一种1,3‑二甲基‑7‑取代喹唑啉‑2,4‑二酮类化合物及其合成方法和应用与流程
本发明涉及化学合成技术领域,具体是一种1,3-二甲基-7-取代喹唑啉-2,4-二酮类化合物及其合成方法和应用。
背景技术:
目前研究发现,能够引起人类疾病的真菌约有270种,可以感染人体的不同部位。浅部真菌仅侵犯人的皮肤、毛发、指甲等角化组织,而深部真菌能侵犯人的骨骼、内脏和中枢神经系统,呈现较高的发病率和死亡率。深度真菌感染已成为艾滋病、急性白血病、器官或骨髓移植等免疫抑制病人死亡的主要原因。随着人类健康意识的提升及对真菌感染类疾病的重视,抗真菌药物的研究已经成为全球药物研究的一个热点。尽管现在有大量的抗真菌药物,如阿莫西林、两性霉素B和唑类,但由于这些抗菌剂具有较窄的光谱活性,而且一些微生物已经产生抗药性,因此,迫切需要设计合成新的抗菌药物。
喹唑啉的环结构是多种生物碱的骨架。喹唑啉酮类药物具有多重生物活性,近年来,喹唑啉酮类化合物在抗艾滋、抗肿瘤、抗癌、抗病毒、抗菌等方面的应用均有报道,这类化合物的研究文章可见:Eur J Med Chem.2009,3046-3055;Expert Opin Ther Pat..2015,789-804;American Journal of PharmTech Research.2013,466-475;Eur J Med Chem.2010,1200-1205;Chin J Chem.2003,339-346;International Research Journal of Pharmacy.2014,476-480;Prog Nat Sci.1998,359-365;Chem Biol Drμg Des.2015,672-684.
这些文献均未覆盖或包括本发明所涉及的新型化合物的结构、合成方法和用途。本发明设计合成了一类1,3-二甲基-7-取代喹唑啉-2,4-二酮类化合物。以多氧霉素B为对照,测定了新化合物对几丁质合成酶的抑制作用,并测定了其在抗真菌,抗细菌方面的活性,拓展了喹唑啉的应用研究。到目前为止,本发明所涉及的新型化合物在抑制几丁质合成酶活性方面还未见报道,所以将其作为几丁质合成酶的抑制剂,开发成新型的抗真菌制剂,为人类的健康做出更大的贡献。
技术实现要素:
本发明的目的在于提供一种1,3-二甲基-7-取代喹唑啉-2,4-二酮类化合物及其合成方法和应用,以解决上述背景技术中提出的问题。
为实现上述目的,本发明提供如下技术方案:
一种1,3-二甲基-7-取代喹唑啉-2,4-二酮类化合物,所述化合物的结构通式为:
式中R1为氢或乙基;R2为苯环、苯环衍生物、杂环或脂肪烃。
进一步的,所述化合物的结构为:
其中R1为H、CH2CH3;
R2为包括但不限于4-CH3OC6H4、4-CH3C6H4、4-FC6H4、4-ClC6H4、4-CF3C6H4、2-ClC6H4、CH2CH3。
进一步的,上述的1,3-二甲基-7-取代喹唑啉-2,4-二酮类化合物为下述化合物的任意一种,
一种所述的1,3-二甲基-7-取代喹唑啉-2,4-二酮类化合物的合成方法,按如下合成方式进行,
具体地说,上述反应条件如下
a)化合物1以DMF为溶剂,在碱性条件下和碘甲烷反应生成化合物2,进一步水解生成化合物3;
b)化合物3和尿素反应生成化合物4,再与碘甲烷反应生成化合物5,进一步水解得中间体6;
c)化合物7a-7u,分别与氯乙酰氯反应生成化合物8a-8u,8a-8u分别于N-甲基乙醇胺反应生成化合物9a-9u;
d)中间体6与化合物9a-9u,经缩合反应生成产物10a-10u。
作为本发明进一步的方案:具体步骤如下:
1)在反应器中,依次加入2-氨基对苯二甲酸二甲酯、氢氧化钠、无水硫酸钠、DMF为溶剂,室温搅拌8~12min,再滴加碘甲烷与DMF的混合液,室温反应55~65min,再加热至50℃反应8~10h;
2)化合物2与氢氧化钠的摩尔比为1:2~3,78~82℃反应1.5~2.5h,水和甲醇为溶剂;
3)化合物3与尿素摩尔比为1:9~11,145~155℃反应5~7h;
4)化合物4、碳酸钾、碘甲烷、无水硫酸钠摩尔比为1:1.8~2.2:1.8~2.2:0.2~0.3,DMF为溶剂,48~52℃反应8~10;
5)化合物5与氢氧化钠摩尔比为1:9~11,48~52℃反应8~10,水和甲醇为溶剂;
6)化合物7a与氯乙酰氯、碳酸氢钠摩尔比为1:1.1~1.3:4.5~5.5,二氯甲烷为溶剂,冰浴反应55~65min;
7)化合物8a、N-甲基乙醇胺、碳酸钾摩尔比为1:1:1.4~1.6,乙腈为溶剂,78~82℃反应2h;
8)化合物6、9a-9u、DMAP、DCC的摩尔比为1:1:0.2~0.3:1,二氯甲烷为溶剂,冰浴反应1.5~2.5h。
一种所述的1,3-二甲基-7-取代喹唑啉-2,4-二酮类化合物在制备抗病原微生物药物中的应用。
进一步的,所述微生物包括但不限于大肠杆菌、金黄色葡萄球菌、耐甲氧西林金黄色葡萄球菌、枯草杆菌、变形杆菌、铜绿色假单胞菌、白色念珠菌、新型隐球菌、黄曲霉菌、烟曲霉菌。
一种所述的1,3-二甲基-7-取代喹唑啉-2,4-二酮类化合物在几丁质合成酶抑制剂药物中的应用。
一种药物组合物,其含有权利要求1、2所述的1,3-二甲基-7-取代喹唑啉-2,4-二酮类化合物和药学上可接受载体。
与现有技术相比,本发明的有益效果是:
经生物活性测试实验证明,本发明的部分化合物对白色念珠菌、黄曲霉菌、新型隐球菌和烟曲霉菌表现出较好的抑制活性。而且对几丁质合成酶抑制活性明显,抑菌效果较好,可用于制备抗病原微生物的药物。
附图说明
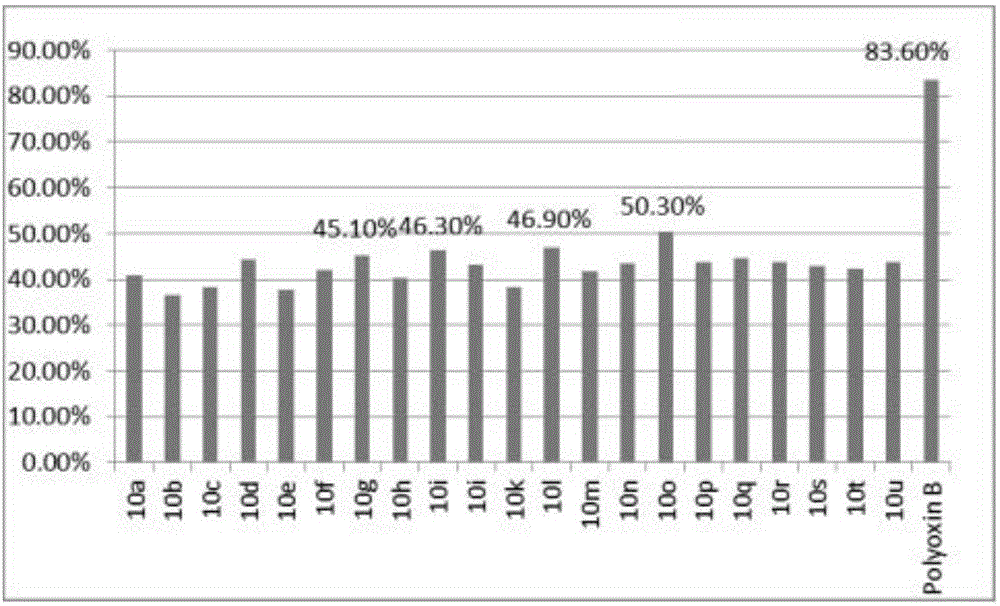
图1为测试化合物浓度为300μg/ml时的几丁质合成酶抑制率的对比图。
具体实施方式
下面将结合本发明实施例中的附图,对本发明实施例中的技术方案进行清楚、完整地描述,显然,所描述的实施例仅仅是本发明一部分实施例,而不是全部的实施例。基于本发明中的实施例,本领域普通技术人员在没有做出创造性劳动前提下所获得的所有其他实施例,都属于本发明保护的范围。
实施例1
在带有干燥管的250mL烧瓶中,加入2-氨基对苯二甲酸二甲酯(10.344g,50mmol),氢氧化钠(3.000g,74mmol),无水硫酸钠(1.776,12mmol)和二甲基甲酰胺(DMF)60mL,室温搅拌十min后再滴加碘甲烷(10.508g,74mmol)与DMF 60mL的混合液,加毕室温反应1h,再加热至50℃反应9h,反应结束后,冷却至室温,加水100mL,用乙酸乙酯萃取(50mL×3),合并乙酸乙酯萃取液,无水硫酸镁干燥,抽滤,滤液旋蒸除去溶剂,然后过柱(乙酸乙酯:石油醚=1:25),即制得化合物2(黄色固体)7.682g,收率68.9%,1H NMR(600MHz,CDCl3):δ3.49(s,3H,CH3),3.90(s,6H,CH3),7.00(s,1H,Ar-3-H),7.17(d,J=6.8Hz,1H,Ar-5-H),7.80(d,J=7.2Hz,1H,Ar-6-H);13C NMR(75MHz,CDCl3)δ:29.63,51.42,111.10,115.11,119.16,130.23,135.21,150.24,165.28,168.59;MS(m/z,%):224(M+1,100)。
实施例2
在250mL烧瓶中,加入化合物2(7.375g,33mmol)、水80mL、甲醇60mL和氢氧化钠(3.330g,83mmol),加热至80℃反应1h,反应结束后,旋蒸除去甲醇,用稀盐酸调节pH=2,析出黄色固体,抽滤,滤饼用水洗涤(30mL×3)后干燥,即制得化合物3(黄色固体)5.920g,收率92%,1H NMR(600MHz,DMSO-d6):δ3.49(s,3H,CH3),7.77(d,J=8.1Hz,1H,Ar-5-H),7.83(s,1H,Ar-3-H),8.09(d,J=8.1Hz,1H,Ar-6-H),11.70(s,1H,COOH),13.58(s,1H,COOH);13C NMR(75MHz,CDCl3);δ29.63,111.76,112.16,119.14,130.22,134.21,150.29,166.38,169.52;MS(m/z,%):196(M+1,100)。
