.enantiomericexcesswasdeterminedbyhplcwithachiralpakas-hcolumn(hexanes∶2-propanol=80∶20,0.8ml/min,254nm,92%ee);minorenantiomertr=10.8min,majorenantiomertr=15.8min.
实施例17:
在氮气气氛下,将(r)-dtbm-segphos(13mg,0.0108mmol,6mol%),agoac(1.6mg,0.0096mmol,5mol%)加入到溶解有查尔酮(3.8mg,0.0182mmol,10mol%)的2ml丙酮溶液中,-40℃下预搅30min后,依次加入亚甲胺叶立德1c(0.2mmol),烯酮类化合物2a(0.182mmol),-40℃下反应12h,tlc监测反应结束后,经硅藻土过滤,萃取,滤液浓缩,经硅胶柱层析纯化,得到无色油状液体3c,产率40%,再经手性高效液相色谱法测得ee值为96%。
该产物的理化指标:1hnmr(400mhz.cdcl3)δ7.57(d,j=7.2hz,2h),7.35(t,j=7.4hz,1h),7.18(t,j=7.5hz,4h),6.96(d,j=7.9hz,2h),4.36-4.30(m,1h),4.27(d,j=8.6hz,1h),4.22(d,j=8.4hz,1h),3.99(q,j=7.1hz,2h),3.84-3.76(m,1h),3.68(s,3h),2.63(s,1h),2.18(s,3h),1.05(t,j=7.1hz,3h).13cnmr(101mhz,cdcl3)δ199.7,172.2,171.7,137.7,137.2,136.6,133.3,129.3,128.7,128.3,127.0,77.5,77.1,76.8,67.9,62.6,61.3,56.9,52.9,52.2,21.0,13.9.hrms(esi)m/z:[m+h]+calculatedforc23h25no5:396.1815,found:396.1809.enantiomericexcesswasdeterminedbyhplcwithachiralpakas-hcolumn(hexanes∶2-propanol=80∶20,0.8ml/min,254nm,96%ee);minorenantiomertr=9.1min,majorenantiomertr=12.6min.
实施例18:
在氮气气氛下,将(r)-dtbm-segphos(13mg,0.0108mmol,6mol%),agoac(1.6mg,0.0096mmol,5mol%)加入到溶解有查尔酮(3.8mg,0.0182mmol,10mol%)的2ml丙酮溶液中,-40℃下预搅30min后,依次加入亚甲胺叶立德1d(0.2mmol),烯酮类化合物2a(0.182mmol),-40℃下反应12h,tlc监测反应结束后,经硅藻土过滤,萃取,滤液浓缩,经硅胶柱层析纯化,得到无色油状液体3d,产率72%,再经手性高效液相色谱法测得ee值为98%。
该产物的理化指标:1hnmr(400mhz,cdcl3)δ7.61(d,j=7.2hz,2h),7.40(t,j=7.4hz,1h),7.29(d,j=8.5hz,2h),7.22(dd,j=18.4,8.2hz,4h),4.41-4.31(m,2h),4.27(d,j=8.4hz,1h),4.01(q,j=7.2hz,2h),3.83(s,1h),3.71(s,3h),2.73(s,1h),1.06(t,j=7.1hz,3h).13cnmr(101mhz,cdcl3)δ199.4,172.3,171.2,139.9,136.5,133.5,131.7,128.8,128.5(d,j=12.0hz),121.7,77.6,77.3,76.9,66.9,62.0,61.3,56.3,52.6,52.1,13.9.hrms(esi)m/z:[m+h]+calculatedforc22h22brno5:460.0759,found:462.0738.enantiomericexcesswasdeterminedbyhplcwithachiralpakas-hcolumn(hexanes∶2-propanol=80∶20,0.8ml/min,254nm,98%ee);minorenantiomertr=11.9min,majorenantiomertr=17.7min.
实施例19:
在氮气气氛下,将(r)-dtbm-segphos(13mg,0.0108mmol,6mol%),agoac(1.6mg,0.0096mmol,5mol%)加入到溶解有查尔酮(3.8mg,0.0182mmol,10mol%)的2ml丙酮溶液中,-40℃下预搅30min后,依次加入亚甲胺叶立德1e(0.2mmol),烯酮类化合物2a(0.182mmol),-40℃下反应12h,tlc监测反应结束后,经硅藻土过滤,萃取,滤液浓缩,经硅胶柱层析纯化,得到无色油状液体3e,产率71%,再经手性高效液相色谱法测得ee值为98%。
该产物的理化指标:1hnmr(400mhz,cdcl3)δ7.57(d,j=7.4hz,2h),7.43-7.35(m,5h),7.20(t,j=7.8hz,2h),4.44(d,j=8.7hz,1h),4.36(t,j=8.3hz,1h),4.26(d,j=8.3hz,1h),3.99(q,j=7.1hz,2h),3.80(t,j=8.1hz,1h),3.69(s,3h),2.75(dd,j=37.5,30.2hz,1h),1.04(t,j=7.1hz,3h).13cnmr(101mhz,cdcl3)δ199.2,172.2,171.1,145.0,136.5,133.6,128.5(d,j=8.7hz),127.5,125.5(q,j=3.7hz),77.4,77.1,76.8,66.8,62.1,61.4,56.3,52.6,52.2,13.9.hrms(esi)m/z:[m+h]+calculatedforc23h22f3no5:450.153,found:450.1525.enantiomericexcesswasdeterminedbyhplcwithachiralpakas-hcolumn(hexanes∶2-propanol=80∶20,0.8ml/min,254nm,98%ee);minorenantiomertr=8.6min.majorenantiomertr=13.0min.
实施例20:
在氮气气氛下,将(r)-dtbm-segphos(13mg,0.0108mmol,6mol%),agoac(1.6mg,0.0096mmol,5mol%)加入到溶解有查尔酮(3.8mg,0.0182mmol,10mol%)的2ml丙酮溶液中,-40℃下预搅30min后,依次加入亚甲胺叶立德1f(0.2mmol),烯酮类化合物2a(0.182mmol),-40℃下反应12h,tlc监测反应结束后,经硅藻土过滤,萃取,滤液浓缩,经硅胶柱层析纯化,得到无色油状液体3f,产率83%,再经手性高效液相色谱法测得ee值为97%。
该产物的理化指标:1hnmr(400mhz,cdcl3)δ7.63(d,j=7.5hz,2h),7.40(t,j=7.2hz,1h),7.24(dd,j=20.1,7.8hz,4h),7.06(d,j=7.8hz,2h),4.42(t,j=8.1hz,1h),4.35(d,j=8.7hz,1h),4.30(d,j=8.4hz,1h),4.06(q,j=7.1hz,2h),3.89(t,j=8.0hz,1h),3.74(s,3h),2.70(s,1h),2.55(q,j=7.5hz,2h),1.18-1.08(m,6h).13cnmr(101mhz,cdcl3)δ199.7,172.2,171.6,144.1,137.5,136.7,133.2,128.6,128.2(d,j=15.2hz),127.1,77.5,77.2,76.9,67.8,62.5,61.2,56.9,52.8,52.1,28.5,15.6,13.9.hrms(esi)m/z:[m+h]+calculatedforc24h27no5:410.1977,found:410.1973.enantiomericexcesswasdeterminedbyhplcwithachiralpakas-hcolumn(hexanes∶2-propanol=80∶20,0.8ml/min,254nm,96%ee);minorenantiomertr=8.0min,majorenantiomertr=10.5min.
