一种2,3′‑螺二吲哚啉‑2‑酮类化合物及其制备方法与流程
本发明涉及螺环杂环化合物和有机化学合成领域,特别涉及一种2,3'-螺二吲哚啉-2-酮类化合物及其制备方法。
背景技术:
螺环氧化吲哚是构成许多药物和生物活性天然产物的核心结构单元。其中,螺吡咯烷-3,2'-氧化吲哚类化合物,其含有吡咯烷单元和氮杂原子与螺季碳中心相毗连的独特结构,广泛存在于药物活性分子与天然生物碱中。因此,螺吡咯烷-3,2'-氧化吲哚类化合物的合成一直是有机化学与药物化学界研究的热点。
目前,螺吡咯烷-3,2'-氧化吲哚类化合物的合成主要通过以下几类反应实现:(1)利用靛红衍生的硝酮与缺电子炔烃的[3+2]环加成反应来制备;(2)利用3-氨基氧化吲哚或靛红衍生的甲亚胺叶立德与缺电子烯烃的[3+2]环加成反应来制备;(3)利用卡宾催化的肉桂醛与靛红亚胺的加成/环化串联反应来制备等。尽管当前螺吡咯烷-3,2'-氧化吲哚类化合物的合成研究已经取得了较大进展,但是现有技术合成获得的此类化合物结构类型还不够广泛。因此,发展更加直接、高效、可供替代的合成技术实现螺吡咯烷-3,2'-氧化吲哚类化合物的结构多样性合成仍具有重要意义。
技术实现要素:
本发明的目的在于提供一种以一价铜盐催化3-氨基氧化吲哚和2-溴甲基芳基溴化物的亲核取代/c(sp2)-n交叉偶联一锅串联反应,高产率合成系列结构多样化的2,3'-螺二吲哚啉-2-酮类化合物的方法。
本方案中的一种2,3'-螺二吲哚啉-2-酮类化合物,结构通式为:
结构通式中:r1选自5-me、5-ome、5-f、5-ocf3、7-f、7-cf3或7-ocf3;
r2选自me、et、npr、nbu、ipr、ph或bn;
r3选自h和叔丁氧羰基(-boc);
r选自me、et、ph;
ar选自苯基、甲氧基取代苯基、[1,3]二氧戊烷并苯基、氟取代苯基、三氟甲基取代苯基、氯取代苯基、萘基或噻吩基。
本发明的2,3'-螺二吲哚啉-2-酮类化合物是一种新型的化合物,具有吲哚类化合物类似的特点,同时还可以作为抗肿瘤和抗菌类药物的活性分子使用。此外,本发明涉及2,3'-螺二吲哚啉-2-酮类化合物的制备方法与其他方法相比,是一种全新的方法,效率相对较高。
2,3'-螺二吲哚啉-2-酮类化合物的制备方法,包括以下步骤:将3-氨基氧化吲哚、2-溴甲基芳基溴化物、一价铜盐催化剂和无机碱加入到有机溶剂中进行反应,反应完毕后经萃取、干燥、柱层析或重结晶处理得到2,3'-螺二吲哚啉-2-酮类化合物,合成路线如下所示:
本发明的工作原理及有益效果:本发明以3-氨基氧化吲哚、2-溴甲基芳基溴化物作为底物,再以一价铜盐催化底物的亲核取代/c(sp2)-n交叉偶联一锅串联反应,合成结构多样性的2,3'-螺二吲哚啉-2-酮类化合物,是对2,3'-螺二吲哚啉-2-酮类化合物合成方法的重要补充;对利用3-氨基氧化吲哚参与的新的串联反应,用于合成其他新结构的螺环氧化吲哚类化合物具有重要的启示作用,而且本方法具有反应进行完全,易于纯化,产率高、操作简单等优点。
优选的,所述一价铜盐催化剂为碘化亚铜、溴化亚铜、氯化亚铜、氰化亚铜或硫酸亚铜。选用这些催化剂,反应的副产物较少,产率较高。
优选的,所述有机溶剂为甲苯、氯苯、氟苯、三氟甲苯、均三甲苯、二甲基亚砜、特戊醇、n,n-二甲基甲酰胺(dmf)、1,4-二氧六环或乙腈。选用这些溶剂,反应的产率较高。
优选的,所述无机碱为碳酸锂、碳酸钠、碳酸钾、碳酸铯、特戊酸钠、磷酸钾、叔丁醇钠、叔丁醇钾或叔丁醇锂。选用这些无机碱,反应的副产物较少,产率较高。
优选的,反应温度为80~170℃,反应时间为6~24h。针对不同的反应物,其反应的活性有所差异,所需反应时间也不同。
进一步优选的,反应完毕后采用柱层析分离方法处理。在生成的产物中,部分不是固体,通过柱层析分离纯化,更为实用。
附图说明
图1为本发明化合物3a的1hnmr谱图;
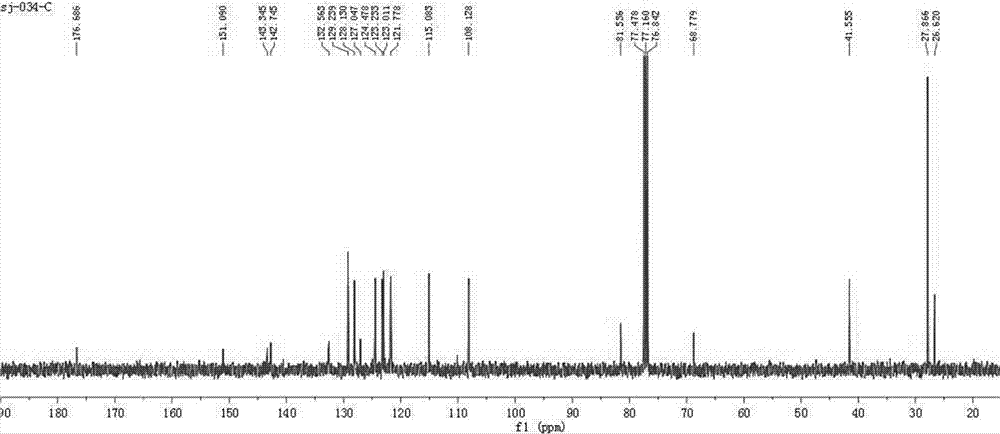
图2为本发明化合物3a的13cnmr谱图;
图3为本发明化合物3b的1hnmr谱图;
图4为本发明化合物3b的13cnmr谱图;
图5为本发明化合物3c的1hnmr谱图;
图6为本发明手性化合物3c的13cnmr谱图。
