二芳基‑β‑内酰胺类化合物及其制备方法和在制药中的用途与流程
本发明属化学制药领域,涉及二芳基-β-内酰胺类化合物,具体涉及具有显著抗肿瘤活性的二芳基-β-内酰胺类化合物、制备方法及其体外、体内抗肿瘤活性,以及该类化合物及其药学盐或以其为成分的复方药物在制备预防和治疗肿瘤相关疾病的药物的应用。
背景技术:
现有技术公开了癌症是目前威胁人类健康的主要疾病之一,其死亡率仅次于心脑血管疾病、位列第二;据估计,到2020年将有1500万的新增病例,死亡人数将达到1000万;目前癌症的治疗方法有手术治疗、放射治疗、化学治疗(药物治疗)和生物治疗等,其中化疗是最为常见的一种手段,即用一种或多种具有细胞毒性的抗肿瘤药物对癌症患者进行治疗。尽管目前已有大量的药物在临床应用,然而由于化疗药物的长期使用或肿瘤细胞自身的突变,许多恶性肿瘤对化疗药物产生耐药性导致化疗效果减弱或消失,以及传统的抗肿瘤药物由于缺乏选择性导致的毒性,使得化疗药物不能满足临床的需要。因此,寻找更为高效、低毒的新型抗肿瘤药物是医药领域的研发热点和急需解决的重要问题。
目前,被批准上市的抗肿瘤药物已有近百种,主要包括以下几种类型:(1)作用于dna的抗肿瘤药物:如烷化剂、金属铂配合物、dna拓扑异构酶抑制剂和抗代谢抗肿瘤药等;(2)作用于激酶的抗肿瘤药物:如酪氨酸激酶抑制剂和丝氨酸/苏氨酸激酶抑制剂等;(3)作用于微管的抗肿瘤药物:如微管聚集抑制剂和微管稳定剂等。其中,作用于微管的抗肿瘤药物是目前治疗前列腺癌、乳腺癌、卵巢癌和其它实体瘤的最有效的化疗药物,是近年来抗肿瘤药物研究的热点之一。
据报道,有关微管蛋白聚集抑制剂的研究尤其是针对康普立停的结构改造研究已经取得了重要进展;所述药物中有抑制剂进入了临床试验,虽然显示出能有效地抑制肿瘤增长,但存在一定毒性,导致最终被批准上市的化合物较少。
基于现有技术的现状,本申请的发明人拟寻找活性更强且副作用更小的新型微管蛋白聚集抑制剂以及血管生成抑制剂,尤其是提供具有显著抗肿瘤活性的新型的二芳基-β-内酰胺类化合物,以适应临床的迫切需求。
技术实现要素:
本发明的目的是提供新型微管蛋白聚集抑制剂以及血管生成抑制剂,具体涉及具有显著抗肿瘤活性的新型的二芳基-β-内酰胺类化合物及其制备方法以及该类化合物及其药学盐或以其为成分的复方药物在制备预防和治疗肿瘤相关疾病的药物的应用。
本发明提供了下述通式结构的二芳基-β-内酰胺类化合物或其药学盐,
其中,r1取自烷氧基、卤素原子、氨基、羟基、酰氧基、甲氧甲酰基、烯丙氧基、炔丙氧基、磺酰氧基、烷氨基、酰氨基、磺酰氨基或者2-3个上述相同或不同基团的组合;
r2取自烷氧基、羟基、卤素、氨基、烷氨基、吗啉基或者2-3个上述相同或不同基团的组合;
r3和r4取自氢原子、烷基、取代烷基、烷氧基、酰氧基、羟基、苯基、取代苯基、吡啶基、环丙基、乙烯基、氨基、烷氨基、吗啉基、磺酰氧基或者r3和r4合并为次甲基或取代次甲基(即=ch2或=chr),所述的取代苯基是指苯环的邻位、间位或对位有且仅有1个硝基、氟原子或甲氧基取代。
本发明中,优选的二芳基-β-内酰胺类化合物具有通式ⅰ结构,
其中,r1取自羟基、氨基、卤素、1-3个碳原子的烷氧基、甲氧甲酰基、烯丙氧基、炔丙氧基、o(ch2)nr2、och2cor3、ocor4、oso2r5、nhcor6、nhso2nh2,其中r2取自卤素、羟基、n,n-二甲基氨基、n-吗啉基,且n=2或3,r3取自1-3个碳原子的烷氧基、氨基,r4取自1-3个碳原子的烷基、苯基、取代苯基、吡啶基、环丙基、乙烯基、n-吗啉基,所述的取代苯基是指苯环的2、3或4位有且仅有1个硝基、氟原子或甲氧基取代,r5取自氨基、4-甲基苯基、n-吗啉基、2-(1,3-二氧代异吲哚-2-基)乙基、4-乙酰氨基苯基、苄基、正丁基、3,4-二甲氧基苯基,r6取自乙烯基、4-硝基苯基、环丙基;其中,更优选的化合物为:
本发明中,还优选的二芳基-β-内酰胺类化合物具有通式ⅱ的结构,
其中,r取自甲氧基,数目是1个或2个,可为3、4、5-位取代。
本发明中,还优选的二芳基-β-内酰胺类化合物具有通式ⅲ的结构,
其特征在于r取自甲基、三甲基硅基、苯基或者叔丁基苯基,烯键的构型为z式或e式。
本发明中,还优选的二芳基-β-内酰胺类化合物具有通式ⅳ或ⅴ的结构,
其中r1独立地选自甲基、乙基、羟甲基、氢原子、烷氧基、酰氧基、羟基、卤素、氨基、苯基氨基、苄基氨基、乙酰氨基、对甲苯磺酰氨基、甲磺酰氨基、苯甲酰氨基、3-氟苯甲酰氨基、甲磺酰氧基、甲氧甲基、n,n-二甲氨甲基、4-羟基苄基、三甲基硅乙基、乙氧羰甲基、羧丙酰氧基,r2独立地取自羟基、氨基、卤素、甲氧基、甲基、氢原子或酰氧基;其中优势化合物为:
本发明中,还优选的二芳基-β-内酰胺类化合物具有通式ⅵ的结构,
其中r1取自氢原子、甲基、乙酰基、丙烯酰基,r2取自氢原子、苄基、酰基、丙烯酰基;其中优选以下化合物:
更具体的,本发明中优选的化合物为:
本发明中所述的“药学上可接受的盐”,具体地可列举为与氢卤酸、硫酸、磷酸、硝酸等无机酸,以及枸橼酸、富马酸、草酸、苹果酸、乳酸、樟脑磺酸等有机酸形成的盐。
本发明的另一个目的是提供上述化合物或这些化合物在药学上可接受的盐以及包含该化合物或其盐的组合物用于制备预防或治疗与肿瘤相关疾病的药物中的应用。
所述的与肿瘤相关疾病具体可列举为甲状腺癌、头颈部鳞状细胞癌、宫颈癌、卵巢癌、乳腺癌、结直肠癌、胰腺癌、食管癌、骨肉瘤、肾癌、胃癌、肺癌、肝癌、黑色素瘤、淋巴瘤、前列腺癌、肾癌、膀胱癌、脑胶质瘤、鼻咽癌,神经内分泌癌、未分化癌、间质肉瘤、绒癌、恶性葡萄胎、恶性畸胎瘤等,以及良性肿瘤,但不受限于此。
本发明提供并证明了具有显著抗肿瘤作用的二芳基-β-内酰胺类化合物或其在药学上可接受的盐,通过抑制微管蛋白聚集抑制肿瘤细胞生长的调控机制,在体外、体内的抗肿瘤实验中对肿瘤的生长具有良好的抑制作用。
附图说明
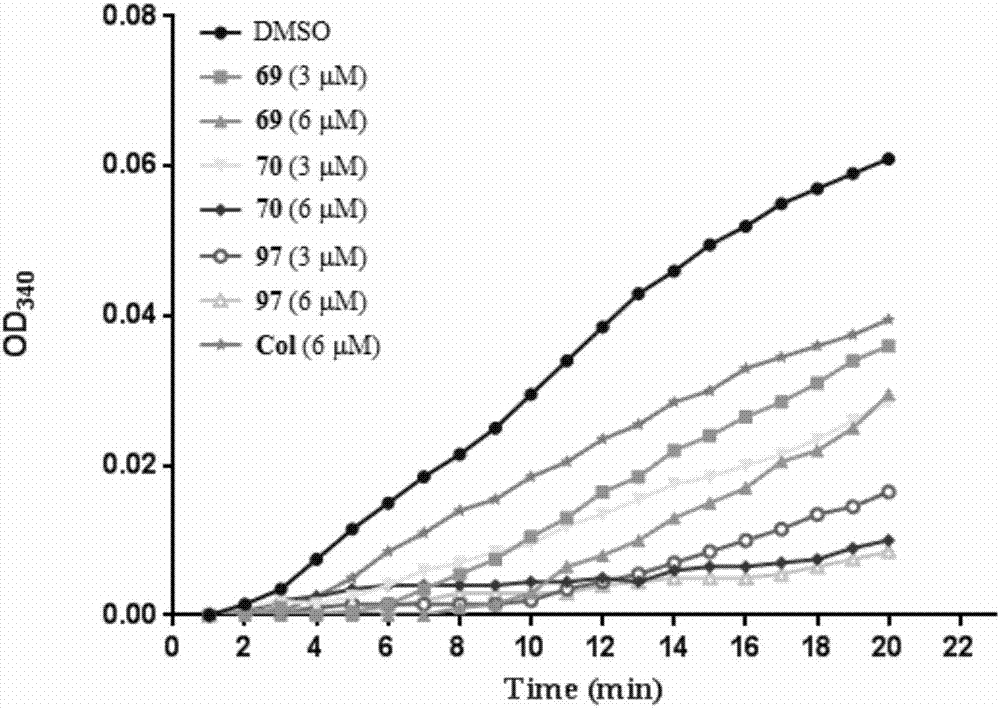
图1是根据吸光度绘制的曲线图,
其中显示了化合物69、70和97明显抑制微管聚集,ic50分别为3.5、1.7和1.6μm。
图2是免疫印迹分析实验,
其中,显示化合物1、69、70和97明显抑制微管聚集,使微管蛋白维持解聚状态。
图3是共聚焦显微镜观察的微管蛋白的形态,
其中,显示化合物1、69、70和97明显抑制微管的聚集。
图4是倒置相差显微镜下观察的毛细血管形成情况,
其中,显示化合物69、70和97明显抑制huvec细胞生成毛细血管样结构。
图5是基质胶塞实验结果,
其中,显示化合物69、70和97明显抑制由vegf介导的新生血管生成。
图6是菌落抑制实验结果,
其中,显示化合物69、70和97明显抑制肿瘤细胞菌落的形成。
图7是体外细胞周期实验结果,
其中,显示化合物69、70和97明显将细胞阻滞于g2/m期。
图8是体外细胞周期相关蛋白检测实验结果,
其中,显示化合物69、70和97明显促进磷酸化组蛋白h3、细胞周期蛋白b1、有丝分裂检验点蛋白bubr1表达。
图9是体外细胞凋亡实验结果,
其中,显示化合物69、70和97能明显促进细胞凋亡。
图10是体外凋亡相关蛋白检测实验结果,
其中,显示化合物69、70和97能明显促进促凋亡蛋白bax、抑癌基因p53、剪切的dna修复酶的表达。
图11是动物水平和组织水平的肿瘤治疗作用和机理研究实验结果,
其中,图11(a)、图11(b)显示化合物70、69和97在体内明显抑制肿瘤生长,对小鼠体重无明显影响;图11(c)组织染色显示化合物70、69和97使肿瘤组织产生坏死区(图中箭头标示),对肝、肾、脾的组织染色并未观察到非正常区域。
具体实施方式
下面结合实施例进一步说明本发明。这些实施例只是用于进一步说明本发明,并不改变本发明的保护范围。本发明目标化合物的制备方法可以进一步用代表性化合物制备过程体现如下:
实施例1化合物(s)-1-(3,4,5-三甲氧基苯基)-4-(3-羟基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(1)的合成
参照文献(wang,x.;meng,f.;wang,y.;han,z.;chen,y.-j.;liu,l.;wang,z.;ding,k.angewandtechemieint.ed.2012,124,9410-9416.)方法,本发明按如下路线合成目标化合物1:
1.13-叔丁基二甲基硅氧基-4-甲氧基苯甲醛(1b)的合成
100ml三口瓶中加入3-羟基-4-甲氧基苯甲醛(1a)(1.58g,10.4mmol)、dmap(25mg,0.2mmol)、无水dcm(50ml)和无水三乙胺(2ml,14.5mmol),冷至冰浴下,tbscl(1.88g,12.5mmol)溶于无水dcm(10ml)中,由滴液漏斗缓慢加入。滴完后撤去冰浴,室温搅拌两小时,加入饱和碳酸氢钠水溶液淬灭反应,分出有机相,水相用dcm萃取三遍,合并有机相,无水硫酸钠干燥;湿法上柱层析分离纯化,收集对应的洗脱液,蒸干溶剂得无色油状化合物(1b)2.66g,收率96%;
1.22-[1-(3-叔丁基二甲基硅氧基-4-甲氧基苯基)-1-羟基甲基]丙烯酸乙酯(1c)的合成
在50ml茄形瓶中,加入化合物4-甲氧基-3-叔丁基二甲基硅氧基苯甲醛(1b)(2.66g,10mmol)、丙烯酸乙酯(1.00g,10mmol)和dabco(1.12g,10mmol),室温搅拌反应2周后,tlc(展开剂:石油醚/乙酸乙酯=9:1)显示原料(rf=0.4)明显减少,有大量新点生成(rf=0.3),停止反应,直接硅胶柱层析,用pe先除去丙烯酸乙酯,继而进行梯度洗脱,收集对应的洗脱液,回收原料1.55g,回收率58%,得无色油状产物(1c)1.12g,扣除回收原料的收率为73%。1hnmr(400mhz,cdcl3)δ6.92(dd,j=8.3,2.1hz,1h),6.85(d,j=2.1hz,1h),6.81(d,j=8.3hz,1h),6.70-6.76(m,1h),6.31(s,1h),5.77(d,j=1.1hz,1h),5.47(d,j=5.0hz,1h),4.17(q,j=7.1hz,2h),3.79(s,3h),1.24(t,j=7.1hz,3h),0.98(s,9h),0.13(s,6h);esi-lrmsm/z(%):367.2[m+h]+.
1.32-[1-(3-叔丁基二甲基硅氧基-4-甲氧基苯基)-1-乙酰氧基甲基]丙烯酸乙酯(1d)的合成
化合物1c(1.12g,3.1mmol)、dmap(45mg,0.31mmol)和tea(0.63g,6.2mmol)溶于20ml无水dcm中,0℃搅拌条件下,缓慢滴加乙酸酐(0.63g,6.2mmol),加完后在冰浴中继续搅拌5min后,tlc(展开剂:石油醚/乙酸乙酯=9:1)显示原料(rf=0.3)完全消失,有新点(rf=0.5)生成,缓慢加入饱和碳酸氢钠水溶液10ml淬灭反应。分液,水层用dcm(3x20ml)萃取,合并有机相,无水硫酸钠干燥。拌硅胶柱层析分离纯化,收集对应的洗脱液,减压蒸干溶剂得无色油状化合物(1d)1.2g,收率96%。1hnmr(400mhz,cdcl3)δ6.93(dd,j=8.3,2.2hz,1h),6.84(d,j=2.2hz,1h),6.79(d,j=8.3hz,1h),6.58(s,1h),6.36(s,1h),5.77(s,1h),4.14(q,j=7.1hz,2h),3.78(s,3h),2.09(s,3h),1.21(t,j=7.1hz,3h),0.98(s,9h),0.13(s,6h);esi-lrmsm/z(%):409.2[m+h]+.