实施例3
在带有干燥管的100mL烧瓶中,加入尿素(17.400g,290mmol),加热至尿素完全溶解后,再加入化合物3(5.732g,29.mmol),加热至150℃反应6h,反应结束后,冷却至100℃,加水80mL,搅拌20min,冷却至室温,加盐酸至PH=2,放置后抽滤,滤饼用水洗涤(50mL×3),干燥,即制得中间体4(肉色固体)5.742g,收率90.0%,1H NMR(600MHz,DMSO):δ3.49(s,3H,CH3)7.77(d,J=8.1Hz,1H,Ar-4-H),7.83(s,1H,Ar-6-H),8.09(d,J=8.1Hz,1H,Ar-3-H),11.70(s,1H,COOH),13.58(s,1H,COOH);13C NMR(75MHz,DMSO-d6)δ30.05,120,86,126.77,127.63,131.13,133.25,136.25,150.29,161.51,166.54;MS(m/z,%):221(M+1,100)
实施例4
在带有干燥管的250mL烧瓶中,加入化合物4(4.400g,20mmol),碳酸钾(2.760g,40mmol),DMF 40mL,无水硫酸钠(0.710g,5mmol),室温搅拌下缓慢向烧瓶中滴加碘甲烷5.680g,40mmol)与DMF 30mL的混合液,滴完后室温反应1h,然后加热至55℃反应6h,停止反应,冷却至室温,抽滤,滤液倒入200mL水中,搅拌,用乙酸乙酯萃取(50mL×4),硫酸镁干燥乙酸乙酯层,抽滤,滤液旋蒸除去溶剂,得到淡黄色固体,用乙酸乙酯重结晶得到化合物5(白色松软固体)4.072g,收率87.0%。1H NMR(600MHz,CDCl3):δ3.50(s,3H,CH3),3.67(s,3H,CH3),3.99(s,3H,CH3),7.90-7.87(m,2H,quinazolone-5,8-H),8.29(s,1H,quinazolone-6-H);13C NMR(75MHz,CDCl3):δ29.01,30.92,52.95,119.83,11.93,123.69,127.16,132.97,136.48,150.8,161.2,165.9;MS(m/z,%):249(M+1,100)。
实施例5
在250mL烧瓶中,加入化合物5(4.031g,16mmol)、水40mL、甲醇30mL和氢氧化钠(6.400g,160mmol),加热至60℃反应2h,反应结束后,旋蒸除去甲醇,用稀盐酸调节pH=2,析出黄色固体,抽滤,滤饼用水洗涤(30mL×3)后干燥,即制得中间体6(黄色固体)3.440g,收率92%,1H NMR(600MHz,DMSO-d6):δ3.32(s,3H,CH3),3.57(s,1H,CH3),7.83(d,J=9.7Hz,2H,quinazolone-5,6-H),8.14(s,1H,quinazolone-8-H),13.55(s,1H,COOH).13C NMR(75MHz,DMSO-d6):δ29.0,30.95,120.81,120.93,126.62,127.13,131.93,136.42,150.8,161.2,165.9;MS(m/z,%):235(M+1,100)。
实施例6
在50mL圆底烧瓶中加入7a(0.456g,5mmol),CH2Cl2(10mL),碳酸氢钠(1.050g,25mmol)室温搅拌10min,冰浴滴加氯乙酰氯(0.672g,6mmol)的二氯甲烷(15mL)溶液,冰浴反应2h,停止反应,加水40mL,用二氯甲烷(15mL×3)萃取,合并有机层,用水(30mL×3)洗有机层,硫酸镁干燥有机层,旋去溶剂得白色固体,用石油醚和乙酸乙酯混合溶剂重结晶得白色粉末状固体8、化合物8b–8u的合成与8a相同.8a-8u的物理常数及光谱数据如下所示。
N-苯基-2-氯乙酰胺8a
白色粉末,产率89%,熔点141.4-142.6℃,1H NMR(600MHz,CDCl3):δ4.19(s,2H,CH2),7.19(t,J=7.4Hz,1H,Ar-4-H),7.36(t,J=7.7Hz,2H,Ar-3,5-H),7.55(d,J=8.1Hz,2H,Ar-2,6-H),8.26(s,1H,CONH);MS(m/z,%):170(M+1,100)。
N-(4-甲氧基苯基)-2-氯-乙酰胺8b
白色粉末,产率90%,熔点101.2-102.1℃,1H NMR(600MHz,CDCl3):δ3.68(s,3H,O-CH3),4.21(s,2H,CH2),6.86(d,J=9.1Hz,2H,Ar-3,5-H),7.46(d,J=9.1Hz,2H,Ar-2,4-H),8.23(s,1H,CONH);MS(m/z,%):200(M+1,100)。
N-(4-(三氟甲基)苯基)-2-氯-乙酰胺8c
白色针状固体,产率92%,熔点161.2-162.3℃,1H NMR(600MHz,CDCl3):δ4.22(s,2H,CH2),7.62(d,J=12Hz,2H,Ar-3,5-H),7.69(d,J=9Hz,2H,Ar-2,4-H),8.36(s,1H,CONH);MS(m/z,%):238(M+1,100)。
N-(2-氯苯基)-2-氯-乙酰胺8d
粉色粉末,产率80%,熔点73.8–74.6℃,1H NMR(600MHz,CDCl3):δ4.21(s,2H,CH2),7.11(t,J=7.5Hz,1H,Ar-4-H),7.30(t,J=12.0Hz,1H,Ar-5-H,),7.36(d,J=9.1Hz,1H,Ar-3-H),8.47(d,J=6.2Hz,1H,Ar-6-H),8.94(s,1H,CONH);MS(m/z,%):204(M+1,100)。
N-(4-氯苯基)-2-氯-乙酰胺8e
白色针状固体,产率82%,熔点180.4–181.3℃,1H NMR(600MHz,CDCl3):δ4.10(s,2H,CH2),7.22(d,J=9.1Hz,2H,Ar-3,5-H),7.53(d,J=12.0Hz,2H,Ar-2,4-H),8.27(s,1H,CONH);MS(m/z,%):204(M+1,100)。
N-(2-甲苯基)-2-氯-乙酰胺8f
白色粉末,产率84%,熔点109.7–111.1℃,1H NMR(600MHz,CDCl3):δ2.87(s,3H,CH3),4.18(s,2H,CH2),7.07(t,J=8.1Hz,1H,Ar-4-H),7.28(t,J=8.1Hz,1H,Ar-5-H),7.37(d,J=8.1Hz,1H,Ar-6-H),7.48(d,J=8.1Hz,1H,Ar-3-H),8.30(s,1H,CONH);MS(m/z,%):184(M+1,100)。
N-(4-甲苯基)-2-氯-乙酰胺8g
白色粉末,产率75%,熔点169.2–170.1℃,1H NMR(600MHz,CDCl3):δ3.43(s,3H,CH3),4.18(s,2H,CH2),6.90(d,J=8.1Hz,2H,Ar-3,5-H),7.56(d,J=8.7Hz,2H,Ar-2,4-H),8.30(s,1H,CONH);MS(m/z,%):184(M+1,100)。
N-(4-氟苯基)-2-氯-乙酰胺8h
白色针状固体,产率83%,熔点129.9–131.0℃,1H NMR(600MHz,CDCl3):δ4.26(s,2H,CH2),7.22(d,J=9.1Hz,2H,Ar-3,5-H),7.63(d,J=12.0Hz,2H,Ar-2,4-H),8.27(s,1H,CONH);MS(m/z,%):188(M+1,100)。