具体实施方式
下面通过具体实施方式对本发明作进一步详细的说明,但具体实施例并不对本发明作任何限定。除非特别说明,实施例中所涉及的试剂、方法均为本领域常用的试剂和方法。
本发明一种2,3'-螺二吲哚啉-2-酮类化合物及其制备方法,合成的化合物3a~c',结构式如下:
制备方法的具体步骤如下:
合成代表性化合物3a的操作步骤:
实施例1:
称取3-氨基氧化吲哚1a(52.5mg,0.2mmol)、2-溴苄溴2a(55.0mg,0.22mmol)、tbuona(0.8mmol,76.8mg,4.0equiv.)、cubr(0.01mmol,1.4mg)于硬质反应管中,然后加入2.0mldmf。反应液在100℃下搅拌反应12h。tlc监测反应进行完全后,向反应液中加入10.0ml水,并用乙酸乙酯萃取(6×5ml)。合并所得的有机相用饱和的食盐水洗涤、无水硫酸钠干燥、过滤和浓缩。最后所得的残留物经柱层析(石油醚/乙酸乙酯=5:1)分离纯化得目标化合物3a(yield86%)。
实施例2:
称取3-氨基氧化吲哚1a(52.5mg,0.2mmol)、2-溴苄溴2a(55.0mg,0.22mmol)、cs2co3(0.8mmol,260.7mg,4.0equiv.)、cui(0.01mmol,1.9mg)于硬质反应管中,然后加入2.0mldmf。反应液在100℃下搅拌反应24h。tlc监测反应进行完全后,向反应液中加入10.0ml水,并用乙酸乙酯萃取(6×5ml)。合并所得的有机相用饱和的食盐水洗涤、无水硫酸钠干燥、过滤和浓缩。最后所得的残留物经柱层析(石油醚/乙酸乙酯=5:1)分离纯化得目标化合物3a(yield76%)。
实施例3:
称取3-氨基氧化吲哚1a(52.5mg,0.2mmol)、2-溴苄溴2a(55.0mg,0.22mmol)、tbuok(0.8mmol,89.8mg,4.0equiv.)、cubr(0.01mmol,1.4mg)于硬质反应管中,然后加入2.0mldmf。反应液在100℃下搅拌反应12h。tlc监测反应进行完全后,向反应液中加入10.0ml水,并用乙酸乙酯萃取(6×5ml)。合并所得的有机相用饱和的食盐水洗涤、无水硫酸钠干燥、过滤和浓缩。最后所得的残留物经柱层析(石油醚/乙酸乙酯=5:1)分离纯化得目标化合物3a(yield77%)。
实施例4:
称取3-氨基氧化吲哚1a(52.5mg,0.2mmol)、2-溴苄溴2a(55.0mg,0.22mmol)、cs2co3(0.8mmol,260.7mg,4.0equiv.)、cubr(0.01mmol,1.4mg)于硬质反应管中,然后加入2.0ml均三甲苯。反应液在100℃下搅拌反应24h。tlc监测反应进行完全后,向反应液中加入10.0ml水,并用乙酸乙酯萃取(6×5ml)。合并所得的有机相用饱和的食盐水洗涤、无水硫酸钠干燥、过滤和浓缩。最后所得的残留物经柱层析(石油醚/乙酸乙酯=5:1)分离纯化得目标化合物3a(yield74%)。
化合物3b~c'的制备:在本实施例中,使用与制备化合物3a相同的反应条件,可分别得到化合物3b~c'。
化合物3a~c'的数据表征如下:
3a:yellowoil;60.3mg,yield86%.1hnmr(400mhz,cdcl3):δ1.12(s,9h),3.21(d,j=16.7hz,1h),3.25(s,3h),3.65(d,j=16.1hz,1h),6.85(d,j=7.7hz,1h),6.98-7.03(m,2h),7.10-7.15(m,2h),7.24-7.27(m,1h),7.30-7.34(m,1h),7.98(d,j=8.1hz,1h);13cnmr(100mhz,cdcl3):δ26.6,27.9,41.6,68.8,81.5,108.1,115.1,121.8,123.0,123.2,124.5,127.0,128.1,129.2,132.6,142.7,143.3,151.1,176.7.hrms(esi-tof)calcd.forc21h22n2nao3[m+na]+373.1523;found:373.1523.