1.4(s)-2-[1-(3-叔丁基二甲基硅氧基-4-甲氧基苯基)-1-(3,4,5-三甲氧基苯基氨基)甲基]丙烯酸乙酯(1f)的合成
氮气氛围下,三(二亚苄基丙酮)二钯(27mg,0.029mmol)和1e(49mg,0.074mmol)分别加入一schlenk管中,加入无水ch2cl2(5ml),室温下搅拌10分钟后,先后加入底物1d(1.2g,2.9mmol),k2co3(1.0m水溶液,5ml,5mmol)和3,4,5-,三氧基苯胺(809mg,0.62mmol);室温下搅拌2h后,tlc(展开剂:石油醚/乙酸乙酯=9:1)显示原料(rf=0.5)完全消失,有新点(rf=0.2)生成,用二氯甲烷萃取(3×10ml),无水硫酸钠干燥,过滤浓缩后,柱层析纯化,得黄色油状物(1f)1.3g,收率84%;1hnmr(400mhz,cdcl3)δ6.91(dd,j=8.3,2.1hz,1h),6.83(d,j=2.1hz,1h),6.80(d,j=8.3hz,1h),6.34(s,1h),5.88(s,1h),5.81(s,2h),5.28(s,1h),4.15(q,j=7.1hz,2h),3.78(s,3h),3.76(s,6h),3.74(s,3h),1.22(t,j=7.1hz,3h),0.96(s,9h),0.11(s,6h).
1.5(s)-1-(3,4,5-三甲氧基苯基)-4-(3-叔丁基二甲基硅氧基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(1g)的合成
化合物1f(1.3g)和sn[n(tms)2]2(1.6g,3.7mmol)加入一schlenk管中,加入无水甲苯(20ml),加热回流4.5小时后,tlc(展开剂:石油醚/乙酸乙酯=3:1)显示原料(rf=0.8)基本消失,有新点(rf=0.5)生成,反应液冷却至室温后,浓缩,柱层析纯化,得无色油状化合物(1g)1.1g,收率92%;[α]d20=+37.3(c1.00,chcl3);1hnmr(400mhz,cdcl3)δ6.91-6.88(m,1h),6.78-6.76(m,2h),6.52(s,2h),5.72(s,1h),5.20(s,1h),5.06(s,1h),3.71(s,3h),3.68(s,3h),3.64(s,6h),0.86(t,j=2.8hz,9h),0.01(d,j=4.8hz,6h);13cnmr(100mhz,cdcl3)δ160.5,153.1,151.1,149.6,145.2,134.2,133.4,128.4,120.1,118.9,111.8,110.1,94.5,63.2,60.5,55.6,55.1,25.3,18.1,-5.01;esi-lrmsm/z:486.2[m+h+].
1.6(s)-1-(3,4,5-三甲氧基苯基)-4-(3-羟基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(1)的合成
化合物1g(1.1g,2.3mmol)溶于25mlthf,冷至冰浴下,tbaf(888mg,3.4mmol)溶于5mlthf,由分液漏斗缓慢滴加,滴完后继续搅拌15min后,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料(rf=0.7)完全消失,有新点(rf=0.5)生成,减压旋干,20ml乙酸乙酯溶解,水洗(20mlx2),饱和食盐水洗(20ml),无水硫酸钠干燥。过滤浓缩后,柱层析纯化,得白色固体(1)597mg,收率71%。mp115–117℃;[α]d20=+38.6(c1.0,chcl3);1hnmr(400mhz,cdcl3):δ6.93(d,j=1.7hz,1h),6.87(dd,j=8.2,1.7hz,1h),6.82(d,j=8.2hz,1h),6.58(s,2h),5.79(s,1h),5.26(s,1h),5.13(s,1h),3.84(s,3h),3.73(s,3h),3.71(s,6h).13cnmr(150mhz,cdcl3):δ160.3,152.9,149.2,146.5,145.6,134.1,133.2,128.9,118.1,112.3,110.3,110.0,94.31,63.0,60.3,55.5,55.4.esi-ms(m/z):372.1(m+h+).esi-hrms(m/z):calcdforc20h21no6+h+[m+h]+,372.1442;found,372.1441.。
实施例2(s)-1-(3,4,5-三甲氧基苯基)-4-(3-甲氧基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(2)的合成
20ml茄形瓶中加入无水dmf(1ml)、化合物1(22mg,0.059mmol)和碳酸钾(15mg,0.108mmol),室温搅拌10min,此时溶液为黄色,加入碘甲烷(17mg,0.12mmol),搅拌反应3h后,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料1(rf=0.3)完全消失,有新点生成(rf=0.5)。停止反应,加入10ml乙酸乙酯,以水(2x10ml)洗涤,饱和食盐水(10ml)洗,无水硫酸钠干燥。拌硅胶柱层析分离纯化,收集对应的洗脱液,蒸干溶剂得白色固体(2)23mg,收率97%。mp138-139℃;[α]d20=+35.6(c0.21,chcl3);1hnmr(400mhz,cdcl3)δ7.00(dd,j=8.2,1.8hz,1h),6.86(d,j=8.2hz,1h),6.83(d,j=1.8hz,1h),6.60(s,2h),5.84(s,1h),5.31(s,1h),5.17(s,1h),3.88(s,3h),3.83(s,3h),3.76(s,3h),3.73(s,6h);13cnmr(100mhz,cdcl3)δ161.3,153.8,150.1,150.0,149.9,134.9,134.2,129.1,120.2,111.4,111.2,109.3,95.1,64.3,61.3,56.3;esi-lrmsm/z(%):386.2[m+h]+;esi-hrmsm/z(%):calcdforc21h24no6[m+h]+386.1598,found386.1598.。