N-(3-氯-4-氟苯基)-2-氯-乙酰胺8i
白色粉末,产率74%,熔点75.9–77.4℃,1H NMR(600MHz,CDCl3):δ4.26(s,2H,CH2),7.40(d,J=6.8Hz,1H,Ar-5-H),7.56(d,J=7.0Hz,1H,Ar-6-H),7.92(s,1H,Ar-2-H),8.28(s,1H,CONH);MS(m/z,%):222(M+1,100)。
N-(3-氯-4-甲基苯基)-2-氯-乙酰胺8j
白色粉末,产率90%,熔点176.7–178.0℃,1H NMR(600MHz,CDCl3):δ4.26(s,2H,CH2),7.40(d,J=6.8Hz,1H,Ar-5-H),7.58(d,J=7.0Hz,1H,Ar-6-H),7.82(s,1H,Ar-2-H),8.30(s,1H,CONH);MS(m/z,%):218(M+1,100)。
N-苄基-2-氯乙酰胺8k
粉色粉末,产率80%,熔点95.1–96.1℃,1H NMR(600MHz,CDCl3):δ4.26(s,2H,CH2),4.49(d,J=6.1Hz,2H,Ar-CH2),6.89(s,1H,CONH),7.29–7.38(m,5H,Ar-H);MS(m/z,%):184(M+1,100)。
N-(3-甲苯基)-2-氯-乙酰胺8l
白色粉末,产率86%,熔点90.2–91.1℃,1H NMR(600MHz,CDCl3),δ:2.37(s,3H,CH3),4.18(s,2H,CH2),6.97(d,J=8.1Hz,1H,Ar-4-H),7.28(t,J=8.1Hz,1H,Ar-5-H),7.37(d,J=8.1Hz,1H,Ar-6-H),7.48(s,1H,Ar-2-H),8.30(s,1H,CONH);MS(m/z,%):184(M+1,100)。
N-(3,5-二(三氟甲基)苯基)-2-氯乙酰胺8m
白色粉末,产率86%,熔点90.2–91.2℃,1H NMR(600MHz,CDCl3):δ4.23(s,2H,CH2),7.68(s,1H,Ar-4-H),8.17(s,2H,Ar-2,6-H),8.30(s,1H,CONH);MS(m/z,%):306(M+1,100)。
N-(4-硝基苯)-2-氯-乙酰胺8n
淡黄色粉末,产率88%,熔点190.6–191.8℃,1H NMR(600MHz,CDCl3):δ4.26(s,2H,CH2),7.26(d,J=6.2Hz,2H,Ar-3,5-H),8.63(d,J=9.0Hz,2H,Ar-2,6-H),8.27(s,1H,CONH);MS(m/z,%):215(M+1,100)。
N-(3,4-二氯苯基)-2-氯-乙酰胺8o
白色粉末,产率76%,熔点75.2–76.8℃,1H NMR(600MHz,CDCl3):δ4.26(s,2H,CH2),7.53(d,J=6.8Hz,1H,Ar-5-H),7.64(d,J=7.0Hz,1H,Ar-6-H),7.92(s,1H,Ar-2-H),8.28(s,1H,CONH);MS(m/z,%):238(M+1,100)。
2-(2-氯乙酰氨基)苯甲酸甲酯8p
白色粉末,产率80%,熔点98.3–99.4℃,1H NMR(600MHz,CDCl3):δ3.96(s,3H,CH3),4.22(s,2H,CH2),7.16(t,J=9.1Hz,1H,Ar-4-H),7.28(s,1H,CONH),7.57(t,J=7.5Hz,1H,Ar-5-H),8.06(d,J=3.2Hz,1H,Ar-3-H),8.70(d,J=9.5Hz,1H,Ar-6-H);MS(m/z,%):228(M+1,100)。
N-(6-甲氧吡啶)-2-氯-乙酰胺8q
白色粉末,产率72%,熔点127.6–128.2℃,1H NMR(600MHz,CDCl3):δ3.72(s,3H,CH3),4.26(s,2H,CH2),7.56(s,1H),8.28(s,1H,CONH),8.57(s,1H);MS(m/z,%):202(M+1,100)。
N-(1-萘)-2-氯-乙酰胺8r
白色粉末,产率68%,熔点162.3–163.1℃,1H NMR(600MHz,CDCl3):δ4.26(s,2H,CH2),6.89(d,J=6.7Hz,1H,naphthalen-2-H),7.37(t,J=7.3Hz,1H,naphthalen-3-H),7.44(t,J=7.6Hz,1H,naphthalen-7-H),7.54(t,J=8.0Hz,1H,Ar-6-H),7.66(d,J=5.1Hz,1H,naphthalen-4-H),8.03(d,J=5.1Hz,1H,naphthalen-5-H),8.15(d,J=8.0Hz,1H,naphthalen-8-H),9.86(s,1H,CONH);MS(m/z,%):220(M+1,100)。
N-乙基-2-氯-乙酰胺8s
淡黄色液体,产率75%,1H NMR(600MHz,CDCl3)δ:1.07(t,J=6.1Hz,3H,CH3),3.11(t,J=6.1Hz,2H,CH2),4.19(s,2H,CH2),8.08(s,1H,CONH);MS(m/z,%):122(M+1,100)。
N,N-二乙基-2-氯-乙酰胺8t
淡黄色液体,产率70%,1H NMR(600MHz,CDCl3)δ:1.17(t,J=6.2Hz,6H,CH3),3.47(t,J=6.2Hz,4H,CH2),4.19(s,2H,CH2);MS(m/z,%):150(M+1,100)。
N-叔丁基-2-氯乙酰胺8u
淡黄色液体,产率82%,1HNMR(600MHz,CDCl3):δ1.37(s,9H,CH3),4.19(s,2H,CH2),8.01(s,1H,CONH);MS(m/z,%):150(M+1,100)。
实施例7
在100mL圆底烧瓶中加入化合物8a(2.535g,15mmol),乙腈(40mL),K2CO3(3.105g,22.5mmol),滴加N-甲基乙醇胺(1.100g,15mmol)的乙腈(10mL)溶液,滴加完后60℃反应4h。反应结束后,减压旋去乙腈,加入乙酸乙酯10mL,有机层用水洗至PH=7,取有机层,旋去溶剂得化合物9a。其他化合物9b–9u的合成方法同上。9a-9u的物理常数及光谱数据如下所示。
N-苯基-2-((2-羟基乙基)(甲基)氨基)乙酰胺9a
淡黄色液体,产率82%,1H NMR(600MHz,CDCl3):δ2.34(s,3H,CH3),2.64(t,J=5.4Hz,2H,CH2),3.32(s,2H,CH2),3.47(t,J=5.4Hz,2H,CH2),7.03(t,J=7.3Hz,1H,Ar-4-H),7.28(t,J=7.8Hz,2H,Ar-3,5-H),7.61(d,J=8.0Hz,2H,Ar-2,6-H),9.78(s,1H,CONH);MS(m/z,%):209(M+1,100)。
N-(4-甲氧基苯基)-2-((2-羟基乙基)(甲基)氨基)乙酰胺9b
淡黄色液体,产率85%,1H NMR(600MHz,CDCl3):δ2.50(s,3H,CH3),3.29(t,J=5.4Hz,2H,CH2),3.32(s,2H,CH2),3.47(t,J=5.4Hz,2H,CH2),3.81(s,3H,CH3),7.08(d,J=7.8Hz,2H,Ar-3,5-H),7.51(d,J=8.0Hz,2H,Ar-2,6-H),9.36(s,1H,CONH);MS(m/z,%):239(M+1,100)。
N-(4-(三氟甲基)苯基)-2-((2-羟基乙基)(甲基)氨基)乙酰胺9c
黄色液体,产率86%,1H NMR(600MHz,CDCl3):δ2.60(s,3H,CH3),3.31(t,J=5.4Hz,2H,CH2),3.33(s,2H,CH2),3.47(t,J=5.4Hz,2H,CH2),7.10(d,J=7.8Hz,2H,Ar-3,5-H),7.51(d,J=8.0Hz,2H,Ar-2,6-H),9.75(s,1H,CONH);MS(m/z,%):277(M+1,100)。