3b:whitesolid;40.1mg,yield55%;mp206.7-207.9℃.1hnmr(400mhz,cdcl3):δ1.11(s,9h),1.33(t,j=7.2hz,3h),3.23(d,j=16.0hz,1h),3.44-3.50(m,1h),3.63(d,j=16.1hz,1h),4.05-4.12(m,1h),6.87(d,j=7.8hz,1h),6.97-7.02(m,2h),7.10-7.14(m,2h),7.23(d,j=7.7hz,1h),7.28-7.32(m,1h),7.98(d,j=7.9hz,1h);13cnmr(100mhz,cdcl3):δ13.1,27.9,35.2,41.7,68.8,81.6,108.2,115.1,121.9,123.0,124.4,127.0,128.1,129.1,132.8,142.0,143.5,151.1,176.2.hrms(esi-tof)calcd.forc22h25n2o3[m+h]+365.1860;found:365.1869.
3c:whitesolid;42.1mg,yield51%;mp202.4-203.6℃.1hnmr(400mhz,cdcl3):δ1.17(s,9h),3.39(d,j=16.1hz,1h),3.75(d,j=16.1hz,1h),7.02-7.09(m,3h),7.16-7.22(m,3h),7.28(s,1h),7.39-7.42(m,1h),7.55-7.56(m,4h),8.04(d,j=7.8hz,1h);13cnmr(100mhz,cdcl3):δ28.1,42.5,69.0,82.0,109.7,115.2,122.2,123.1,123.8,124.5,125.6,126.8,127.8,128.2,129.0,129.6,132.7,134.5,142.2,143.5,151.2,175.8.hrms(esi-tof)calcd.forc26h24n2nao3[m+na]+435.1679;found:435.1686.
3d:yellowoil;40.1mg,yield47%.1hnmr(400mhz,cdcl3):δ1.12(s,9h),3.31(d,j=16.0hz,1h),3.73(d,j=16.0hz,1h),4.41(d,j=15.4hz,1h),5.42(d,j=15.4hz,1h),6.78(d,j=7.1hz,1h),6.96-7.01(m,1h),7.03(d,j=7.3hz,1h),7.12-7.17(m,2h),7.21(d,j=7.2hz,1h),7.28(d,j=10.0hz,2h),7.36(s,4h),8.02(d,j=7.4hz,1h);13cnmr(100mhz,cdcl3):δ27.8,41.8,44.1,68.8,81.7,109.0,115.1,121.8,123.0,123.2,124.5,126.9,127.6,127.9,128.1,129.0,129.1,132.7,135.9,141.9,143.5,151.1,176.8.hrms(esi-tof)calcd.forc27h26n2nao3[m+na]+449.1836;found:449.1851.
3e:colourlessoil;51.0mg,yield70%.1hnmr(400mhz,cdcl3):δ1.13(s,9h),2.24(s,3h),3.19(d,j=16.1hz,1h),3.22(s,3h),3.63(d,j=16.1hz,1h),6.73(d,j=7.8hz,1h),6.91(s,1h),6.99-7.03(m,1h),7.10(d,j=7.7hz,1h),7.14(d,j=7.4hz,1h),7.23-7.27(m,1h),7.98(d,j=8.1hz,1h);13cnmr(100mhz,cdcl3):δ21.1,26.6,27.9,41.6,68.9,81.5,107.9,115.1,122.5,123.0,124.5,127.2,128.1,129.4,132.6,132.9,140.3,143.4,151.2,176.6.hrms(esi-tof)calcd.forc22h24n2nao3[m+na]+387.1679;found:387.1686.