实施例21:
在氮气气氛下,将(r)-dtbm-segphos(13mg,0.0108mmol,6mol%),agoac(1.6mg,0.0096mmol,5mol%)加入到溶解有查尔酮(3.8mg,0.0182mmol,10mol%)的2ml丙酮溶液中,-40℃下预搅30min后,依次加入亚甲胺叶立德1a(0.2mmol),烯酮类化合物2b(0.182mmol),-40℃下反应12h,tlc监测反应结束后,经硅藻土过滤,萃取,滤液浓缩,经硅胶柱层析纯化,得到无色油状液体3g,产率55%,再经手性高效液相色谱法测得ee值为92%。
该产物的理化指标:1hnmr(400mhz,cdcl3)δ7.62(d,j=8.8hz,2h),7.36(d,j=7.0hz,2h),7.29-7.16(m,3h),6.72(d,j=8.8hz,2h),4.36(d,j=3.7hz,2h),4.31(d,j=8.4hz,1h),4.07(q,j=7.2hz,2h),3.90(dd,j=8.1,3.8hz,1h),3.75(d,j=2.2hz,6h),2.59(s,1h),1.14(t,j=7.1hz,3h).13cnmr(101mhz,cdcl3)δ197.8,172.3,171.7,163.8,140.5,131.0,129.6,128.7,128.0,127.1,113.6,77.5,77.1,76.8,68.0,62.6,61.2,56.5,55.4,52.8,52.1,14.0.hrms(esi)m/z:[m+h]+calculatedforc23h25no6:412.1773,found:412.1763.enantiomericexcesswasdeterminedbyhplcwithachiralpakas-hcolumn(hexanes∶2-propanol=80∶20,0.8ml/min,254nm,92%ee);minorenantiomertr=13.6min.majorenantiomertr=18.8min.
实施例22:
在氮气气氛下,将(r)-dtbm-segphos(13mg,0.0108mmol,6mol%),agoac(1.6mg,0.0096mmol,5mol%)加入到溶解有查尔酮(3.8mg,0.0182mmol,10mol%)的2ml丙酮溶液中,-40℃下预搅30min后,依次加入亚甲胺叶立德1c(0.2mmol),烯酮类化合物2b(0.182mmol),-40℃下反应12h,tlc监测反应结束后,经硅藻土过滤,萃取,滤液浓缩,经硅胶柱层析纯化,得到无色油状液体3h,产率71%,再经手性高效液相色谱法测得ee值为96%。
该产物的理化指标:1hnmr(400mhz,cdcl3)δ7.63(d,j=8.9hz,2h),7.24(d,j=8.0hz,2h),7.02(d,j=7.9hz,2h),6.70(d,j=8.8hz,2h),4.33(d,j=6.7hz,2h),4.27(d,j=8.4hz,1h),4.05(q,j=7.1hz,2h),3.88-3.81(m,1h),3.72(d,j=5.0hz,6h),2.68(s,1h),2.24(s,3h),1.12(t,j=7.1hz,3h).13cnmr(101mhz,cdcl3)δ197.9,172.2,171.8,163.7,137.5(d,j=10.7hz),131.0,129.7,129.3,127.0,113.5,77.6,77.2,76.9,67.8,62.6,61.2,56.5,55.3,52.9,52.1,21.0,14.0.hrms(esi)m/z:[m+h]+calculatedforc24h27no6:426.1917,found:426.1916.enantiomericexcesswasdeterminedbyhplcwithachiralpakas-hcolumn(hexanes∶2-propanol=80∶20,0.8ml/min,254nm,96%ee);minorenantiomertr=13.1min,majorenantiomertr=17.9min.
实施例23:
在氮气气氛下,将(r)-dtbm-segphos(13mg,0.0108mmol,6mol%),agoac(1.6mg,0.0096mmol,5mol%)加入到溶解有查尔酮(3.8mg,0.0182mmol,10mol%)的2ml丙酮溶液中,-40℃下预搅30min后,依次加入亚甲胺叶立德1f(0.2mmol),烯酮类化合物2c(0.182mmol),-40℃下反应12h,tlc监测反应结束后,经硅藻土过滤,萃取,滤液浓缩,经硅胶柱层析纯化,得到无色油状液体3i,产率69%,再经手性高效液相色谱法测得ee值为96%。
该产物的理化指标:1hnmr(400mhz,cdcl3)δ7.47(d,j=8.6hz,2h),7.18(d,j=8.1hz,2h),7.14(d,j=8.6hz,2h),7.01(d,j=8.0hz,2h),4.25(dd,j=14.5,7.8hz,3h),4.01(q,j=7.1hz,2h),3.84(t,j=8.1hz,1h),3.70(s,3h),2.51(q,j=7.6hz,2h),1.11(t,j=5.5hz,3h),1.08(t,j=5.0hz,3h).13cnmr(101mhz,cdcl3)δ198.5,172.4,171.4,144.4,139.8,137.3,135.0,130.1,128.6,128.3,127.0,77.4,77.1,76.8,68.0,62.3,61.4,56.9,52.7,52.2,28.5,15.6,14.0.hrms(esi)m/z:[m+h]+calculatedforc24h26clno5:444.1582,found:444.1578.enantiomericexcesswasdeterminedbyhplcwithachiralpakas-hcolumn(hexanes∶2-propanol=80∶20,0.8ml/min,254nm,96%ee);minorenantiomertr=8.8min.majorenantiomertr=11.2min.
实施例24:
在氮气气氛下,将(r)-dtbm-segphos(13mg,0.0108mmol,6mol%),agoac(1.6mg,0.0096mmol,5mol%)加入到溶解有查尔酮(3.8mg,0.0182mmol,10mol%)的2ml丙酮溶液中,-40℃下预搅30min后,依次加入亚甲胺叶立德1g(0.2mmol),烯酮类化合物2c(0.182mmol),-40℃下反应12h,tlc监测反应结束后,经硅藻土过滤,萃取,滤液浓缩,经硅胶柱层析纯化,得到无色油状液体3j,产率57%,再经手性高效液相色谱法测得ee值为98%。
该产物的理化指标:1hnmr(400mhz,cdcl3)δ7.60(d,j=8.6hz,2h),7.29(dd,j=11.6,8.5hz,4h),7.23(d,j=8.4hz,2h),4.37(d,j=7.3hz,2h),4.33(d,j=8.3hz,1h),4.08(q,j=7.1hz,2h),3.89(t,j=7.8hz,1h),3.78(s,3h),2.84-2.52(m,1h),1.14(t,j=7.1hz,3h).13cnmr(101mhz,cdcl3)δ198.3,172.4,171.1,140.1,139.1,134.9,133.8,123.0,128.8(d,j=7.5hz),128.4,77.4,77.1,76.8,67.0,61.9,61.4,56.3,52.6,52.2,13.9.hrms(esi)m/z:[m+h]+calculatedforc22h21cl2no5:450.0875,found:450.0869.enantiomericexcesswasdeterminedbyhplcwithachiralpakas-hcolumn(hexanes∶2-propanol=80∶20,0.8ml/min,254nm,98%ee);minorenantiomertr=11.1min,majorenantiomertr=16.1min.
实施例25:
在氮气气氛下,将(r)-dtbm-segphos(13mg,0.0108mmol,6mol%),agoac(1.6mg,0.0096mmol,5mol%)加入到溶解有查尔酮(3.8mg,0.0182mmol,10mol%)的2ml丙酮溶液中,-40℃下预搅30min后,依次加入亚甲胺叶立德1d(0.2mmol),烯酮类化合物2c(0.182mmol),-40℃下反应12h,tlc监测反应结束后,经硅藻土过滤,萃取,滤液浓缩,经硅胶柱层析纯化,得到无色油状液体3k,产率64%,再经手性高效液相色谱法测得ee值为94%。
该产物的理化指标:1hnmr(400mhz,cdcl3)δ7.60(d,j=8.6hz,2h),7.37(d,j=8.4hz,2h),7.26(dd,j=13.1,8.5hz,4h),4.36(d,j=6.1hz,2h),4.32(d,j=8.4hz,1h),4.07(q,j=7.1hz,2h),3.88(t,j=8.2hz,1h),3.77(s,3h),2.78(dd,j=18.0,6.1hz,1h),1.13(t,j=7.1hz,3h).13cnmr(101mhz,cdcl3)δ198.3,172.3,171.0,140.1,139.6,134.9,131.8,123.0,128.8(d,j=4.4hz),121.9,77.5,77.2,76.8,67.0,61.9,61.4,56.2,52.7,52.2,13.9.hrms(esi)m/z:[m+h]+calculatedforc22h21brclno5:494.0369,found:496.0346.enantiomericexcesswasdeterminedbyhplcwithachiralpakas-hcolumn(hexanes∶2-propanol=80∶20,0.8ml/min,254nm,94%ee);minorenantiomertr=12.1min,majorenantiomertr=17.8min.