N-(2-氯苯基)-2-((2-羟基乙基)(甲基)氨基)乙酰胺9d
淡黄色液体,产率85%,1H NMR(600MHz,CDCl3):δ2.52(s,3H,CH3),2.64(t,J=5.3Hz,2H,CH2),3.32(s,2H,CH2),3.47(t,J=5.4Hz,2H,CH2),7.03(t,J=7.5Hz,1H,Ar-4-H),7.17(t,J=7.8Hz,1H,Ar-5-H),7.53(d,J=7.6Hz,1H,Ar-3-H),8.02(d,J=8.0Hz,1H,Ar-6-H),9.75(s,1H,CONH);MS(m/z,%):243(M+1,100)。
N-(4-氯苯基)-2-((2-羟基乙基)(甲基)氨基)乙酰胺9e
淡黄色液体,产率82%,1H NMR(600MHz,CDCl3):δ2.63(s,3H,CH3),3.32(t,J=5.4Hz,2H,CH2),3.35(s,2H,CH2),3.47(t,J=5.4Hz,2H,CH2),7.37(d,J=8.0Hz,2H,Ar-3,5-H),7.63(d,J=7.6Hz,2H,Ar-2,6-H),9.69(s,1H,CONH);MS(m/z,%):243(M+1,100)。
N-(2-甲苯基)-2-((2-羟基乙基)(甲基)氨基)乙酰胺9f
淡黄色液体,产率88%,1H NMR(600MHz,CDCl3):δ2.12(s,3H,CH3),2.60(s,3H,CH3),3.29(t,J=5.4Hz,2H,CH2),3.35(s,2H,CH2),3.47(t,J=5.5Hz,2H,CH2),6.93(t,J=7.2Hz,1H,Ar-4-H),7.17(t,J=7.3Hz,1H,Ar-5-H),7.33(d,J=7.6Hz,1H,Ar-6-H),7.43(d,J=8.0Hz,1H,Ar-3-H),9.86(s,1H,CONH);MS(m/z,%):223(M+1,100)。
N-(4-甲苯基)-2-((2-羟基乙基)(甲基)氨基)乙酰胺9g
淡黄色液体,产率84%,1H NMR(600MHz,CDCl3):δ2.22(s,3H,CH3),2.44(s,3H,CH3),3.27(t,J=5.4Hz,2H,CH2),3.32(s,2H,CH2),3.47(t,J=5.5Hz,2H,CH2),7.11(t,J=8.2Hz,2H,Ar-3,5-H),7.46(t,J=8.3Hz,2H,Ar-2,6-H),9.76(s,1H,CONH);MS(m/z,%):223(M+1,100)。
N-(4-氟苯基)-2-((2-羟基乙基)(甲基)氨基)乙酰胺9h
淡黄色液体,产率82%,1H NMR(600MHz,CDCl3):δ2.16(s,3H,CH3),3.27(t,J=5.4Hz,2H,CH2),3.31(s,2H,CH2),3.47(t,J=5.5Hz,2H,CH2),7.12(t,J=7.6Hz,2H,Ar-3,5-H),7.50(t,J=7.7Hz,2H,Ar-2,6-H),9.85(s,1H,CONH);MS(m/z,%):223(M+1,100)。
N-(3-氯-4-氟苯基)-2-((2-羟基乙基)(甲基)氨基)乙酰胺9i
淡黄色液体,产率76%,1H NMR(600MHz,CDCl3):δ2.26(s,3H,CH3),3.27(t,J=5.4Hz,2H,CH2),3.31(s,2H,CH2),3.47(t,J=5.5Hz,2H,CH2),7.29(d,J=8.6Hz,1H,Ar-5-H),7.50(d,J=8.7Hz,1H,Ar-6-H),7.85(s,1H,Ar-2-H),9.73(s,1H,CONH);MS(m/z,%):261(M+1,100)。
N-(3-氯-4-甲基苯基)-2-((2-羟基乙基)(甲基)氨基)乙酰胺9j
淡黄色液体,产率83%,1H NMR(600MHz,CDCl3):δ2.23(s,3H,CH3),2.44(s,3H,CH3),3.27(t,J=5.4Hz,2H,CH2),3.33(s,2H,CH2),3.47(t,J=5.5Hz,2H,CH2),7.09(d,J=7.0Hz,1H,Ar-5-H),7.34(d,J=7.2Hz,1H,Ar-6-H),7.87(s,1H,Ar-2-H),9.85(s,1H,CONH);MS(m/z,%):257(M+1,100)。
N-苄基-2-((2-羟基乙基)(甲基)氨基)乙酰胺9k
淡黄色液体,产率70%,1H NMR(600MHz,CDCl3):δ2.60(s,3H,CH3),3.29(t,J=5.4Hz,2H,CH2),,4.32(s,2H,CH2),3.47(t,J=5.4Hz,2H,CH2),4.31(s,2H,CH2),7.03(d,J=7.3Hz,2H,Ar-2,6-H),7.18(t,J=7.5Hz,1H,Ar-4-H),7.28(d,J=8.0Hz,2H,Ar-3,5-H),9.86(s,1H,CONH);MS(m/z,%):223(M+1,100)。
N-(3-甲苯基)-2-((2-羟基乙基)(甲基)氨基)乙酰胺9l
淡黄色液体,产率87%,1H NMR(600MHz,CDCl3):δ2.16(s,3H,CH3),2.24(s,3H,CH3),3.27(t,J=5.4Hz,2H,CH2),3.31(s,2H,CH2),3.47(t,J=5.5Hz,2H,CH2),6.92(d,J=5.0Hz,1H,Ar-4-H),7.24(t,J=7.2Hz,1H,Ar-5-H),7.42(d,J=5.0Hz,1H,Ar-6-H),7.64(s,1H,Ar-2-H),9.85(s,1H,CONH);MS(m/z,%):223(M+1,100)。
N-(3,5-二(三氟甲基)苯基)-2-((2-羟基乙基)(甲基)氨基)乙酰胺9m
淡黄色液体,产率76%,1H NMR(600MHz,CDCl3)δ:2.16(s,3H,CH3),3.27(t,J=5.4Hz,2H,CH2),3.31(s,2H,CH2),3.47(t,J=5.5Hz,2H,CH2),7.75(s,1H,Ar-4-H),8.14(s,2H,Ar-2,6-H),9.85(s,1H,CONH);MS(m/z,%):345(M+1,100)。
N-(4-硝基苯基)-2-((2-羟基乙基)(甲基)氨基)乙酰胺9n
淡黄色液体,产率67%,1H NMR(600MHz,CDCl3):δ2.26(s,3H,CH3),3.27(t,J=5.4Hz,2H,CH2),3.32(s,2H,CH2),3.47(t,J=5.5Hz,2H,CH2),7.82(t,J=8.0Hz,2H,Ar-2,6-H),8.24(t,J=7.7Hz,2H,Ar-3,5-H),9.85(s,1H,CONH);MS(m/z,%):254(M+1,100)。
N-(3,4-二氯苯基)-2-((2-羟基乙基)(甲基)氨基)乙酰胺9o
淡黄色液体,产率77%,1H NMR(600MHz,CDCl3):δ2.23(s,3H,CH3),3.27(t,J=5.4Hz,2H,CH2),3.34(s,2H,CH2),3.49(t,J=5.5Hz,2H,CH2),7.39(d,J=8.3Hz,1H,Ar-5-H),7.50(t,J=8.7Hz,1H,Ar-6-H),7.93(s,1H,Ar-2-H),9.85(s,1H,CONH);MS(m/z,%):277(M+1,100)。
2-(2-((2-羟基乙基)(甲基)氨基)乙酰氨基)苯甲酸甲酯9p