3f:whitesolid;54.0mg,yield71%;mp163.2-164.5℃.1hnmr(400mhz,cdcl3):δ1.14(s,9h),3.20(d,j=16.2hz,1h),3.22(s,3h),3.64(d,j=16.2hz,1h),3.70(s,3h),6.71(d,j=1.9hz,1h),6.74(d,j=8.5hz,1h),6.82-6.84(m,1h),6.98-7.02(m,1h),7.13(d,j=7.3hz,1h),7.23(d,j=7.7hz,1h),7.97(d,j=8.1hz,1h);13cnmr(100mhz,cdcl3):δ26.6,27.9,41.6,56.0,69.1,81.5,108.6,109.0,113.6,115.1,123.0,124.5,127.0,128.1,133.7,136.2,143.3,151.0,156.6,176.4.hrms(esi-tof)calcd.forc22h24n2nao4[m+na]+403.1628;found:403.1626.
3g:whitesolid;59.0mg,yield80%;mp190.3-192.1℃.1hnmr(400mhz,cdcl3):δ1.15(s,9h),3.19(d,j=16.5hz,1h),3.24(s,3h),3.65(d,j=16.0hz,1h),6.76-6.78(m,1h),6.87(d,j=6.5hz,1h),7.00-7.04(m,2h),7.14(d,j=7.0hz,1h),7.24-7.27(m,1h),7.97(d,j=7.8hz,1h);13cnmr(100mhz,cdcl3):δ26.8,27.9,41.6,68.9,81.8,108.7(d,j=7.6hz,1c),110.1(d,j=24.8hz,1c),115.2,115.4(d,j=23.4hz,1c),123.2,124.5,126.6,128.3,134.0,138.7,143.2,150.8,159.6(d,j=240.9hz,1c),176.4.hrms(esi-tof)calcd.forc21h21fn2nao3[m+na]+391.1428;found:391.1436.
3h:yellowsolid;41.3mg,yield56%;mp161.2-162.7℃.1hnmr(400mhz,cdcl3):δ1.18(s,9h),3.20(d,j=16.1hz,1h),3.46(s,3h),3.64(d,j=16.0hz,1h),6.88-6.94(m,2h),6.99-7.07(m,2h),7.13(d,j=7.4hz,1h),7.23-7.27(m,1h),7.97(d,j=8.1hz,1h);13cnmr(100mhz,cdcl3):δ27.9,28.4,41.7,68.8,81.8,115.1,117.1(d,j=19.2hz,1c),117.7,123.1,123.9(d,j=6.2hz,1c),124.5,126.7,128.2,129.8,135.4,143.2,147.7(d,j=242.3hz,1c),150.9,176.3.hrms(esi-tof)calcd.forc21h21fn2nao3[m+na]+391.1428;found:391.1424.
3i:yellowoil;49.4mg,yield59%.1hnmr(400mhz,cdcl3):δ1.14(s,9h),3.19(d,j=16.1hz,1h),3.47(s,3h),3.65(d,j=15.9hz,1h),7.03-7.08(m,2h),7.15(d,j=6.3hz,1h),7.28(s,2h),7.61(d,j=7.4hz,1h),7.98(d,j=7.5hz,1h);13cnmr(100mhz,cdcl3):δ27.8,28.4,42.1,67.1,82.1,112.6(q,j=33.2hz,1c),115.1,122.3,122.6,123.3,124.6,125.2,126.5,127.0(q,j=5.9hz,1c),128.4,135.2,140.5,143.2,150.7,176.3.hrms(esi-tof)calcd.forc22h21f3n2nao3[m+na]+441.1397;found:441.1401.
3j:yellowsolid;31.0mg,yield62%;mp259.9-262.1℃.1hnmr(400mhz,cdcl3):δ3.23(s,3h),3.25(d,j=15.8hz,1h),3.61(d,j=15.8hz,1h),4.18(s,1h),6.71(d,j=7.6hz,1h),6.79-6.86(m,2h),6.99-7.02(m,1h),7.08-7.14(m,2h),7.24(d,j=7.7hz,1h),7.29-7.33(m,1h);13cnmr(100mhz,cdcl3):δ26.6,41.9,68.3,108.5,110.0,119.8,122.8,123.4,124.7,126.7,127.9,129.6,133.1,142.6,149.9,178.5.hrms(esi-tof)calcd.forc16h15n2o[m+h]+251.1179;found:251.1182.