实施例3(s)-1-(3,4,5-三甲氧基苯基)-4-(3-乙氧基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(3)的合成
20ml茄形瓶中,加入无水dmf1ml、化合物1(22mg,0.059mmol)和碳酸钾(15mg,0.108mmol),室温搅拌10min,此时溶液为黄色,加入溴乙烷(12mg,0.12mmol),过夜反应,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料1(rf=0.3)完全消失,有新点生成(rf=0.5),停止反应,加入10ml乙酸乙酯,以水洗涤(3x10ml),无水硫酸钠干燥。直接拌硅胶柱层析分离纯化,收集对应的洗脱液,蒸干溶剂得白色固体(3)23mg,收率97%。mp92-93℃;[α]d20=+34.0(c0.27,chcl3).1hnmr(400mhz,cdcl3)δ6.98(dd,j=8.2,2.0hz,1h),6.86(d,j=8.2hz,1h),6.83(d,j=1.9hz,1h),6.60(s,2h),5.83(s,1h),5.29(s,1h),5.16(s,1h),4.02(q,j=7.0hz,2h),3.86(s,3h),3.75(s,3h),3.72(s,6h),1.41(t,j=7.0hz,3h);13cnmr(100mhz,cdcl3)δ161.2,153.6,150.0,149.9,149.1,134.8,134.0,128.9,112.0,111.5,111.1,110.6,94.9,64.6,64.2,61.2,56.2,56.1,14.8;esi-lrmsm/z(%):400.2[m+h]+;esi-hrmsm/z(%):calcdforc22h26no6[m+h]+400.1755,found400.1756.。
实施例4(s)-1-(3,4,5-三甲氧基苯基)-4-(3-丙氧基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(4)的合成
20ml茄形瓶中加入无水dmf1ml,化合物1(20mg,0.054mmol)和碳酸钾(15mg,0.108mmol),室温搅拌10min(此时溶液为黄色)后加入溴丙烷(13mg,0.108mmol),室温7h后,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料1(rf=0.3)约40%剩余,有新点生成(rf=0.5),升温至50℃过夜,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料1(rf=0.3)完全消失,有新点生成(rf=0.2),停止反应,冷至室温后加入10ml乙酸乙酯,以水洗涤(2x10ml),饱和食盐水(10ml)洗,无水硫酸钠干燥3h,拌硅胶柱层析分离纯化,收集对应的洗脱液,蒸干溶剂得白色固体(4)19mg,收率85%;mp90-91℃;[α]d20=+40.0(c0.07,chcl3);1hnmr(400mhz,cdcl3)δ6.98(dd,j=8.2hz,j=1.4hz,1h),6.86(d,j=8.2hz,1h),6.84(d,j=1.4hz,1h),6.60(s,2h),5.84(s,1h),5.30(s,1h),5.17(s,1h),3.90(t,j=6.8hz,2h),3.86(s,3h),3.76(s,3h),3.73(s,6h),1.87–1.76(m,2h),1.00(t,j=7.4hz,3h);13cnmr(100mhz,cdcl3)δ161.4,153.8,150.3,150.1,149.5,134.9,134.2,129.0,120.1,111.8,111.1,110.9,95.1,70.8,64.4,61.3,56.3,22.7,10.7;esi-lrmsm/z(%):436.2[m+na]+;esi-hrmsm/z(%):calcdforc23h28no6[m+h]+414.1911,found414.1912.。
实施例5(s)-1-(3,4,5-三甲氧基苯基)-4-(3-异丙氧基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(5)的合成
20ml茄形瓶中加入无水thf/dmso(3/1,1.5ml),化合物1(20mg,0.054mmol)和碳酸钾(37mg,0.27mmol),室温搅拌10min(此时溶液为黄色)后加入2-溴丙烷(15mg,0.12mmol),过夜反应后,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料1(rf=0.3)完全消失,有新点生成(rf=0.2),停止反应,加入10ml乙酸乙酯,以水洗涤(3x10ml),无水硫酸钠干燥3h,直接拌硅胶柱层析分离纯化,收集对应的洗脱液,蒸干溶剂得白色固体(5)21mg,收率98%;mp146-147℃;[α]d20=+13.1(c0.21,chcl3);1hnmr(400mhz,cdcl3)δ6.99(dd,j=8.2,2.0hz,1h),6.93–6.85(m,2h),6.61(s,2h),5.84(t,j=1.7hz,1h),5.31(s,1h),5.18(s,1h),4.55–4.42(m,1h),3.86(s,3h),3.77(s,3h),3.73(s,6h),1.33(d,j=6.1hz,3h),1.28(d,j=6.1hz,3h);13cnmr(100mhz,cdcl3)δ160.4,152.9,150.4,149.3,147.2,134.1,133.2,128.0,119.5,133.3,111.4,110.1,70.1,63.3,60.3,55.4,55.3,21.3,21.2;esi-lrmsm/z(%):436.2[m+na]+;esi-hrmsm/z(%):calcdforc23h28no6[m+h]+414.1911,found414.1916.。