淡黄色液体,产率85%,1H NMR(600MHz,CDCl3):δ2.12(s,3H,CH3),3.29(t,J=5.4Hz,2H,CH2),3.35(s,2H,CH2),3.47(t,J=5.5Hz,2H,CH2),3.87(s,3H,CH3),7.20(t,J=7.2Hz,1H,Ar-4-H),7.47(t,J=7.3Hz,1H,Ar-5-H),7.83(d,J=7.6Hz,1H,Ar-3-H),8.23(d,J=8.0Hz,1H,Ar-6-H),9.86(s,1H,CONH);MS(m/z,%):267(M+1,100)。
N-(6-甲氧吡啶)-2-((2-羟基乙基)(甲基)氨基)乙酰胺9q
淡黄色液体,产率67%,1H NMR(600MHz,CDCl3):δ2.16(s,3H,CH3),3.27(t,J=5.4Hz,2H,CH2),3.36(s,2H,CH2),3.47(t,J=5.5Hz,2H,CH2),3.86(s,3H,CH3),7.46(s,1H,aromatic-H),9.30(s,1H,aromatic-H),9.85(s,1H,CONH);MS(m/z,%):241(M+1,100)。
N-(1-萘)-2-((2-羟基乙基)(甲基)氨基)乙酰胺9r
淡黄色液体,产率72%,1H NMR(600MHz,CDCl3):δ2.12(s,3H,CH3),3.29(t,J=5.4Hz,2H,CH2),3.41(s,2H,CH2),3.47(t,J=5.5Hz,2H,CH2),6.89(d,J=6.7Hz,1H,naphthalen-2-H),7.37(t,J=7.3Hz,1H,naphthalen-3-H),7.44(t,J=7.6Hz,1H,naphthalen-7-H),7.54(t,J=8.0Hz,1H,Ar-6-H),7.66(d,J=5.1Hz,1H,naphthalen-4-H),8.06(d,J=5.1Hz,1H,naphthalen-5-H),8.13(d,J=8.0Hz,1H,naphthalen-8-H),9.86(s,1H,CONH);MS(m/z,%):259(M+1,100)。
N-乙基-2-((2-羟基乙基)(甲基)氨基)乙酰胺9s
淡黄色液体,产率79%,1H NMR(600MHz,CDCl3):δ1.04(t,J=6.7Hz,3H,CH3),2.12(s,3H,CH3),3.29(t,J=5.4Hz,2H,CH2),3.31(s,2H,CH2),3.33(m,2H,CH2)3.47(t,J=5.5Hz,2H,CH2),8.86(s,1H,CONH);MS(m/z,%):161(M+1,100)。
N,N-二乙基-2-((2-羟基乙基)(甲基)氨基)乙酰胺9t
淡黄色液体,产率78%,1H NMR(600MHz,CDCl3):δ1.04(t,J=6.7Hz,6H,CH3),2.12(s,3H,CH3),3.29(t,J=5.4Hz,2H,CH2),3.30(s,2H,CH2),3.34(m,4H,CH2),3.47(t,J=5.5Hz,2H,CH2);MS(m/z,%):189(M+1,100)。
N-叔丁基-2-((2-羟基乙基)(甲基)氨基)乙酰胺9u
淡黄色液体,产率79%,1H NMR(600MHz,CDCl3):δ1.34(s,9H,CH3),2.12(s,3H,CH3),3.29(t,J=5.4Hz,2H,CH2),3.32(s,2H,CH2),3.48(t,J=5.5Hz,2H,CH2),8.86(s,1H,CONH);MS(m/z,%):189(M+1,100)。
实施例8
在100mL圆底烧瓶中加入化合物6(1.200g,2.5mmol),CH2Cl2(15mL),DMAP(0.060g,0.003mmol),DCC(1.038g,2.5mmol),冰浴下滴加9a(1.045g,2.5mmol)的CH2Cl2(10mL)溶液,室温反应6h。反应结束后,-15℃冷却2h。过滤,干法过柱(乙酸乙酯:石油醚=1:1)得产物10a.化合物10b–10u合成方法同上。
上述制得的化合物,经X-6精密显微熔点测定仪测定熔点(未校正);1H CNMR谱用BrukerAV-300型傅立叶变换核磁共振仪测定,使用Shimadzu LC-MS-2010A/EV测定,其具体分析数据如下:
白色粉末,产率68%,熔点156.5-157.3℃,1H NMR(600MHz,CDCl3):δ2.44(s,3H,CH3),2.93(t,J=5.0Hz,2H,CH2),3.20(s,2H,CH2),3.44(s,3H,CH3),3.48(s,3H,CH3),4.49(t,J=5.2Hz,2H,CH2),6.95(t,J=7.3Hz,1H,Ar-4-H),7.11(t,J=7.8Hz,2H,Ar-3,5-H),7.31(d,J=7.9Hz,2H,Ar-2,6-H),7.70(s,1H,CONH),7.76(d,J=8.1Hz,1H,quinolin-6-H),8.16(d,J=8.1Hz,1H,quinolin-5-H),8.93(s,1H,quinolin-8-H);13C NMR(75MHz,CDCl3):δ29.81,30.80,43.52,58.06,61.64,62.28,119.63,119.92,121.68,125.64,127.59,128.03,128.91,133.72,136.15,138.58,151.30,161.92,165.93,168.52;MS(m/z,%):425(M+1,100)。
黄色粉末,产率80%,熔点133.3-134.8℃,1H NMR(600MHz,CDCl3):δ2.51(s,3H,CH3),2.98(t,J=4.7Hz,2H,CH2),3.25(s,2H,CH2),3.50(s,3H,CH3),3.51(s,3H,CH3),3.73(s,1H,CH3),4.55(t,J=5.2Hz,2H,CH2),6.68(d,J=8.7Hz,2H,Ar-3,5-H),7.24(d,J=8.8Hz,2H,Ar-2,6-H),7.73(s,1H,quinolin-8-H),7.86(d,J=8.1Hz,1H,quinolin-6-H),8.23(d,J=8.1Hz,1H,quinolin-5-H),8.87(s,1H,CONH);13C NMR(75MHz,CDCl3):δ28.64,30.88,43.65,55.44,56.81,62.17,63.12,113.96,115.04,118.59,121.06,123.16,129.29,130.60,135.48,140.40,150.90,156.34,161.26,165.41,168.14;MS(m/z,%):455(M+1,100)。
黄色粉末,产率68%,熔点133.1-134.0℃,1H NMR(600MHz,CDCl3):δ2.54(s,3H,CH3),3.02(t,J=4.7Hz,2H,CH2),3.31(s,2H,CH2),3.49(s,3H,CH3),3.53(s,3H,CH3),4.58(t,J=5.2Hz,2H,CH2),7.42(d,J=8.5Hz,2H,Ar-3,5-H),7.53(d,J=8.4Hz,2H,Ar-2,6-H),7.73(s,1H,CONH),7.83(d,J=8.1Hz,1H,quinolin-6-H),8.26(d,J=8.1Hz,1H,quinolin-5-H),9.24(s,1H,quinolin-8-H);13C NMR(75MHz,CDCl3):δ29.60,30.84,43.77,56.81,62.14,63.17,114.96,118.64,119.95,123.96,125.36,125.61,129.32,132.10,133.71,135.29,140.60,150.48,161.12,165.39,168.80;MS(m/z,%):493(M+1,100)。