3k:yellowsolid;44.9mg,yield85%;mp252.1-253.2℃.1hnmr(400mhz,cdcl3):δ1.30(t,j=7.1hz,3h),3.25(d,j=15.7hz,1h),3.62(d,j=15.8hz,1h),3.78-3.81(m,2h),4.19(s,1h),6.71(d,j=7.6hz,1h),6.78-6.83(m,1h),6.88(d,j=7.6hz,1h),6.98-7.01(m,1h),7.09-7.14(m,2h),7.25(d,j=7.4hz,1h),7.29-7.32(m,1h);13cnmr(100mhz,cdcl3):δ12.8,35.1,42.0,68.3,108.6,110.1,119.8,123.0,123.2,124.7,126.8,127.9,129.5,133.4,141.7,149.9,178.1.hrms(esi-tof)calcd.forc17h17n2o[m+h]+265.1335;found:265.1339.
3l:whitesolid;29.4mg,yield47%;mp223.8-224.6℃.1hnmr(400mhz,cdcl3):δ3.39(d,j=15.8hz,1h),3.75(d,j=15.7hz,1h),4.35(s,1h),6.75(d,j=7.5hz,1h),6.82-6.88(m,2h),7.03-7.07(m,1h),7.11-7.17(m,2h),7.23(d,j=7.6hz,1h),7.33(d,j=7.4hz,1h),7.40-7.47(m,3h),7.52-7.55(m,2h);13cnmr(100mhz,cdcl3):δ42.6,68.4,109.8,110.1,119.9,123.2,123.9,124.8,126.5,126.6,128.0,128.2,129.5,129.8,133.0,134.4,142.4,149.9,177.9.hrms(esi-tof)calcd.forc21h17n2o[m+h]+313.1335;found:313.1347.
3m:yellowsolid;48.3mg,yield74%;mp211.9-213.4℃.1hnmr(400mhz,cdcl3):δ3.32(d,j=15.7hz,1h),3.69(d,j=15.8hz,1h),4.26(s,1h),4.89(d,j=15.5hz,1h),4.96(d,j=15.6hz,1h),6.73-6.76(m,2h),6.81-6.85(m,1h),6.95-6.99(m,1h),7.10-7.21(m,3h),7.25-7.29(m,2h),7.33(s,4h);13cnmr(100mhz,cdcl3):δ42.2,44.1,68.3,109.5,110.1,119.9,123.0,123.4,124.8,126.7,127.5,127.9,128.0,129.0,129.5,133.1,135.8,141.7,149.9,178.7.hrms(esi-tof)calcd.forc22h18n2nao[m+na]+349.1311;found:349.1323.
3n:yellowsolid;40.2mg,yield76%;mp251.8-252.4℃.1hnmr(400mhz,cdcl3):δ2.26(s,3h),3.21(s,3h),3.24(d,j=15.8hz,1h),3.61(d,j=15.8hz,1h),4.17(s,1h),6.70-6.75(m,2h),6.80-6.83(m,1h),7.06(s,1h),7.09-7.12(m,3h);13cnmr(100mhz,cdcl3):δ21.1,26.6,42.0,68.3,108.2,110.0,119.8,123.6,124.7,126.8,127.9,129.8,133.1,133.2,140.2,149.9,178.5.hrms(esi-tof)calcd.forc17h17n2o[m+h]+265.1335;found:265.1339.
3o:whitesolid;39.2mg,yield73%;mp190.9-192.8℃.1hnmr(400mhz,cdcl3):δ3.22(s,3h),3.23(d,j=15.8hz,1h),3.61(d,j=15.8hz,1h),4.21(s,1h),6.71(d,j=7.6hz,1h),6.76-6.83(m,2h),6.98-7.02(m,2h),7.08-7.13(m,2h);13cnmr(100mhz,cdcl3):δ26.7,42.0,68.5,109.1(d,j=7.9hz,1c),110.1,111.1(d,j=24.7hz,1c),115.7(d,j=23.4hz,1c),120.0,124.8,126.3,128.1,134.6(d,j=7.6hz,1c),138.4,149.6,159.7(d,j=240.9hz,1c),178.2.hrms(esi-tof)calcd.forc16h13fn2nao[m+na]+291.0904;found:291.0911.
3p:yellowsolid;50.2mg,yield66%;mp168.7-169.5℃.1hnmr(400mhz,cdcl3):δ1.11(s,9h),3.16(d,j=16.2hz,1h),3.24(s,3h),3.61(d,j=16.1hz,1h),3.78(s,3h),6.72(s,1h),6.78(d,j=8.5hz,1h),6.83(d,j=7.6hz,1h),6.98-7.01(m,1h),7.10(d,j=7.0hz,1h),7.29-7.33(m,1h),7.87(d,j=8.6hz,1h);13cnmr(100mhz,cdcl3):δ26.6,27.9,41.6,55.8,68.9,81.3,108.1,110.9,112.7,115.5,121.8,123.2,128.4,129.2,132.6,137.0,142.8,151.1,156.0,176.6.hrms(esi-tof)calcd.forc22h24n2nao4[m+na]+403.1628;found:403.1636.