实施例6(s)-1-(3,4,5-三甲氧基苯基)-4-(3-烯丙氧基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(6)的合成
20ml茄形瓶中加入dmf2ml,化合物1(20mg,0.054mmol),碳酸铯(35mg,0.108mmol),搅拌0.5h后,加入3-溴丙烯(13mg,0.054mmol),过夜反应后,tlc(展开剂:石油醚/乙酸乙酯=3:2)显示原料1(rf=0.6)完全消失,有新点生成(rf=0.3),停止反应,加入10mldcm,以水(2x10ml)洗涤,饱和食盐水(10ml)洗,无水硫酸钠干燥3h,拌硅胶柱层析分离纯化,收集对应的洗脱液,蒸干溶剂得白色固体(6)21mg,收率98%;m.p.92~93℃;[α]d20=+40.0(c0.39,chcl3);1hnmr(400mhz,cdcl3)δ6.99(dd,j=8.2,1.2hz,1h),6.87(d,j=8.2hz,1h),6.85(s,1h),6.59(s,2h),6.00(ddd,j=17.4,10.5,5.5hz,1h),5.83(s,1h),5.33(d,j=17.4hz,1h),5.29(s,1h),5.22(d,j=10.5hz,1h),5.16(s,1h),4.55(d,j=5.5hz,2h),3.87(s,3h),3.76(s,3h),3.73(s,6h);13cnmr(100mhz,cdcl3)δ161.0,153.5,150.1,149.8,148.6,134.7,133.8,132.8,128.7,120.1,118.3,111.7,111.5,110.7,94.9,69.9,64.0,61.0,56.1,56.0;esi-lrmsm/z(%):412.2[m+h]+;esi-hrmsm/z(%):calcdforc23h26no6[m+h]+412.1755,found412.1756.。
实施例7(s)-1-(3,4,5-三甲氧基苯基)-4-(3-炔丙氧基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(7)的合成
20ml茄形瓶中加入dmf2ml,化合物1(20mg,0.054mmol),碳酸铯(35mg,0.108mmol),搅拌0.5h后,加入3-溴丙炔(13mg,0.054mmol),过夜反应后,tlc(展开剂:石油醚/乙酸乙酯=3:2)显示原料1(rf=0.6)完全消失,有新点生成(rf=0.3),停止反应,加入10mldcm,以水(2x10ml)洗涤,饱和食盐水(10ml)洗,无水硫酸钠干燥3h,拌硅胶柱层析分离纯化,收集对应的洗脱液,蒸干溶剂得白色固体(7)18mg,收率85%;m.p.106~107℃;[α]d20=+50.5(c0.33,chcl3);1hnmr(400mhz,cdcl3)δ7.04(d,j=8.2hz,1h),7.02(s,1h),6.90(d,j=8.2hz,1h),6.60(s,2h),5.84(s,1h),5.33(s,1h),5.17(s,1h),4.73(d,j=2.2hz,2h),3.87(s,3h),3.76(s,3h),3.73(s,6h),2.34(t,j=2.2hz,1h);13cnmr(100mhz,cdcl3)δ160.9,153.5,150.3,149.8,147.2,134.7,133.8,128.7,121.0,112.9,111.9,110.8,95.0,78.0,76.0,63.8,60.96,56.9,56.1,56.0;esi-lrmsm/z(%):410.2[m+h]+;esi-hrmsm/z(%):calcdforc23h24no6[m+h]+410.1598,found410.1600.。
实施例8(s)-1-(3,4,5-三甲氧基苯基)-4-(3-羟乙氧基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(8)的合成
20ml茄形瓶中加入无水dmso1ml,化合物1(20mg,0.054mmol)和碳酸钾(37mg,0.27mmol),室温搅拌10min(此时溶液为黄色)后加入2-氯乙醇(5mg,0.12mmol),70℃过夜反应,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料1(rf=0.3)完全消失,有新点生成(rf=0.1),停止反应,加入10ml乙酸乙酯,以水洗涤(3x10ml),无水硫酸钠干燥3h,直接拌硅胶柱层析分离纯化,收集对应的洗脱液,蒸干溶剂得白色固体(8)12mg,收率54%;[α]d20=+22.0(c0.25,chcl3);1hnmr(400mhz,cdcl3)δ7.03(dd,j=4.0hz,j=8.0hz,1h),6.87(d,j=8.2hz,2h),6.58(s,2h),5.83(s,1h),5.29(s,1h),5.15(s,1h),4.05(t,j=4.0hz,2h),3.92(t,j=4.0hz,2h),3.85(s,3h),3.75(s,3h),3.72(s,6h),2.81(s,1h);13cnmr(100mhz,cdcl3)δ160.3,152.9,149.7,149.1,148.2,134.1,133.2,128.4,120.2,111.6,111.1,110.3,94.2,70.7,63.2,60.5,60.3,55.4,55.3;esi-lrmsm/z(%):416.2[m+h]+;esi-hrmsm/z(%):calcdforc22h26no7[m+h]+416.1704,found416.1704.。
实施例9(s)-1-(3,4,5-三甲氧基苯基)-4-(3-羟丙氧基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(9)的合成