黄色固体,产率68%,熔点197.8-199.0℃,1H NMR(600MHz,CDCl3):δ2.54(s,3H,CH3),3.02(t,J=5.0Hz,2H,CH2),3.29(s,2H,CH2),3.49(s,3H,CH3),3.53(s,3H,CH3),4.58(t,J=5.2Hz,2H,CH2),7.12(t,J=7.5Hz,1H,Ar-4-H),7.33(t,J=8.4Hz,1H,Ar-5-H),7.63(d,J=6.4Hz,1H,Ar-3-H),7.79(d,J=6.2Hz,1H,Ar-6-H),7.83(s,1H,CONH),7.96(d,J=8.1Hz,1H,quinolin-6-H),8.26(d,J=8.1Hz,1H,quinolin-5-H),9.24(d,J=8.1Hz,1H,quinolin-8-H);13C NMR(75MHz,CDCl3)δ:28.63,30.84,43.77,56.81,62.14,63.20,114.96,118.64,118.95,123.06,125.96,129.32,135.29,140.60,150.48,161.12,165.39,168.80;MS(m/z,%):459(M+1,100)。
黄色粉末,产率77%,熔点135.4-136.7℃,1H NMR(600MHz,CDCl3):δ2.52(s,3H,CH3),2.99(t,J=5.2Hz,2H,CH2),3.26(s,2H,CH2),3.50(s,3H,CH3),3.51(s,3H,CH3),4.55(t,J=5.2Hz,2H,CH2),7.11(d,J=8.7Hz,2H,Ar-3,5-H),7.32(d,J=8.8Hz,2H,Ar-2,6-H),7.72(s,1H,CONH),7.82(d,J=8.1Hz,1H,quinolin-6-H),8.23(d,J=8.1Hz,1H,quinolin-5-H),9.03(s,1H,quinolin-8-H);13C NMR(75MHz,CDCl3):δ28.65,30.85,43.73,56.81,62.17,63.17,114.96,118.04,120.59,123.06,128.16,129.09,130.10,135.48,136.01,140.90,150.85,161.16,165.41,168.4;MS(m/z,%):459(M+1,100)。
白色粉末,产率79%,熔点130.3-131.5℃,1H NMR(600MHz,CDCl3):δ2.07(s,3H,CH3),2.56(s,3H,CH3),3.02(t,J=5.3Hz,2H,CH2),3.31(s,2H,CH2),3.49(s,3H,CH3),3.53(s,3H,CH3),4.56(t,J=5.3Hz,2H,CH2),6.97(t,J=7.5Hz,1H,Ar-4-H),7.03(t,J=7.3Hz,1H,Ar-5-H),7.15(d,J=7.4Hz,1H,Ar-6-H),7.74(s,1H,CONH),7.77(d,J=8.2Hz,1H,Ar-3-H),7.86(d,J=8.1Hz,1H,quinolin-6-H),8.16(d,J=8.1Hz,1H,quinolin-5-H),9.04(s,1H,quinolin-8-H);13C NMR(75MHz,CDCl3)δ:17.43,28.63,30.84,43.77,56.81,62.14,63.20,114.96,118.52,121.95,123.06,124.96,126.32,127.29,129.20,130.60,135.39,139.44,140.30,150.48,161.12,165.39,168.80;MS(m/z,%):439(M+1,100)。
黄色粉末,产率80%,熔点137.6-139.0℃,1H NMR(600MHz,CDCl3):δ2.51(s,3H,CH3),2.64(s,3H,CH3),2.98(t,J=4.7Hz,2H,CH2),3.25(s,2H,CH2),3.50(s,3H,CH3),3.73(s,1H,CH3),4.55(t,J=5.2Hz,2H,CH2),7.21(d,J=8.7Hz,2H,Ar-3,5-H),7.44(d,J=8.8Hz,2H,Ar-2,6-H),7.73(s,1H,CONH),7.86(d,J=8.0Hz,1H,quinolin-6-H),8.23(d,J=8.1Hz,1H,quinolin-5-H),8.87(s,1H,quinolin-8-H);13C NMR(75MHz,CDCl3):δ21.64,30.88,43.65,55.44,56.81,62.17,63.12,113.96,115.04,118.59,121.06,123.16,129.29,130.60,135.48,140.40,150.90,156.34,161.26,165.41,168.14;MS(m/z,%):439(M+1,100)
黄色粉末,产率68%,熔点113.7-114.5℃,1H NMR(600MHz,CDCl3):δ2.39(s,3H,CH3),2.74(s,3H,CH3),2.86(t,J=4.7Hz,5H,CH2,CH3),3.16(s,2H,CH2),4.36(m,2H,CH2),,6.33(s,1H,CONH),6.85–6.79(m,2H,Ar-3,5-H),7.13(d,J=8.0Hz,2H,Ar-2,6-H),7.25(d,J=8.1Hz,1H,quinolin-6-H),7.36(d,J=8.1Hz,1H,quinolin-5-H),9.04(s,1H,quinolin-8-H);13CNMR(75MHz,CDCl3)δ28.60,30.84,43.77,56.81,62.14,63.17,114.96,119.63,119.91,120.35,125.69,127.64,133.75,134.11,136.12,151.38,161.97,162.95,165.85,168.53;MS(m/z,%):443(M+1,100)
黄色粉末,产率77%,熔点131.2-132.0℃,1H NMR(600MHz,CDCl3):δ2.26(s,3H,CH3),2.89(t,J=5.1Hz,2H,CH2),3.30(s,2H,CH2),3.36(s,3H,CH3),3.51(s,3H,CH3),4.65(t,J=5.1Hz,2H,CH2),7.11(d,J=8.6Hz,1H,Ar-5-H),7.23(s,1H,CONH),7.32(d,J=8.8Hz,1H,Ar-6-H),7.72(s,1H,quinolin-6-H),7.85(s,1H,Ar-2-H),8.23(d,J=8.1Hz,1H,quinolin-5-H),9.03(s,1H,quinolin-8-H);13C NMR(75MHz,CDCl3):δ28.65,30.85,43.73,56.71,62.23,63.17,114.96,118.04,119.90,120.59,123.06,124.21,125.63,127.65,133.10,135.48,136.01,149.90,154.85,161.16,165.41,168.48;MS(m/z,%):477(M+1,100)
黄色粉末,产率73%,熔点129.7-131.0℃,1H NMR(600MHz,CDCl3):δ2.26(s,3H,CH3),2.37(s,3H,CH3)2.89(t,J=5.1Hz,2H,CH2),3.30(s,2H,CH2),3.36(s,3H,CH3),3.51(s,3H,CH3),4.65(t,J=5.1Hz,2H,CH2),7.01(d,J=8.3Hz,1H,Ar-5-H),7.13(s,1H,CONH),7.32(d,J=8.8Hz,1H,Ar-6-H),7.64(s,1H,quinolin-6-H),7.85(s,1H,Ar-2-H),8.12(d,J=8.1Hz,1H,quinolin-5-H),9.03(s,1H,quinolin-8-H);13C NMR(75MHz,CDCl3):δ26.72,28.65,30.85,43.73,56.71,62.23,63.17,114.96,118.04,120.59,123.06,128.16,129.09,130.10,135.48,136.01,138.90,150.85,161.16,165.41,168.48;MS(m/z,%):473(M+1,100)