3q:yellowsolid;51.3mg,yield65%;mp228.0-229.6℃.1hnmr(400mhz,cdcl3):δ1.10(s,9h),3.09(d,j=15.8hz,1h),3.23(s,3h),3.54(d,j=15.7hz,1h),5.93(d,j=7.6hz,2h),6.61(s,1h),6.83(d,j=7.4hz,1h),7.00-7.02(m,1h),7.13(d,j=6.8hz,1h),7.29-7.31(m,1h),7.63(s,1h);13cnmr(100mhz,cdcl3):δ26.6,27.9,41.3,69.4,81.4,98.4,101.4,104.9,108.1,118.7,121.7,123.2,129.3,132.5,137.6,142.7,143.5,147.5,151.0,176.5.hrms(esi-tof)calcd.forc22h22n2nao5[m+na]+417.1421;found:417.1428.
3r:whitesolid;48.0mg,yield65%;mp162.8-164.0℃.1hnmr(400mhz,cdcl3):δ1.12(s,9h),3.23(d,j=16.4hz,1h),3.25(s,3h),3.62(d,j=16.4hz,1h),6.71-6.75(m,1h),6.85(d,j=7.7hz,1h),7.01-7.05(m,1h),7.14(d,j=7.6hz,1h),7.20-7.24(m,1h),7.31-7.35(m,1h),7.76(d,j=8.0hz,1h);13cnmr(100mhz,cdcl3):δ26.7,27.8,42.8,69.3,82.0,108.3,109.9(d,j=19.4hz,1c),111.0,113.9,121.9,123.4,125.8,129.5,130.1(d,j=7.6hz,1c),132.2,142.8,150.9,158.9(d,j=245.1hz,1c),176.2.hrms(esi-tof)calcd.forc21h21fn2nao3[m+na]+391.1428;found:391.1436.
3s:whitesolid;58.2mg,yield79%;mp191.8-192.8℃.1hnmr(400mhz,cdcl3):δ1.11(s,9h),3.18(d,j=16.4hz,1h),3.24(s,3h),3.62(d,j=16.4hz,1h),6.84-6.87(m,2h),6.92-6.96(m,1h),7.00-7.04(m,1h),7.11(d,j=7.2hz,1h),7.31-7.35(m,1h),7.91-7.94(m,1h);13cnmr(100mhz,cdcl3):δ26.6,27.9,41.3,69.1,81.7,108.2,111.8(d,j=24.4hz,1c),114.3(d,j=22.7hz,1c),115.7(d,j=8.1hz,1c),121.8,123.3,128.8(d,j=8.6hz,1c),129.4,132.2,139.4,142.7,151.0,159.2(d,j=239.3hz,1c),176.4.hrms(esi-tof)calcd.forc21h21fn2nao3[m+na]+391.1428;found:391.1441.
3t:yellowoil;58.2mg,yield79%.1hnmr(400mhz,cdcl3):δ1.11(s,9h),3.16(d,j=15.9hz,1h),3.24(s,3h),3.58(d,j=15.9hz,1h),6.67-6.72(m,1h),6.85(d,j=7.7hz,1h),7.00-7.06(m,2h),7.11(d,j=7.2hz,1h),7.31-7.34(m,1h),7.74(d,j=10.2hz,1h);13cnmr(100mhz,cdcl3):δ26.6,27.8,40.8,69.6,82.0,103.5(d,j=29.6hz,1c),108.2,109.4(d,j=22.8hz,1c),121.8,122.3,123.3,124.8(d,j=9.9hz,1c),129.4,132.2,142.7,144.7(d,j=12.3hz,1c),150.9,163.1(d,j=241.0hz,1c),176.3.hrms(esi-tof)calcd.forc21h21fn2nao3[m+na]+391.1428;found:391.1437.
3u:yellowsolid;52.0mg,yield62%;mp169.7-170.8℃.1hnmr(400mhz,cdcl3):δ1.13(s,9h),3.26(s,3h),3.26(d,j=16.4hz,1h),3.67(d,j=16.4hz,1h),6.87(d,j=7.7hz,1h),7.01-7.05(m,1h),7.11(d,j=7.1hz,1h),7.32-7.36(m,1h),7.39(s,1h),7.53(d,j=8.4hz,1h),8.06(d,j=8.2hz,1h);13cnmr(100mhz,cdcl3):δ26.6,27.8,41.1,69.1,82.3,108.4,114.8,121.6,121.7,123.4,124.5(q,j=270.0hz,1c),125.0(q,j=32.0hz,1c),125.8(q,j=4.0hz,1c),127.9,129.6,131.9,142.8,146.3,150.8,176.1.hrms(esi-tof)calcd.forc22h22f3n2o3[m+h]+419.1577;found:419.1573.