实施例26:
在氮气气氛下,将(r)-dtbm-segphos(13mg,0.0108mmol,6mol%),agoac(1.6mg,0.0096mmol,5mol%)加入到溶解有查尔酮(3.8mg,0.0182mmol,10mol%)的2ml丙酮溶液中,-40℃下预搅30min后,依次加入亚甲胺叶立德1f(0.2mmol),烯酮类化合物2d(0.182mmol),-40℃下反应12h,tlc监测反应结束后,经硅藻土过滤,萃取,滤液浓缩,经硅胶柱层析纯化,得到无色油状液体3l,产率54%,再经手性高效液相色谱法测得ee值为95%。
该产物的理化指标:1hnmr(400mhz,cdcl3)δ7.60(d,j=8.5hz,2h),7.31(d,j=8.5hz,2h),7.25(d,j=8.1hz,2h),7.08(d,j=8.0hz,2h),4.32(dd,j=11.3,8.1hz,3h),4.08(q,j=7.1hz,2h),3.91(t,j=8.0hz,1h),3.77(s,3h),3.30(s,1h),2.59(q,j=7.6hz,2h),1.17(dt,j=13.0,7.4hz,6h).13cnmr(101mhz,cdcl3)δ199.1,172.3,171.4,144.4,137.6,137.3,135.9,123.0,128.3,127.0,101.6,77.4,77.1,76.8,68.0,62.3,61.4,56.8,52.7,52.2,28.5,15.7,14.0.hrms(esi)m/z:[m+h]+calculatedforc24h26ino5:536.0938,found:536.0932.enantiomericexcesswasdeterminedbyhplcwithachiralpakas-hcolumn(hexanes∶2-propanol=80∶20,0.8ml/min,254nm,96%ee);minorenantiomertr=19.0min,majorenantiomertr=29.1min.
实施例27:
在氮气气氛下,将(r)-dtbm-segphos(13mg,0.0108mmol,6mol%),agoac(1.6mg,0.0096mmol,5mol%)加入到溶解有查尔酮(3.8mg,0.0182mmol,10mol%)的2ml丙酮溶液中,-40℃下预搅30min后,依次加入亚甲胺叶立德1g(0.2mmol),烯酮类化合物2d(0.182mmol),-40℃下反应12h,tlc监测反应结束后,经硅藻土过滤,萃取,滤液浓缩,经硅胶柱层析纯化,得到无色油状液体3m,产率61%,再经手性高效液相色谱法测得ee值为96%。
该产物的理化指标:1hnmr(400mhz,cdcl3)δ7.67(d,j=8.5hz,2h),7.36(d,j=8.6hz,2h),7.30(d,j=8.5hz,2h),7.22(d,j=8.5hz,2h),4.38(d,j=8.8hz,1h),4.35(d,j=7.8hz,1h),4.33-4.30(m,1h),4.07(q,j=7.2hz,2h),3.87(t,j=8.1hz,1h),3.77(s,3h),2.72(dd,j=15.0,7.4hz,1h),1.13(t,j=7.1hz,3h).13cnmr(101mhz,cdcl3)δ198.8,172.3,171.0,139.1,137.8,135.8,133.8,129.9,128.9,128.4,101.9,77.5,77.2,76.9,66.9,61.9,61.4,56.2,52.7,52.2,14.0.hrms(esi)m/z:[m+h]+calculatedforc22h21clino5:542.0234,found:542.0229.enantiomericexcesswasdeterminedbyhplcwithachiralpakas-hcolumn(hexanes∶2-propanol=80∶20,0.8ml/min,254nm,97%ee);minorenantiomertr=28.6min,majorenantiomertr=44.4min.
实施例28:
在氮气气氛下,将(r)-dtbm-segphos(13mg,0.0108mmol,6mol%),agoac(1.6mg,0.0096mmol,5mol%)加入到溶解有查尔酮(3.8mg,0.0182mmol,10mol%)的2ml丙酮溶液中,-40℃下预搅30min后,依次加入亚甲胺叶立德1d(0.2mmol),烯酮类化合物2d(0.182mmol),-40℃下反应12h,tlc监测反应结束后,经硅藻土过滤,萃取,滤液浓缩,经硅胶柱层析纯化,得到无色油状液体3n,产率68%,再经手性高效液相色谱法测得ee值为95%。
该产物的理化指标:1hnmr(400mhz,cdcl3)δ7.68(d,j=8.6hz,2h),7.37(t,j=9.0hz,4h),7.24(d,j=8.4hz,2h),4.38(d,j=8.7hz,1h),4.33(dd,j=10.3,8.1hz,2h),4.07(q,j=7.1hz,2h),3.86(t,j=8.1hz,1h),3.78(s,3h),2.88-2.63(m,1h),1.14(t,j=7.1hz,3h).13cnmr(101mhz,cdcl3)δ198.9,172.3,171.0,139.6,137.8,135.8,131.8,129.9,128.8,122.0,101.9,77.5,77.2,76.9,67.0,61.9,615,56.1,52.7,52.3,14.0.hrms(esi)m/z:[m+h]+calculatedforc22h21brino5:585.9723,found:587.9702.enantiomericexcesswasdeterminedbyhplcwithachiralpakas-hcolumn(hexanes∶2-propanol=80∶20,0.8ml/min,254nm,95%ee);minorenantiomertr=30.9min,majorenantiomertr=45.9min.
实施例29:
在氮气气氛下,将(r)-dtbm-segphos(13mg,0.0108mmol,6mol%),agoac(1.6mg,0.0096mmol,5mol%)加入到溶解有查尔酮(3.8mg,0.0182mmol,10mol%)的2ml丙酮溶液中,-40℃下预搅30min后,依次加入亚甲胺叶立德1c(0.2mmol),烯酮类化合物2e(0.182mmol),-40℃下反应12h,tlc监测反应结束后,经硅藻土过滤,萃取,滤液浓缩,经硅胶柱层析纯化,得到无色油状液体3o,产率58%,再经手性高效液相色谱法测得ee值为97%。
该产物的理化指标:1hnmr(400mhz,cdcl3)δ7.57(d,j=8.2hz,2h),7.27(d,j=8.0hz,2h),7.07(dd,j=7.9,3.9hz,4h),4.42-4.33(m,2h),4.30(d,j=8.3hz,1h),4.08(q,j=7.1hz,2h),3.87(dd,j=8.2,6.9hz,1h),3.76(s,3h),2.64(s,1h),2.29(d,j=11.8hz,6h),1.15(t,j=7.1hz,3h).13cnmr(101mhz,cdcl3)δ199.2,172.2,171.8,144.2,137.6,137.3,134.2,129.3,129.1,128.8,127.0,77.5,77.2,76.9,67.8,62.7,61.2,56.7,53.0,52.1,21.5,21.1,14.0.hrms(esi)m/z:[m+h]+calculatedforc24h27no5:408.1812,found:410.1968.enantiomericexcesswasdeterminedbyhplcwithachiralpakas-hcolumn(hexanes∶2-propanol=80∶20,0.8ml/min,254nm,98%ee);minorenantiomertr=8.3min,majorenantiomertr=10.7min.