白色粉末,产率79%,熔点158.2-159.7℃,1H NMR(600MHz,CDCl3):δ2.37(s,3H,CH3),2.83(t,J=5.0Hz,2H,CH2)3.03(s,3H,CH3),3.48(s,3H,CH3),,3.17(s,2H,CH2),4.33(t,J=5.2Hz,2H,CH2),4.38(d,J=6.2Hz,2H,CH2),6.87(s,1H,CONH),7.05(t,J=7.3Hz,1H,Ar-4-H),7.11(t,J=7.8Hz,2H,Ar-2,6-H),7.21(t,J=7.8Hz,2H,Ar-3,5-H),7.36(d,J=8.1Hz,1H,quinolin-6-H),7.48(d,J=8.1Hz,1H,quinolin-5-H),8.03(s,1H,quinolin-8-H);13C NMR(75MHz,CDCl3)δ:28.65,30.85,43.73,44.67,56.71,62.23,63.17,114.96,118.04,120.59,123.06,128.16,129.09,130.10,135.48,136.01,138.90,150.85,161.16,165.41,168.48;MS(m/z,%):439(M+1,100)
黄色粉末,产率73%,熔点126.5-128.7℃,1H NMR(600MHz,CDCl3):δ2.31(s,3H,CH3),2.44(s,3H,CH3),2.98(t,J=4.7Hz,2H,CH2),3.25(s,2H,CH2),3.50(s,3H,CH3),3.73(s,1H,CH3),4.55(t,J=5.2Hz,2H,CH2),6.96(d,J=8.7Hz,1H,Ar-4-H),7.23(s,1H,CONH),7.31(t,J=8.6Hz,1H,Ar-2-H),7.64(d,J=8.0Hz,1H,quinolin-6-H),8.23(d,J=8.1Hz,1H,quinolin-5-H),8.87(s,1H,quinolin-8-H);13C NMR(75MHz,CDCl3)δ:21.64,28.36,30.88,43.65,56.81,62.17,63.12,118.96,119.04,119.59,121.06,123.16,124.53,128.06,129.29,135.48,137.21,138.56,150.90,160.34,161.26,165.41,168.14;MS(m/z,%):439(M+1,100)
黄色粉末,产率77%,熔点153.2-154.0℃,1H NMR(600MHz,CDCl3):δ2.26(s,3H,CH3),2.89(t,J=5.1Hz,2H,CH2),3.30(s,2H,CH2),3.26(s,3H,CH3),3.51(s,3H,CH3),4.67(t,J=5.1Hz,2H,CH2),7.23(s,1H,CONH),7.64(s,1H,Ar-4-H),7.72(s,1H,quinolin-6-H),8.12(s,2H,Ar-2,6-H),8.23(d,J=8.1Hz,1H,quinolin-5-H),9.03(s,1H,quinolin-8-H);13C NMR(75MHz,CDCl3)δ28.65,30.85,43.73,56.71,62.23,63.17,114.96,118.04,120.59,123.06,124.4,128.16,129.09,130.10,135.48,136.01,149.90,150.85,161.16,165.41,168.48;MS(m/z,%):561(M+1,100)
黄色粉末,产率68%,熔点158.2-159.1℃,1H NMR(600MHz,CDCl3):δ2.63(s,3H,CH3),3.02(t,J=4.7Hz,2H,CH2),3.31(s,2H,CH2),3.49(s,3H,CH3),3.53(s,3H,CH3),4.58(t,J=5.2Hz,2H,CH2),7.73(s,1H,CONH),7.82(d,J=8.5Hz,2H,Ar-2,6-H),7.85(d,J=8.1Hz,1H,quinolin-6-H),8.14(d,J=8.4Hz,2H,Ar-3,5-H),8.26(d,J=8.1Hz,1H,quinolin-5-H),9.04(s,1H,quinolin-8-H);13C NMR(75MHz,CDCl3):δ28.60,30.84,43.77,56.81,62.14,63.17,118.96,118.64,119.05,123.06,125.96,129.32,135.29,144.60,150.48,161.12,165.39,168.80;MS(m/z,%):470(M+1,100)
黄色粉末,产率77%,熔点130.2-131.1℃,1H NMR(600MHz,CDCl3):δ2.47(s,3H,CH3),2.84(t,J=5.1Hz,2H,CH2),2.97(s,3H,CH3),2.98(s,3H,CH3),3.15(s,2H,CH2),4.65(t,J=5.1Hz,2H,CH2),6.92(s,1H,CONH),7.11(d,J=8.6Hz,1H,Ar-5-H),7.32(d,J=8.8Hz,1H,Ar-6-H),7.43(s,1H,quinolin-6-H),7.85(s,1H,Ar-2-H),8.23(d,J=8.1Hz,1H,quinolin-5-H),9.03(s,1H,quinolin-8-H);13C NMR(75MHz,CDCl3)δ28.65,30.85,43.73,56.71,62.23,63.17,115.06,117.64,118.79,119.86,123.16,129.49,130.10,131.60,134.01,135.90,136.25,140.28,151.45,161.16,165.41,168.48;MS(m/z,%):493(M+1,100)
白色粉末,产率79%,熔点138.5-139.7℃,1H NMR(600MHz,CDCl3):δ2.57(s,3H,CH3),3.01(s,2H,CH2),3.32(t,J=5.3Hz,2H,CH2),3.51(s,3H,CH3),3.59(s,3H,CH3),3.83(s,3H,CH3),4.66(t,J=5.3Hz,2H,CH2),7.01(t,J=7.5Hz,1H,Ar-4-H),7.50(t,J=7.3Hz,1H,Ar-5-H),7.74(s,1H,CONH),7.80(d,J=8.1Hz,1H,quinolin-6-H),7.87(d,J=8.2Hz,1H,Ar-3-H),8.16(d,J=7.4Hz,1H,Ar-6-H)),8.66(d,J=8.1Hz,1H,quinolin-5-H),10.04(s,1H,quinolin-8-H);13C NMR(75MHz,CDCl3)28.63,30.84,43.77,51.5,56.81,62.14,63.20,115.36,119.52,121.95,123.06,124.26,126.32,127.29,129.20,133.60,136.39,139.44,141.30,149.48,161.12,165.39,168.80,169.20;MS(m/z,%):483(M+1,100)