3v:whitesolid;56.5mg,yield74%;mp190.9-192.8℃.1hnmr(400mhz,cdcl3):δ1.11(s,9h),3.18(d,j=16.4hz,1h),3.24(s,3h),3.62(d,j=16.3hz,1h),6.85(d,j=7.7hz,1h),7.00-7.04(m,1h),7.09-7.12(m,2h),7.22(d,j=8.7hz,1h),7.31-7.35(m,1h),7.91(d,j=8.6hz,1h);13cnmr(100mhz,cdcl3):δ26.7,27.9,41.2,69.0,81.9,108.3,116.0,121.8,123.4,124.6,128.0,128.1,129.0,129.5,132.1,142.1,142.8,150.9,176.3.hrms(esi-tof)calcd.forc21h21cln2nao3[m+na]+407.1133;found:407.1141.
3w:yellowoil;44.1mg,yield55%.1hnmr(400mhz,cdcl3):δ1.28(s,9h),3.27(d,j=16.0hz,1h),3.31(s,3h),3.92(d,j=16.0hz,1h),6.88(d,j=7.8hz,1h),6.94-6.98(m,1h),7.06(d,j=7.4hz,1h),7.29-7.34(m,2h),7.43-7.48(m,2h),7.64(d,j=8.2hz,1h),7.84(d,j=7.4hz,1h),8.04(d,j=7.6hz,1h);13cnmr(100mhz,cdcl3):δ26.7,27.8,43.0,70.6,82.0,108.2,122.1,123.2,123.3,124.9,125.2,125.3,125.5,125.6,128.4,129.4,132.6,134.6,138.4,142.5,152.0,176.3.hrms(esi-tof)calcd.forc25h24n2nao3[m+na]+423.1679;found:423.1697.
3x:yellowoil;48.1mg,yield66%,dr4:1.1hnmr(400mhz,cdcl3):δ(major)0.93(d,j=7.1hz,3h),1.13(s,9h),3.25(s,3h),3.90(q,j=6.9hz,1h),6.84-6.95(m,3h),7.01-7.10(m,2h),7.20-7.23(m,1h),7.28-7.32(m,1h),7.97(d,j=7.8hz,1h);13cnmr(100mhz,cdcl3):δ(major)13.3,26.7,27.8,45.1,74.3,81.4,108.0,114.8,122.6,122.8,123.0,123.3,128.9,129.4,131.0,132.7,142.8,143.2,151.2,177.1.hrms(esi-tof)calcd.forc22h24n2nao3[m+na]+387.1679;found:387.1681.
3y:yellowsolid;39.8mg,yield52%,dr2:1;mp108.7-110.2℃.1hnmr(400mhz,cdcl3):δ(major+minor)0.91(d,j=7.0hz,3h),1.12(s,9h),3.21(s,1h),3.25(s,2h),3.84(q,j=7.1hz,1h),6.69-6.73(m,1h),6.82-6.90(m,2h),6.94-7.01(m,2h),7.30-7.34(m,1h),7.74(d,j=10.2hz,1h);13cnmr(100mhz,cdcl3):δ(major+minor)13.5,14.5,26.7,27.8,44.6,74.2,74.9,82.0,103.2(d,j=29.5hz,1c),103.3(d,j=29.4hz,1c),108.1,109.3(d,j=22.8hz,1c),122.1,122.7,123.2,123.3,123.4,127.5,128.1,129.1,129.6,143.2,143.4,144.1,144.2,151.0,151.2,163.2(d,j=241.2hz,1c),173.9,176.7.hrms(esi-tof)calcd.forc22h23fn2nao3[m+na]+405.1585;found:405.1592.
3z:whitesolid;55.8mg,yield70%,dr3:1;mp79.3-81.2℃.1hnmr(400mhz,cdcl3):δ(major+minor)0.91(d,j=7.1hz,3h),1.12(s,9h),3.20(s,0.75h),3.25(s,2.25h),3.84(q,j=7.0hz,1h),6.84-6.89(m,2h),6.93-6.99(m,3h),7.30-7.33(m,1h),8.02(s,1h);13cnmr(100mhz,cdcl3):δ(major+minor)13.4,14.3,26.7,27.8,44.7,74.0,74.6,82.0,108.2,115.2,115.3,122.0,122.7,122.9,123.0,123.2,123.3,123.6,127.4,129.2,129.6,131.3,134.0,143.2,143.4,143.9,151.0,173.8,176.7.hrms(esi-tof)calcd.forc22h23cln2nao3[m+na]+421.1289;found:421.1297.