20ml茄形瓶中加入无水乙腈2ml,化合物1(20mg,0.054mmol),碳酸钾(15mg,0.108mmol),碘化钾(1.5mg,0.0081mmol),搅拌条件下加入3-溴丙醇(9mg,0.065mmol),升温至50摄氏度过夜反应后,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料1(rf=0.3)完全消失,有新点生成(rf=0.1),停止反应,加入10mldcm,以水(2x10ml)洗涤,饱和食盐水(10ml)洗,无水硫酸钠干燥3h,拌硅胶柱层析分离纯化,收集对应的洗脱液,蒸干溶剂得白色固体(9)22mg,收率95%;[α]d20=+18.7(c0.27,chcl3);1hnmr(400mhz,cdcl3)δ7.01(d,j=8.2hz,1h),6.91–6.79(m,2h),6.61(s,2h),5.85(s,1h),5.31(s,1h),5.18(s,1h),4.14(t,j=5.8hz,2h),3.86–3.84(m,5h),3.77(s,3h),3.74(s,6h),2.59(brd,1h),2.08–2.03(m,2h);13cnmr(100mhz,cdcl3)δ160.4,152.9,149.4,149.2,148.3,134.1,133.2,128.2,119.8,110.7,110.2,110.1,94.2,67.7,63.3,60.7,60.3,55.5,55.3,31.0,29.1;esi-lrmsm/z(%):431.2[m+h]+;esi-hrmsm/z(%):calcdforc23h28no7[m+h]+430.1860,found430.1861.。
实施例10(s)-1-(3,4,5-三甲氧基苯基)-4-(3-氯乙氧基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(10)的合成
20ml茄形瓶中加入2-丁酮2ml,化合物1(20mg,0.054mmol),碳酸钾(15mg,0.108mmol),搅拌条件下加入2-溴氯乙烷(31mg,0.162mmol),回流反应14h后,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料1(rf=0.3)基本消失,有新点生成(rf=0.4),停止反应,加入10mldcm,以水(2x10ml)洗涤,饱和食盐水(10ml)洗,无水硫酸钠干燥3h,拌硅胶柱层析分离纯化,收集对应的洗脱液,蒸干溶剂得淡黄色固体(10)17mg,收率73%;m.p.91~92℃;[α]d20=+36.4(c0.33,chcl3);1hnmr(400mhz,cdcl3)δ7.05(dd,j=8.2,1.8hz,1h),6.90–6.88(m,2h),6.59(s,2h),5.84(s,1h),5.30(s,1h),5.16(s,1h),4.22(t,j=6.0hz,2h),3.87(s,3h),3.80(t,j=6.0hz,2h),3.76(s,3h),3.73(s,6h);13cnmr(100mhz,cdcl3)δ161.2,153.7,150.6,149.9,148.5,134.9,134.0,129.1,121.4,112.7,112.3,111.2,95.0,69.7,64.0,61.2,56.3,42.0;esi-lrmsm/z(%):434.1[m+h]+;esi-hrmsm/z(%):calcdforc22h25clno6[m+h]+434.1365,found434.1366.。
实施例11(s)-1-(3,4,5-三甲氧基苯基)-4-(3-氯丙氧基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(11)的合成
20ml茄形瓶中加入丙酮2ml,化合物1(20mg,0.054mmol),碳酸钾(15mg,0.108mmol),搅拌条件下加入3-溴氯丙烷(34mg,0.216mmol),回流过夜反应后,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料1(rf=0.3)完全消失,有新点生成(rf=0.5),停止反应,加入10mldcm,以水(2x10ml)洗涤,饱和食盐水(10ml)洗,无水硫酸钠干燥3h,拌硅胶柱层析分离纯化,收集对应的洗脱液,蒸干溶剂得白色固体(11)18mg,收率77%;m.p.72~73℃;[α]d20=+20.1(c0.15,chcl3);1hnmr(400mhz,cdcl3)δ7.01(d,j=8.2hz,1h),6.88–6.86(m,2h),6.60(s,2h),5.84(s,1h),5.30(s,1h),5.17(s,1h),4.18–4.01(m,2h),3.85(s,3h),3.76–3.73(m,11h),2.27–2.22(m,2h);13cnmr(100mhz,cdcl3)δ161.2,153.7,150.3,150.0,149.0,134.8,134.0,129.0,120.6,112.0,111.6,111.1,95.0,65.9,64.1,61.2,56.3,56.2,41.7,32.4;esi-lrmsm/z(%):449.1[m+h]+;esi-hrmsm/z(%):calcdforc23h27clno6[m+h]+448.1521,found448.1523.。
实施例12(s)-1-(3,4,5-三甲氧基苯基)-4-(3-乙氧酰基甲氧基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(12)的合成