黄色粉末,产率73%,熔点169.3-170.0℃,1H NMR(600MHz,CDCl3):δ2.31(s,3H,CH3),2.44(s,3H,CH3),2.98(t,J=4.7Hz,2H,CH2),3.25(s,2H,CH2),3.50(s,3H,CH3),3.73(s,1H,CH3),4.55(t,J=5.2Hz,2H,CH2),7.56(s,1H,Ar-6-H),7.64(d,J=8.0Hz,1H,quinolin-6-H),8.23(d,J=8.1Hz,1H,quinolin-5-H),8.33(s,1H,CONH),8.87(s,1H,quinolin-8-H),9.01(s,1H);13C NMR(75MHz,CDCl3),δ:28.36,30.88,43.65,54.36,56.81,62.17,63.12,101.4,119.59,119.97,125.06,127.16,134.53,136.10,150.90,151.3,160.34,161.26,165.41,168.14,169.72;MS(m/z,%):457(M+1,100)
白色粉末,产率79%,熔点166.6-168.3℃,1H NMR(600MHz,CDCl3):δ2.67(s,3H,CH3),3.07(s,2H,CH2),3.15(s,3H,CH3),3.41(s,2H,CH2),3.50(s,3H,CH3),4.60(t,J=5.3Hz,2H,CH2),7.11(t,J=7.5Hz,1H,naphthalen-2-H),7.28(t,J=7.3Hz,1H,naphthalen-3-H),7.37(s,1H,CONH),7.40(t,J=6.3Hz,1H,naphthalen-5-H),7.48(d,J=8.1Hz,1H,quinolin-6-H),7.63(d,J=8.3Hz,1H,naphthalen-6-H),7.58(t,J=8.0Hz,1H,naphthalen-7-H),7.63(t,J=7.3Hz,1H,naphthalen-4-H),7.83(d,J=7.1Hz,1H,naphthalen-4-H),8.46(d,J=8.1Hz,1H,quinolin-5-H),9.62(s,1H,quinolin-8-H);13C NMR(75MHz,CDCl3)28.63,30.84,43.77,58.5,62.14,63.20,105.36,119.02,119.65,119.91,121.02,124.26,125.32,125.69,126.20,127.31,128.59,133.60,134.39,135.44,136.30,151.12,161.39,165.80,168.20;MS(m/z,%):475(M+1,100)
黄色粉末,产率77%,熔点101.2-103.0℃,1H NMR(600MHz,CDCl3):δ1.04(t,J=7.1Hz,3H,CH3),2.47(s,3H,CH3),2.84(t,J=5.1Hz,2H,CH2),2.90(m,2H,CH2),2.97(s,3H,CH3),2.98(s,3H,CH3),3.15(s,2H,CH2),4.65(t,J=5.1Hz,2H,CH2),6.92(s,1H,CONH),7.43(s,1H,quinolin-6-H),8.23(d,J=8.1Hz,1H,quinolin-5-H),9.03(s,1H,quinolin-8-H);13C NMR(75MHz,CDCl3)δ15.70,29.65,30.85,33.86,43.73,58.71,62.03,63.17,119.56,119.90,125.16,127.49,133.10,136.60,151.45,161.16,165.41,168.48;MS(m/z,%):377(M+1,100)
黄色粉末,产率77%,熔点110.7-118.8℃,1H NMR(600MHz,CDCl3):δ1.24(t,J=7.1Hz,6H,CH3),2.47(s,3H,CH3),2.84(t,J=5.1Hz,2H,CH2),2.90(m,4H,CH2),2.97(s,3H,CH3),3.06(s,3H,CH3),3.15(s,2H,CH2),4.65(t,J=5.1Hz,2H,CH2),7.43(d,J=8.1Hz,1H,quinolin-6-H),8.23(d,J=8.1Hz,1H,quinolin-5-H),9.13(s,1H,quinolin-8-H);13C NMR(75MHz,CDCl3)δ14.70,29.65,30.85,33.86,43.73,58.71,62.03,63.17,119.56,119.90,125.16,127.49,133.10,136.60,151.45,161.16,165.41,168.48;MS(m/z,%):405(M+1,100)
黄色粉末,产率77%,熔点104.7-105.6℃,1H NMR(600MHz,CDCl3):δ1.26(s,9H,CH3),2.47(s,3H,CH3),2.94(t,J=5.1Hz,2H,CH2),2.97(s,2H,CH2),3.51(s,3H,CH3),3.76(s,3H,CH3),4.65(t,J=5.1Hz,2H,CH2),7.07(s,1H,CONH),7.83(d,1H,J=8.1Hz,quinolin-6-H),7.90(d,J=8.1Hz,1H,quinolin-5-H),8.45(s,1H,quinolin-8-H);13C NMR(75MHz,CDCl3)δ29.20,29.65,30.85,33.86,43.73,58.71,62.03,63.17,119.56,119.90,125.16,127.49,133.10,136.60,151.45,161.16,165.41,168.48;MS(m/z,%):405(M+1,10
实施例9:1,3-二甲基-7-取代喹唑啉-2,4-二酮类化合物抗微生物活性实验
本发明化合物用DMSO溶解后,用高压灭菌的蒸馏水制成溶液备用,将96孔板,西林瓶,枪头等物品高压灭菌,用移液枪移取稀释好的菌液溶液100uL到96孔板中,,用氧氟沙星,链霉素;氟康唑,多氧霉素B作为参比对照。细菌,真菌在37度培养24h,观察现象。结果见附表。
附表1、本发明1,3-二甲基-7-取代喹唑啉-2,4-二酮类化合物抗细菌微生物活性数据(MICμg/mL)
附表2 1,3-二甲基-7-取代喹唑啉-2,4-二酮类化合物抗真菌微生物活性数据(MICμg/mL)
上述实验以链霉素,氧氟沙星,氟康唑,多氧霉素B为对照药,属抗生素药物,是公认的抗微生物活性很强的抗生素,但它目前已出现微生物耐药现象。本发明新化合物无耐药性,上述活性数据结果表明,本发明新化合物具有较明显的抑菌作用,特别地,化合物10h对细菌均表现出显著的抑制作用,其对变形杆菌MIC可达到64μg/mL,对大肠杆菌MIC可达到32μg/mL。化合物10l对真菌黄曲霉菌MIC可达到32μg/mL,优于多氧霉素B 64μg/mL。
实施例10:1,3-二甲基-7-取代喹唑啉-2,4-二酮类化合物几丁质酶抑制活性实验
几丁质合成酶来源于啤酒酵母细胞膜,酶与底物在微孔板上一起孵化反应,产生的几丁质与板上包被的WGA结合在一起而被固定,然后加入WGA-HRP,WGA-HRP可与固定在板上的几丁质结合,将多余的试剂用蒸馏水洗掉,然后使用TMB底物溶液检测HRP的活性,使用2M的硫酸终止反应后在450nm处检测其OD,计算出化合物的IC50值。
其结果见附图1。
对于本领域技术人员而言,显然本发明不限于上述示范性实施例的细节,而且在不背离本发明的精神或基本特征的情况下,能够以其他的具体形式实现本发明。因此,无论从哪一点来看,均应将实施例看作是示范性的,而且是非限制性的,本发明的范围由所附权利要求而不是上述说明限定,因此旨在将落在权利要求的等同要件的含义和范围内的所有变化囊括在本发明内。不应将权利要求中的任何附图标记视为限制所涉及的权利要求。
- 还没有人留言评论。精彩留言会获得点赞!