实施例30:
在氮气气氛下,将(r)-dtbm-segphos(13mg,0.0108mmol,6mol%),agoac(1.6mg,0.0096mmol,5mol%)加入到溶解有查尔酮(3.8mg,0.0182mmol,10mol%)的2ml丙酮溶液中,-40℃下预搅30min后,依次加入亚甲胺叶立德1f(0.2mmol),烯酮类化合物2e(0.182mmol),-40℃下反应12h,tlc监测反应结束后,经硅藻土过滤,萃取,滤液浓缩,经硅胶柱层析纯化,得到无色油状液体3p,产率49%,再经手性高效液相色谱法测得ee值为90%。
该产物的理化指标:1hnmr(400mhz,cdcl3)δ7.55(d,j=8.2hz,2h),7.28(d,j=8.1hz,2h),7.07(dd,j=9.6,8.4hz,4h),4.38(d,j=6.9hz,2h),4.30(d,j=8.3hz,1h),4.08(q,j=7.1hz,2h),3.88(dd,j=8.0,6.9hz,1h),3.77(s,3h),2.58(q,j=7.6hz,2h),2.31(s,3h),1.18(t,j=5.4hz,3h),1.15(t,j=5.0hz,3h).13cnmr(101mhz,cdcl3)δ199.2,172.2,171.8,144.1(d,j=7.2hz),137.5,134.2,128.9(d,j=19.8hz),128.1,127.1,77.4,77.1,76.8,67.8,62.7,61.2,56.8,52.9,52.1,28.5,21.5,15.6,14.0.hrms(esi)m/z:[m+h]+calculatedforc25h29no5:424.2128,found:424.2123.enantiomericexcesswasdeterminedbyhplcwithachiralpakas-hcolumn(hexanes∶2-propanol=80∶20,0.8ml/min,254nm,90%ee);minorenantiomertr=7.7min,majorenantiomertr=9.8min.
实施例31:
在氮气气氛下,将(r)-dtbm-segphos(13mg,0.0108mmol,6mol%),agoac(1.6mg,0.0096mmol,5mol%)加入到溶解有查尔酮(3.8mg,0.0182mmol,10mol%)的2ml丙酮溶液中,-40℃下预搅30min后,依次加入亚甲胺叶立德1g(0.2mmol),烯酮类化合物2e(0.182mmol),-40℃下反应12h,tlc监测反应结束后,经硅藻土过滤,萃取,滤液浓缩,经硅胶柱层析纯化,得到无色油状液体3q,产率67%,再经手性高效液相色谱法测得ee值为95%。
该产物的理化指标:1hnmr(400mhz,cdcl3)δ7.57(d,j=8.2hz,2h),7.32(d,j=8.5hz,2h),7.20(d,j=8.5hz,2h),7.09(d,j=8.1hz,2h),4.39(d,j=4.9hz,2h),4.32(d,j=8.2hz,1h),4.07(q,j=7.1hz,2h),3.87(t,j=7.9hz,1h),3.76(s,3h),2.74(s,1h),2.31(s,3h),1.12(t,j=7.1hz,3h).13cnmr(101mhz,cdcl3)δ198.9,172.3,171.3,144.4,139.4,134.1,133.5,129.2,128.8-128.4(m),77.5,77.2,76.9,66.9,62.2,61.3,56.3,52.7,52.1,21.5,13.9.hrms(esi)m/z:[m+h]+calculatedforc23h24clno5:430.1423,found:430.1419.enantiomericexcesswasdeterminedbyhplcwithachiralpakas-hcolumn(hexanes∶2-propanol=80∶20,0.8ml/min,254nm,96%ee);minorenantiomertr=9.9min,majorenantiomertr=12.9min.
实施例32:
在氮气气氛下,将(r)-dtbm-segphos(13mg,0.0108mmol,6mol%),agoac(1.6mg,0.0096mmol,5mol%)加入到溶解有查尔酮(3.8mg,0.0182mmol,10mol%)的2ml丙酮溶液中,-40℃下预搅30min后,依次加入亚甲胺叶立德1d(0.2mmol),烯酮类化合物2e(0.182mmol),-40℃下反应12h,tlc监测反应结束后,经硅藻土过滤,萃取,滤液浓缩,经硅胶柱层析纯化,得到无色油状液体3r,产率71%,再经手性高效液相色谱法测得ee值为97%。
该产物的理化指标:1hnmr(400mhz,cdcl3)δ7.57(d,j=8.2hz,2h),7.35(d,j=8.4hz,2h),7.26(d,j=8.4hz,2h),7.09(d,j=8.1hz,2h),4.38(d,j=4.6hz,2h),4.31(d,j=8.3hz,1h),4.06(q,j=7.1hz,2h),3.89-3.83(m,1h),3.76(s,3h),2.71(s,1h),2.32(s,3h),1.12(t,j=7.1hz,3h).13cnmr(101mhz,cdcl3)δ198.9,172.3,171.3,144.5,139.9,134.1,131.7,129.2,128.8(d,j=12.7hz),121.7,77.5,77.2,76.9,66.9,62.2,61.3,56.2,52.7,52.1,21.5,13.9.hrms(esi)m/z:[m+h]+calculatedforc23h24brno5:474.0916,found:476.0894.enantiomericexcesswasdeterminedbyhplcwithachiralpakas-hcolumn(hexanes∶2-propanol=80∶20,0.8ml/min,254nm,98%ee);minorenantiomertr=10.4min,majorenantiomertr=13.6min.
实施例33:
在氮气气氛下,将(r)-dtbm-segphos(13mg,0.0108mmol,6mol%),agoac(1.6mg,0.0096mmol,5mol%)加入到溶解有查尔酮(3.8mg,0.0182mmol,10mol%)的2ml丙酮溶液中,-40℃下预搅30min后,依次加入亚甲胺叶立德1g(0.2mmol),烯酮类化合物2f(0.182mmol),-40℃下反应12h,tlc监测反应结束后,经硅藻土过滤,萃取,滤液浓缩,经硅胶柱层析纯化,得到无色油状液体3s,产率73%,再经手性高效液相色谱法测得ee值为98%。
该产物的理化指标:1hnmr(400mhz,cdcl3)δ7.49(d,j=7.0hz,1h),7.28(d,j=8.4hz,2h),7.16(dd,j=7.1,4.1hz,5h),4.34(d,j=8.9hz,1h),4.27(t,j=8.1hz,2h),4.10(q,j=7.1hz,2h),3.91(t,j=8.0hz,1h),3.76(s,3h),2.67(s,1h),1.19(t,j=7.2hz,3h).13cnmr(101mhz,cdcl3)δ201.1,172.0,171.3,139.9,138.7,133.8(d,j=19.3hz),132.1,129.1,128.6(d,j=6.8hz),127.1,119.3,77.5,77.1,76.8,66.2,62.2,61.5,60.7,52.3,51.3,14.0.hrms(esi)m/z:[m+h]+calculatedforc22h21brclno5:494.0369,found:496.0346.enantiomericexcesswasdeterminedbyhplcwithachiralpakas-hcolumn(hexanes∶2-propanol=80∶20,0.8ml/min,254nm,98%ee);minorenantiomertr=12.1min,majorenantiomertr=16.8min.
实施例34:
在氮气气氛下,将(r)-dtbm-segphos(13mg,0.0108mmol,6mol%),agoac(1.6mg,0.0096mmol,5mol%)加入到溶解有查尔酮(3.8mg,0.0182mmol,10mol%)的2ml丙酮溶液中,-40℃下预搅30min后,依次加入亚甲胺叶立德1d(0.2mmol),烯酮类化合物2f(0.182mmol),-40℃下反应12h,tlc监测反应结束后,经硅藻土过滤,萃取,滤液浓缩,经硅胶柱层析纯化,得到无色油状液体3t,产率65%,再经手性高效液相色谱法测得ee值为98%。
该产物的理化指标:1hnmr(400mhz,cdcl3)δ7.49(d,j=7.0hz,1h),7.31(d,j=8.3hz,2h),7.22(d,j=8.3hz,2h),7.17(dd,j=10.0,5.1hz,3h),4.33(d,j=8.9hz,1h),4.27(t,j=7.3hz,2h),4.09(q,j=7.1hz,2h),3.91(t,j=8.0hz,1h),3.75(s,3h),2.69(s,1h),1.18(t,j=7.1hz,3h).13cnmr(101mhz,cdcl3)δ201.0,172.0,171.2,139.9,139.3,133.8,132.1,131.6,129.1,128.9,127.1,121.8,119.3,77.5,77.2,76.9,66.1,62.1,61.4,60.7,52.2,51.3,14.0.hrms(esi)m/z:[m+h]+calculatedforc22h21br2no5:537.9869,found:539.9848.enantiomericexcesswasdeterminedbyhplcwithachiralpakas-hcolumn(hexanes∶2-propanol=80∶20,0.8ml/min,254nm,98%ee);minorenantiomertr=12.9min,majorenantiomertr=18.1min.