3a':yellowsolid;52.2mg,yield69%,dr3:1;mp86.6-88.1℃.1hnmr(400mhz,cdcl3):δ(major+minor)0.93(d,j=7.0hz,3h),1.13(s,9h),2.20(s,2.25h),2.29(s,0.75h),3.18(s,0.75h),3.23(s,2.25h),3.89(q,j=6.8hz,1h),6.68(s,1h),6.73(d,j=8.0hz,1h),7.04(d,j=9.0hz,1h),7.10(d,j=6.7hz,2h),7.23(d,j=7.4hz,1h),7.98(d,j=7.6hz,1h);13cnmr(100mhz,cdcl3):δ(major+minor)13.3,14.7,21.0,26.7,27.8,45.1,73.5,74.4,81.4,107.8,114.6,114.8,122.7,122.8,123.0,123.9,127.8,128.2,129.0,129.6,132.2,132.8,140.8,141.0,142.9,151.3,174.0,177.1.hrms(esi-tof)calcd.forc23h26n2nao3[m+na]+401.1836;found:401.1842.
3b':yellowoil;49.7mg,yield63%,dr4:1.1hnmr(400mhz,cdcl3):δ(major)0.95(d,j=7.3hz,3h),1.15(s,9h),3.23(s,3h),3.67(s,3h),3.90(q,j=6.8hz,1h),6.49(s,1h),6.75(d,j=8.9hz,1h),6.83(d,j=9.7hz,1h),7.03(d,j=7.1hz,1h),7.09(d,j=7.0hz,1h),7.23(d,j=7.5hz,1h),7.96(d,j=7.6hz,1h);13cnmr(100mhz,cdcl3):δ(major)13.3,26.7,27.9,45.1,56.0,74.6,81.5,108.3,110.9,113.4,114.8,122.9,123.1,128.3,128.9,131.0,136.8,142.8,151.2,156.0,176.8.hrms(esi-tof)calcd.forc23h26n2nao4[m+na]+417.1785;found:417.1779.
3c':yellowsolid;41.8mg,yield49%,dr3:1;mp107.3-108.8℃.1hnmr(400mhz,cdcl3):δ(major)1.06(d,j=7.0hz,3h),1.19(s,9h),3.99(q,j=7.0hz,1h),6.94-6.98(m,1h),7.02-7.08(m,2h),7.09-7.14(m,2h),7.40-7.41(m,1h),7.48-7.57(m,6h),8.01-8.05(m,1h);13cnmr(100mhz,cdcl3):δ(major)13.8,28.1,46.2,74.5,82.0,109.4,109.7,114.7,114.9,122.9,123.6,125.5,127.9,128.7,129.2,129.6,129.7,132.4,133.1,142.5,143.0,151.4,176.2.hrms(esi-tof)calcd.forc27h26n2nao3[m+na]+449.1836;found:449.1839.
抽取化合物3a、3b、3c进行谱图分析,nmr谱图如图1~6所示。
以上所述的仅是本发明的实施例,方案中公知的具体结构及特性等常识在此未作过多描述。应当指出,对于本领域的技术人员来说,在不脱离本发明结构的前提下,还可以作出若干变形和改进,这些也应该视为本发明的保护范围,这些都不会影响本发明实施的效果和专利的实用性。本申请要求的保护范围应当以其权利要求的内容为准,说明书中的具体实施方式等记载可以用于解释权利要求的内容。
- 还没有人留言评论。精彩留言会获得点赞!
- 双吲哚甲烷衍生物促进CuCl催化Sonogashira交叉偶联反应的方法
- 吲哚-3-甲醇、二吲哚甲烷及其衍生物用于治疗过敏性咽炎药物的应用
- 吲哚-3-甲醇、二吲哚甲烷及其衍生物用于治疗接触性皮炎药物的应用
- 吲哚-3-甲醇、二吲哚甲烷及其衍生物用于治疗银屑病药物的应用
- 吲哚-3-甲醇、二吲哚甲烷及其衍生物用于治疗顽固性牙周炎药物的应用
- 吲哚-3-甲醇、二吲哚甲烷及其衍生物用于治疗慢性食管炎药物的应用
- 吲哚-3-甲醇、二吲哚甲烷及其衍生物用于治疗胃溃疡药物的应用
- 吲哚-3-甲醇、二吲哚甲烷及其衍生物用于治疗前列腺炎药物的应用
- 吲哚-3-甲醇、二吲哚甲烷及其衍生物用于治疗肾小球肾炎药物的应用
- 一种制备二吲哚甲烷类化合物的方法