实施例35:
在氮气气氛下,将(r)-dtbm-segphos(21.9mg,0.0182mmol,10mol%),agoac(1.6mg,0.0096mmol,5mol%)加入到溶解有查尔酮(3.8mg,0.0182mmol,10mol%)的2ml丙酮溶液中,-40℃下预搅30min后,依次加入亚甲胺叶立德1a(0.2mmol),烯酮类化合物2a(0.182mmol),-40℃下反应12h,tlc监测反应结束后,经硅藻土过滤,萃取,滤液浓缩,经硅胶柱层析纯化,得到无色油状液体3a,产率66%,再经手性高效液相色谱法测得ee值为95%。
该产物的理化指标:1hnmr(400mhz,cdcl3)δ7.65(d,j=7.4hz,2h),7.45(t,j=7.4hz,1h),7.38(d,j=6.7hz,2h),7.27(dd,j=11.3,7.7hz,5h),4.42(dd,j=17.5,8.1hz,2h),4.34(d,j=8.4hz,1h),4.09(q,j=7.1hz,2h),3.93(t,j=7.9hz,1h),3.79(s,3h),2.59(s,1h),1.15(t,j=7.1hz,3h).13cnmr(101mhz,cdcl3)δ199.7,172.3,171.6,140.3,136.6,133.4,128.7(d,j=7.4hz),128.4,128.1,127.1,77.4,77.1,76.8,68.0,62.5,61.4,56.9,52.8,52.2,14.0.hrms(esi)m/z:[m+h]+calculatedforc22h23no5:382.1657,found:382.1652.enantiomericexcesswasdeterminedbyhplcwithachiralpakas-hcolumn(hexanes∶2-propanol=90∶10,1.0ml/min,254nm,95%ee);minorenantiomertr=12.5min,majorenantiomertr=20.0min.
实施例36:
在氮气气氛下,将(r)-dtbm-segphos(2.2mg,0.00182mmol,1mol%),agoac(1.6mg,0.0096mmol,5mol%)加入到溶解有查尔酮(0.38mg,0.00182mmol,1mol%)的2ml丙酮溶液中,-40℃下预搅30min后,依次加入亚甲胺叶立德1a(0.2mmol),烯酮类化合物2a(0.182mmol),-40℃下反应12h,tlc监测反应结束后,经硅藻土过滤,萃取,滤液浓缩,经硅胶柱层析纯化,得到无色油状液体3a,产率59%,再经手性高效液相色谱法测得ee值为97%。
该产物的理化指标:1hnmr(400mhz,cdcl3)δ7.65(d,j=7.4hz,2h),7.45(t,j=7.4hz,1h),7.38(d,j=6.7hz,2h),7.27(dd,j=11.3,7.7hz,5h),4.42(dd,j=17.5,8.1hz,2h),4.34(d,j=8.4hz,1h),4.09(q,j=7.1hz,2h),3.93(t,j=7.9hz,1h),3.79(s,3h),2.59(s,1h),1.15(t,j=7.1hz,3h).13cnmr(101mhz,cdcl3)δ199.7,172.3,171.6,140.3,136.6,133.4,128.7(d,j=7.4hz),128.4,128.1,127.1,77.4,77.1,76.8,68.0,62.5,61.4,56.9,52.8,52.2,14.0.hrms(esi)m/z:[m+h]+calculatedforc22h23no5:382.1657,found:382.1652.enantiomericexcesswasdeterminedbyhplcwithachiralpakas-hcolumn(hexanes∶2-propanol=90∶10,1.0ml/min,254nm,97%ee);minorenantiomertr=12.5min,majorenantiomertr=20.0min.
实施例37:
在氮气气氛下,将xing-phos(6.5mg,0.0108mmol,6mol%),agoac(1.6mg,0.0096mmol,5mol%)加入到溶解有查尔酮(3.8mg,0.0182mmol,10mol%)的2ml丙酮溶液中,-20℃下预搅30min后,依次加入亚甲胺叶立德1a(0.2mmol),烯酮类化合物2a(0.182mmol),-20℃下反应10h,tlc监测反应结束后,经硅藻土过滤,萃取,滤液浓缩,经硅胶柱层析纯化,得到无色油状液体3a,产率62%,再经手性高效液相色谱法测得ee值为95%。
该产物的理化指标:1hnmr(400mhz,cdcl3)δ7.65(d,j=7.4hz,2h),7.45(t,j=7.4hz,1h),7.38(d,j=6.7hz,2h),7.27(dd,j=11.3,7.7hz,5h),4.42(dd,j=17.5,8.1hz,2h),4.34(d,j=8.4hz,1h),4.09(q,j=7.1hz,2h),3.93(t,j=7.9hz,1h),3.79(s,3h),2.59(s,1h),1.15(t,j=7.1hz,3h).13cnmr(101mhz,cdcl3)δ199.7,172.3,171.6,140.3,136.6,133.4,128.7(d,j=7.4hz),128.4,128.1,127.1,77.4,77.1,76.8,68.0,62.5,61.4,56.9,52.8,52.2,14.0.hrms(esi)m/z:[m+h]+calculatedforc22h23no5:382.1657,found:382.1652.enantiomericexcesswasdeterminedbyhplcwithachiralpakas-hcolumn(hexanes∶2-propanol=90∶10,1.0ml/min,254nm,95%ee);minorenantiomertr=12.5min,majorenantiomertr=20.0min.
实施例38:
在氮气气氛下,将fei-phos(7.1mg,0.0108mmol,6mol%),agoac(1.6mg,0.0096mmol,5mol%)加入到溶解有查尔酮(3.8mg,0.0182mmol,10mol%)的2ml丙酮溶液中,-20℃下预搅30min后,依次加入亚甲胺叶立德1a(0.2mmol),烯酮类化合物2a(0.182mmol),-20℃下反应15h,tlc监测反应结束后,经硅藻土过滤,萃取,滤液浓缩,经硅胶柱层析纯化,得到无色油状液体3a,产率49%,再经手性高效液相色谱法测得ee值为93%。
该产物的理化指标:1hnmr(400mhz,cdcl3)δ7.65(d,j=7.4hz,2h),7.45(t,j=7.4hz,1h),7.38(d,j=6.7hz,2h),7.27(dd,j=11.3,7.7hz,5h),4.42(dd,j=17.5,8.1hz,2h),4.34(d,j=8.4hz,1h),4.09(q,j=7.1hz,2h),3.93(t,j=7.9hz,1h),3.79(s,3h),2.59(s,1h),1.15(t,j=7.1hz,3h).13cnmr(101mhz,cdcl3)δ199.7,172.3,171.6,140.3,136.6,133.4,128.7(d,j=7.4hz),128.4,128.1,127.1,77.4,77.1,76.8,68.0,62.5,61.4,56.9,52.8,52.2,14.0.hrms(esi)m/z:[m+h]+calculatedforc22h23no5:382.1657,found:382.1652.enantiomericexcesswasdeterminedbyhplcwithachiralpakas-hcolumn(hexanes∶2-propanol=90∶10,1.0ml/min,254nm,93%ee);minorenantiomertr=12.5min,majorenantiomertr=20.0min.
实施例39:
在氮气气氛下,将tao-phos(6.3mg,0.0108mmol,6mol%),agoac(1.6mg,0.0096mmol,5mol%)加入到溶解有查尔酮(3.8mg,0.0182mmol,10mol%)的2ml四氢呋喃溶液中,0℃下预搅30min后,依次加入亚甲胺叶立德1a(0.2mmol),烯酮类化合物2a(0.182mmol),0℃下反应10h,tlc监测反应结束后,经硅藻土过滤,萃取,滤液浓缩,经硅胶柱层析纯化,得到无色油状液体3a,产率50%,再经手性高效液相色谱法测得ee值为91%。
该产物的理化指标:1hnmr(400mhz,cdcl3)δ7.65(d,j=7.4hz,2h),7.45(t,j=7.4hz,1h),7.38(d,j=6.7hz,2h),7.27(dd,j=11.3,7.7hz,5h),4.42(dd,j=17.5,8.1hz,2h),4.34(d,j=8.4hz,1h),4.09(q,j=7.1hz,2h),3.93(t,j=7.9hz,1h),3.79(s,3h),2.59(s,1h),1.15(t,j=7.1hz,3h).13cnmr(101mhz,cdcl3)δ199.7,172.3,171.6,140.3,136.6,133.4,128.7(d,j=7.4hz),128.4,128.1,127.1,77.4,77.1,76.8,68.0,62.5,61.4,56.9,52.8,52.2,14.0.hrms(esi)m/z:[m+h]+calculatedforc22h23no5:382.1657,found:382.1652.enantiomericexcesswasdeterminedbyhplcwithachiralpakas-hcolumn(hexanes∶2-propanol=90∶10,1.0ml/min,254nm,91%ee);minorenantiomertr=12.5min,majorenantiomertr=20.0min.
实施例40:
在氮气气氛下,将hznu(6.5mg,0.0108mmol,6mol%),agoac(1.6mg,0.0096mmol,5mol%)加入到溶解有查尔酮(3.8mg,0.0182mmol,10mol%)的2ml四氢呋喃溶液中,-20℃下预搅30min后,依次加入亚甲胺叶立德1a(0.2mmol),烯酮类化合物2a(0.182mmol),-20℃下反应10h,tlc监测反应结束后,经硅藻土过滤,萃取,滤液浓缩,经硅胶柱层析纯化,得到无色油状液体3a,产率52%,再经手性高效液相色谱法测得ee值为92%。
该产物的理化指标:1hnmr(400mhz,cdcl3)δ7.65(d,j=7.4hz,2h),7.45(t,j=7.4hz,1h),7.38(d,j=6.7hz,2h),7.27(dd,j=11.3,7.7hz,5h),4.42(dd,j=17.5,8.1hz,2h),4.34(d,j=8.4hz,1h),4.09(q,j=7.1hz,2h),3.93(t,j=7.9hz,1h),3.79(s,3h),2.59(s,1h),1.15(t,j=7.1hz,3h).13cnmr(101mhz,cdcl3)δ199.7,172.3,171.6,140.3,136.6,133.4,128.7(d,j=7.4hz),128.4,128.1,127.1,77.4,77.1,76.8,68.0,62.5,61.4,56.9,52.8,52.2,14.0.hrms(esi)m/z:[m+h]+calculatedforc22h23no5:382.1657,found:382.1652.enantiomericexcesswasdeterminedbyhplcwithachiralpakas-hcolumn(hexanes∶2-propanol=90∶10,1.0ml/min,254nm,92%ee);minorenantiomertr=12.5min,majorenantiomertr=20.0min.
实施例41:
在氮气气氛下,将(r)-dtbm-segphos(13mg,0.0108mmol,6mol%),agoac(1.6mg,0.0096mmol,5mol%)加入到溶解有丙烯酸乙酯(1.8mg,0.0182mmol,10mol%)的2ml丙酮溶液中,-40℃下预搅30min后,依次加入亚甲胺叶立德1a(0.2mmol),烯酮类化合物2a(0.182mmol),-40℃下反应12h,tlc监测反应结束后,经硅藻土过滤,萃取,滤液浓缩,经硅胶柱层析纯化,得到无色油状液体3a,产率61%,再经手性高效液相色谱法测得ee值为87%。
该产物的理化指标:1hnmr(400mhz,cdcl3)δ7.65(d,j=7.4hz,2h),7.45(t,j=7.4hz,1h),7.38(d,j=6.7hz,2h),7.27(dd,j=11.3,7.7hz,5h),4.42(dd,j=17.5,8.1hz,2h),4.34(d,j=8.4hz,1h),4.09(q,j=7.1hz,2h),3.93(t,j=7.9hz,1h),3.79(s,3h),2.59(s,1h),1.15(t,j=7.1hz,3h).13cnmr(101mhz,cdcl3)δ199.7,172.3,171.6,140.3,136.6,133.4,128.7(d,j=7.4hz),128.4,128.1,127.1,77.4,77.1,76.8,68.0,62.5,61.4,56.9,52.8,52.2,14.0.hrms(esi)m/z:[m+h]+calculatedforc22h23no5:382.1657,found:382.1652.enantiomericexcesswasdeterminedbyhplcwithachiralpakas-hcolumn(hexanes∶2-propanol=90∶10,1.0ml/min,254nm,87%ee);minorenantiomertr=12.5min,majorenantiomertr=20.0min.
实施例42:
在氮气气氛下,将(r)-dtbm-segphos(13mg,0.0108mmol,6mol%),agoac(1.6mg,0.0096mmol,5mol%)加入到溶解有l-苯基-2-硝基丙烯(3.0mg,0.0182mmol,10mol%)的2ml丙酮溶液中,-40℃下预搅30min后,依次加入亚甲胺叶立德1a(0.2mmol),烯酮类化合物2a(0.182mmol),-40℃下反应12h,tlc监测反应结束后,经硅藻土过滤,萃取,滤液浓缩,经硅胶柱层析纯化,得到无色油状液体3a,产率50%,再经手性高效液相色谱法测得ee值为79%。
该产物的理化指标:1hnmr(400mhz,cdcl3)δ7.65(d,j=7.4hz,2h),7.45(t,j=7.4hz,1h),7.38(d,j=6.7hz,2h),7.27(dd,j=11.3,7.7hz,5h),4.42(dd,j=17.5,8.1hz,2h),4.34(d,j=8.4hz,1h),4.09(q,j=7.1hz,2h),3.93(t,j=7.9hz,1h),3.79(s,3h),2.59(s,1h),1.15(t,j=7.1hz,3h).13cnmr(101mhz,cdcl3)δ199.7,172.3,171.6,140.3,136.6,133.4,128.7(d,j=7.4hz),128.4,128.1,127.1,77.4,77.1,76.8,68.0,62.5,61.4,56.9,52.8,52.2,14.0.hrms(esi)m/z:[m+h]+calculatedforc22h23no5:382.1657,found:382.1652.enantiomericexcesswasdeterminedbyhplcwithachiralpakas-hcolumn(hexanes∶2-propanol=90∶10,1.0ml/min,254nm,79%ee);minorenantiomertr=12.5min,majorenantiomertr=20.0min.
实施例43:
在氮气气氛下,将(r)-dtbm-segphos(13mg,0.0108mmol,6mol%),agoac(1.6mg,0.0096mmol,5mol%)加入到溶解有β-硝基苯乙烯(2.7mg,0.0182mmol,10mol%)的2ml丙酮溶液中,-40℃下预搅30min后,依次加入亚甲胺叶立德1a(0.2mmol),烯酮类化合物2a(0.182mmol),-40℃下反应12h,tlc监测反应结束后,经硅藻土过滤,萃取,滤液浓缩,经硅胶柱层析纯化,得到无色油状液体3a,产率57%,再经手性高效液相色谱法测得ee值为66%。
该产物的理化指标:1hnmr(400mhz,cdcl3)δ7.65(d,j=7.4hz,2h),7.45(t,j=7.4hz,1h),7.38(d,j=6.7hz,2h),7.27(dd,j=11.3,7.7hz,5h),4.42(dd,j=17.5,8.1hz,2h),4.34(d,j=8.4hz,1h),4.09(q,j=7.1hz,2h),3.93(t,j=7.9hz,1h),3.79(s,3h),2.59(s,1h),1.15(t,j=7.1hz,3h).13cnmr(101mhz,cdcl3)δ199.7,172.3,171.6,140.3,136.6,133.4,128.7(d,j=7.4hz),128.4,128.1,127.1,77.4,77.1,76.8,68.0,62.5,61.4,56.9,52.8,52.2,14.0.hrms(esi)m/z:[m+h]+calculatedforc22h23no5:382.1657,found:382.1652.enantiomericexcesswasdeterminedbyhplcwithachiralpakas-hcolumn(hexanes∶2-propanol=90∶10,1.0ml/min,254nm,66%ee);minorenantiomertr=12.5min,majorenantiomertr=20.0min.
实施例44:
在氮气气氛下,将(r)-dtbm-segphos(13mg,0.0108mmol,6mol%),agoac(1.6mg,0.0096mmol,5mol%)加入到溶解有n-苯基马来酰亚胺(3.2mg,0.0182mmol,10mol%)的2ml丙酮溶液中,-40℃下预搅30min后,依次加入亚甲胺叶立德1a(0.2mmol),烯酮类化合物2a(0.182mmol),-40℃下反应12h,tlc监测反应结束后,经硅藻土过滤,萃取,滤液浓缩,经硅胶柱层析纯化,得到无色油状液体3a,产率59%,再经手性高效液相色谱法测得ee值78%。
该产物的理化指标:1hnmr(400mhz,cdcl3)δ7.65(d,j=7.4hz,2h),7.45(t,j=7.4hz,1h),7.38(d,j=6.7hz,2h),7.27(dd,j=11.3,7.7hz,5h),4.42(dd,j=17.5,8.1hz,2h),4.34(d,j=8.4hz,1h),4.09(q,j=7.1hz,2h),3.93(t,j=7.9hz,1h),3.79(s,3h),2.59(s,1h),1.15(t,j=7.1hz,3h).13cnmr(101mhz,cdcl3)δ199.7,172.3,171.6,140.3,136.6,133.4,128.7(d,j=7.4hz),128.4,128.1,127.1,77.4,77.1,76.8,68.0,62.5,61.4,56.9,52.8,52.2,14.0.hrms(esi)m/z:[m+h]+calculatedforc22h23no5:382.1657,found:382.1652.enantiomericexcesswasdeterminedbyhplcwithachiralpakas-hcolumn(hexanes∶2-propanol=90∶10,1.0ml/min,254nm,78%ee);minorenantiomertr=12.5min,majorenantiomertr=20.0min
实施例45:
在氮气气氛下,将(r)-dtbm-segphos(13mg,0.0108mmol,6mol%),agoac(1.6mg,0.0096mmol,5mol%)加入到溶解有2,6-二氯-ω-硝基苯乙烯(4.0mg,0.0182mmol,10mol%)的2ml丙酮溶液中,-40℃下预搅30min后,依次加入亚甲胺叶立德1a(0.2mmol),烯酮类化合物2a(0.182mmol),-40℃下反应12h,tlc监测反应结束后,经硅藻土过滤,萃取,滤液浓缩,经硅胶柱层析纯化,得到无色油状液体3a,产率46%,再经手性高效液相色谱法测得ee值为62%。
该产物的理化指标:1hnmr(400mhz,cdcl3)δ7.65(d,j=7.4hz,2h),7.45(t,j=7.4hz,1h),7.38(d,j=6.7hz,2h),7.27(dd,j=11.3,7.7hz,5h),4.42(dd,j=17.5,8.1hz,2h),4.34(d,j=8.4hz,1h),4.09(q,j=7.1hz,2h),3.93(t,j=7.9hz,1h),3.79(s,3h),2.59(s,1h),1.15(t,j=7.1hz,3h).13cnmr(101mhz,cdcl3)δ199.7,172.3,171.6,140.3,136.6,133.4,128.7(d,j=7.4hz),128.4,128.1,127.1,77.4,77.1,76.8,68.0,62.5,61.4,56.9,52.8,52.2,14.0.hrms(esi)m/z:[m+h]+calculatedforc22h23no5:382.1657,found:382.1652.enantiomericexcesswasdeterminedbyhplcwithachiralpakas-hcolumn(hexanes∶2-propanol=90∶10,1.0ml/min,254nm,62%ee);minorenantiomertr=12.5min,majorenantiomertr=20.0min.
实施例46:
在氮气气氛下,将(r)-dtbm-segphos(21.9mg,0.0182mmol,10mol%),agoac(3.0mg,0.0182mmol,10mol%)加入到溶解有查尔酮(3.8mg,0.0182mmol,10mol%)的2ml丙酮溶液中,-40℃下预搅30min后,依次加入亚甲胺叶立德1a(0.2mmol),烯酮类化合物2a(0.182mmol),-40℃下反应12h,tlc监测反应结束后,经硅藻土过滤,萃取,滤液浓缩,经硅胶柱层析纯化,得到无色油状液体3a,产率62%,再经手性高效液相色谱法测得ee值为91%。
该产物的理化指标:1hnmr(400mhz,cdcl3)δ7.65(d,j=7.4hz,2h),7.45(t,j=7.4hz,1h),7.38(d,j=6.7hz,2h),7.27(dd,j=11.3,7.7hz,5h),4.42(dd,j=17.5,8.1hz,2h),4.34(d,j=8.4hz,1h),4.09(q,j=7.1hz,2h),3.93(t,j=7.9hz,1h),3.79(s,3h),2.59(s,1h),1.15(t,j=7.1hz,3h).13cnmr(101mhz,cdcl3)δ199.7,172.3,171.6,140.3,136.6,133.4,128.7(d,j=7.4hz),128.4,128.1,127.1,77.4,77.1,76.8,68.0,62.5,61.4,56.9,52.8,52.2,14.0.hrms(esi)m/z:[m+h]+calculatedforc22h23no5:382.1657,found:382.1652.enantiomericexcesswasdeterminedbyhplcwithachiralpakas-hcolumn(hexanes∶2-propanol=90∶10,1.0ml/min,254nm,91%ee);minorenantiomertr=12.5min,majorenantiomertr=20.0min.
实施例47:
在氮气气氛下,将(r)-dtbm-segphos(2.2mg,0.00182mmol,1mol%),agoac(0.3mg,0.00182mmol,1mol%)加入到溶解有查尔酮(3.8mg,0.0182mmol,10mol%)的2ml丙酮溶液中,-40℃下预搅30min后,依次加入亚甲胺叶立德1a(0.2mmol),烯酮类化合物2a(0.182mmol),-40℃下反应12h,tlc监测反应结束后,经硅藻土过滤,萃取,滤液浓缩,经硅胶柱层析纯化,得到无色油状液体3a,产率62%,再经手性高效液相色谱法测得ee值为94%。
该产物的理化指标:1hnmr(400mhz,cdcl3)δ7.65(d,j=7.4hz,2h),7.45(t,j=7.4hz,1h),7.38(d,j=6.7hz,2h),7.27(dd,j=11.3,7.7hz,5h),4.42(dd,j=17.5,8.1hz,2h),4.34(d,j=8.4hz,1h),4.09(q,j=7.1hz,2h),3.93(t,j=7.9hz,1h),3.79(s,3h),2.59(s,1h),1.15(t,j=7.1hz,3h).13cnmr(101mhz,cdcl3)δ199.7,172.3,171.6,140.3,136.6,133.4,128.7(d,j=7.4hz),128.4,128.1,127.1,77.4,77.1,76.8,68.0,62.5,61.4,56.9,52.8,52.2,14.0.hrms(esi)m/z:[m+h]+calculatedforc22h23no5:382.1657,found:382.1652.enantiomericexcesswasdeterminedbyhplcwithachiralpakas-hcolumn(hexanes∶2-propanol=90∶10,1.0ml/min,254nm,94%ee);minorenantiomertr=12.5min,majorenantiomertr=20.0min.
实施例48:
在氮气气氛下,将(r)-dtbm-segphos(13mg,0.0108mmol,6mol%),cu(oac)2与agoac复合金属催化剂(1.7mg,0.0096mmol,5mol%)加入到溶解有查尔酮(3.8mg,0.0182mmol,10mol%)的2ml丙酮溶液中,-40℃下预搅30min后,依次加入亚甲胺叶立德1a(0.2mmol),烯酮类化合物2a(0.182mmol),-40℃下反应12h,tlc监测反应结束后,经硅藻土过滤,萃取,滤液浓缩,经硅胶柱层析纯化,得到无色油状液体3a,产率43%,再经手性高效液相色谱法测得ee值为89%。
该产物的理化指标:1hnmr(400mhz,cdcl3)δ7.65(d,j=7.4hz,2h),7.45(t,j=7.4hz,1h),7.38(d,j=6.7hz,2h),7.27(dd,j=11.3,7.7hz,5h),4.42(dd,j=17.5,8.1hz,2h),4.34(d,j=8.4hz,1h),4.09(q,j=7.1hz,2h),3.93(t,j=7.9hz,1h),3.79(s,3h),2.59(s,1h),1.15(t,j=7.1hz,3h).13cnmr(101mhz,cdcl3)δ199.7,172.3,171.6,140.3,136.6,133.4,128.7(d,j=7.4hz),128.4,128.1,127.1,77.4,77.1,76.8,68.0,62.5,61.4,56.9,52.8,52.2,14.0.hrms(esi)m/z:[m+h]+calculatedforc22h23no5:382.1657,found:382.1652.enantiomericexcesswasdeterminedbyhplcwithachiralpakas-hcolumn(hexanes∶2-propanol=90∶10,1.0ml/min,254nm,89%ee);minorenantiomertr=12.5min,majorenantiomertr=20.0min.
实施例49:
在氮气气氛下,将(r)-dtbm-segphos(13mg,0.0108mmol,6mol%),agoac(1.6mg,0.0096mmol,5mol%)加入到溶解有查尔酮(3.8mg,0.0182mmol,10mol%)的2ml丙酮溶液中,-40℃下预搅30min后,依次加入亚甲胺叶立德1a(0.218mmol),烯酮类化合物2a(0.182mmol),-40℃下反应12h,tlc监测反应结束后,经硅藻土过滤,萃取,滤液浓缩,经硅胶柱层析纯化,得到无色油状液体3a,产率66%,再经手性高效液相色谱法测得ee值为91%。
该产物的理化指标:1hnmr(400mhz,cdcl3)δ7.65(d,j=7.4hz,2h),7.45(t,j=7.4hz,1h),7.38(d,j=6.7hz,2h),7.27(dd,j=11.3,7.7hz,5h),4.42(dd,j=17.5,8.1hz,2h),4.34(d,j=8.4hz,1h),4.09(q,j=7.1hz,2h),3.93(t,j=7.9hz,1h),3.79(s,3h),2.59(s,1h),1.15(t,j=7.1hz,3h).13cnmr(101mhz,cdcl3)δ199.7,172.3,171.6,140.3,136.6,133.4,128.7(d,j=7.4hz),128.4,128.1,127.1,77.4,77.1,76.8,68.0,62.5,61.4,56.9,52.8,52.2,14.0.hrms(esi)m/z:[m+h]+calculatedforc22h23no5:382.1657,found:382.1652.enantiomericexcesswasdeterminedbyhplcwithachiralpakas-hcolumn(hexanes∶2-propanol=90∶10,1.0ml/min,254nm,91%ee);minorenantiomertr=12.5min,majorenantiomertr=20.0min.
实施例50:
在氮气气氛下,将(r)-dtbm-segphos(13mg,0.0108mmol,6mol%),agoac(1.6mg,0.0096mmol,5mol%)加入到溶解有查尔酮(3.8mg,0.0182mmol,10mol%)的2ml丙酮溶液中,-40℃下预搅30min后,依次加入亚甲胺叶立德1a(0.182mmol),烯酮类化合物2a(0.182mmol),-40℃下反应12h,tlc监测反应结束后,经硅藻土过滤,萃取,滤液浓缩,经硅胶柱层析纯化,得到无色油状液体3a,产率59%,再经手性高效液相色谱法测得ee值为93%。
该产物的理化指标:1hnmr(400mhz,cdcl3)δ7.65(d,j=7.4hz,2h),7.45(t,j=7.4hz,1h),7.38(d,j=6.7hz,2h),7.27(dd,j=11.3,7.7hz,5h),4.42(dd,j=17.5,8.1hz,2h),4.34(d,j=8.4hz,1h),4.09(q,j=7.1hz,2h),3.93(t,j=7.9hz,1h),3.79(s,3h),2.59(s,1h),1.15(t,j=7.1hz,3h).13cnmr(101mhz,cdcl3)δ199.7,172.3,171.6,140.3,136.6,133.4,128.7(d,j=7.4hz),128.4,128.1,127.1,77.4,77.1,76.8,68.0,62.5,61.4,56.9,52.8,52.2,14.0.hrms(esi)m/z:[m+h]+calculatedforc22h23no5:382.1657,found:382.1652.enantiomericexcesswasdeterminedbyhplcwithachiralpakas-hcolumn(hexanes∶2-propanol=90∶10,1.0ml/min,254nm,93%ee);minorenantiomertr=12.5min,majorenantiomertr=20.0min.
对比例1
制备方法与实施例1相同,不添加查尔酮得到无色油状液体3a,产率58%,经手性高效液相色谱法测得ee值为96%,同时产生一定量无色油状副产物3b,产率27%,经手性高效液相色谱法测得ee值为89%。
副产物3b的理化指标:1hnmr(400mhz,cdcl3)δ8.00(d,j=7.2hz,1h),7.64-7.54(m,2h),7.51(d,j=5.6hz,1h),7.44-7.35(m,1h),7.30-7.23(m,2h),7.05(d,j=1.4hz,4h),4.81(d,j=8.3hz,1h),4.69-4.59(m,1h),4.39(d,j=8.7hz,1h),4.33(t,j=7.2hz,1h),4.28(d,j=8.0hz,1h),4.22(d,j=7.1hz,1h),3.89(s,2h),3.82(dd,j=7.9,6.3hz,1h),3.74-3.66(m,1h),3.47(s,1h),2.62(s,1h),1.34(d,j=7.2hz,1h),1.30(t,j=7.1hz,2h),1.23(t,j=7.0hz,1h),1.10(t,j=7.1hz,1h).13cnmr(101mhz,cdcl3)δ198.7,172.6,172.4,137.8,137.1,132.9,128.7(d,j=13.8hz),128.4-128.0(m),127.8,127.3(d,j=11.1hz),77.4,77.1,76.8,66.6,63.5,61.4,55.5,52.6,51.4,14.2.hrms(esi)m/z:[m+h]+calculatedforc22h23no5:382.1657,found:382.1652.enantiomericexcesswasdeterminedbyhplcwithachiralpakia-hcolumn(hexanes∶2-propanol=90∶10,1.0ml/min,254nm,89%ee);minorenantiomertr=12.5min,majorenantiomertr=20.0min.
说明添加了添加剂之后可抑制副产物的生成,同时提高了目标产物的产率。
上述化合物3a结构式的核磁共振氢谱图如图1所示;化合物3a结构式的碳谱图如图2所示;化合物3b结构式的核磁共振氢谱图如图3所示;化合物3b结构式的碳谱图如4所示。
总之,以上所述仅为本发明的较佳实施例,凡依本发明申请专利范围所作的均等变化与修饰,皆应属本发明专利的涵盖范围。