20ml茄形瓶中加入无水dmf1.5ml,化合物1(20mg,0.054mmol)和碳酸铯(35mg,0.108mmol),冰浴条件下搅拌0.5h(此时溶液为黄色,有不溶物)后加入溴乙酸乙酯(18mg,0.108mmol)(溶液由黄色变为无色,仍有不溶物),升温至90摄氏度反应6h,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料1(rf=0.3)完全消失,有新点生成(rf=0.5),停止反应,冷却至室温后加入10ml乙酸乙酯,以水洗涤(3x10ml),饱和食盐水(10ml)洗,无水硫酸钠干燥3h,直接拌硅胶柱层析分离纯化,收集对应的洗脱液,蒸干溶剂得无色油状物(12)33mg,收率95%;1hnmr(400mhz,cdcl3)δ7.05(dd,j=8.3,1.9hz,1h),6.90(d,j=8.3hz,1h),6.81(d,j=1.9hz,1h),6.58(s,2h),5.82(s,1h),5.29(s,1h),5.14(s,1h),4.64(s,2h),4.17–4.11(m,2h),3.88(s,3h),3.76(s,3h),3.73(s,6h),1.22(t,j=7.2hz,3h);esi-lrmsm/z(%):458.0[m+h]+.。
实施例13(s)-1-(3,4,5-三甲氧基苯基)-4-(3-叔丁氧酰基甲氧基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(13)的合成
20ml茄形瓶中加入化合物1(20mg,0.054mmol)和碳酸铯(35mg,0.108mmol),溴乙酸叔丁酯(21mg,0.108mmol),无水dmf1.5ml,室温搅拌反应5h,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料1(rf=0.3)完全消失,有新点生成(rf=0.5),停止反应,冷却至室温后加入10ml乙酸乙酯,以水洗涤(3x10ml),饱和食盐水(10ml)洗,无水硫酸钠干燥3h,直接拌硅胶柱层析分离纯化,收集对应的洗脱液,蒸干溶剂得淡黄色油状物(13)24mg,收率92%;[α]d20=+37.8(c0.12,chcl3);1hnmr(400mhz,cdcl3)δ7.03(d,j=8.2hz,1h),6.89(d,j=8.2hz,1h),6.76(s,1h),6.58(s,2h),5.81(s,1h),5.27(s,1h),5.13(s,1h),4.53(s,2h),3.88(s,3h),3.75(s,3h),3.72(s,6h),1.39(s,9h);13cnmr(150mhz,cdcl3)δ160.9,153.5,150.2,149.9,148.9,134.8,133.8,128.9,120.4,111.7,111.6,110.7,94.9,67.3,63.9,60.9,58.0,56.1,55.9,45.9;esi-lrmsm/z(%):486.2[m+h]+;esi-hrmsm/z(%):calcdforc26h31nnao8[m+na]+508.1942,found508.1944.。
实施例14(s)-1-(3,4,5-三甲氧基苯基)-4-(3-二甲氨基乙氧基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(14)的合成
20ml茄形瓶中加入无水乙腈2ml,化合物1(30mg,0.081mmol),碳酸钾(45mg,0.323mmol),2-氯乙基二甲胺盐酸盐(14mg,0.097mmol),回流反应8h后,tlc(展开剂:二氯甲烷/甲醇=15:1)显示原料1(rf=0.8)完全消失,有新点生成(rf=0.5),停止反应,加入10mldcm,以水(2x10ml)洗涤,饱和食盐水(10ml)洗,无水硫酸钠干燥3h,拌硅胶柱层析分离纯化,收集对应的洗脱液,蒸干溶剂得白色固体(14)27mg,收率88%;[α]d20=+37.8(c0.12,chcl3);1hnmr(400mhz,cdcl3)δ7.03(d,j=8.2hz,1h),6.89(d,j=8.2hz,1h),6.76(s,1h),6.58(s,2h),5.81(s,1h),5.27(s,1h),5.13(s,1h),4.53(s,2h),3.88(s,3h),3.75(s,3h),3.72(s,6h),1.39(s,9h);13cnmr(150mhz,cdcl3)δ160.9,153.5,150.2,149.9,148.9,134.8,133.8,128.9,120.4,111.7,111.6,110.7,94.9,67.3,63.9,60.9,58.0,56.1,55.9,45.9;esi-lrmsm/z(%):486.2[m+h]+;esi-hrmsm/z(%):calcdforc26h31nnao8[m+na]+508.1942,found508.1944.。
实施例15(s)-1-(3,4,5-三甲氧基苯基)-4-(3-吗啉基乙氧基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(15)的合成
20ml茄形瓶中加入无水乙腈2ml,化合物1(20mg,0.054mmol),碳酸钾(30mg,0.216mmol),n-(2-氯乙基)吗啉盐酸盐(12mg,0.065mmol),回流反应9h后,tlc(展开剂:石油醚/乙酸乙酯=1:2)显示原料1(rf=0.7)完全消失,有新点生成(rf=0.2),停止反应,加入10mldcm,以水(2x10ml)洗涤,饱和食盐水(10ml)洗,无水硫酸钠干燥3h,拌硅胶柱层析分离纯化,收集对应的洗脱液,蒸干溶剂得白色固体(15)23mg,收率88%;mp86-87℃;[α]d20=+115.4(c0.13,chcl3);1hnmr(400mhz,cdcl3)δ7.01(d,j=8.2hz,1h),6.86(d,j=8.2hz,1h),6.84(d,j=0.9hz,1h),6.59(s,2h),5.83(s,1h),5.29(s,1h),5.15(s,1h),4.12–3.97(m,2h),3.85(s,3h),3.76(s,3h),3.74–3.67(m,10h),2.78(t,j=6.0hz,2h),2.55(s,4h);13cnmr(150mhz,cdcl3)δ160.4,152.9,149.5,149.2,148.3,134.1,133.2,128.3,119.9,111.0,110.4,110.2,94.2,66.2,66.0,63.3,60.3,56.8,55.4,55.3,53.4;esi-lrmsm/z(%):485.2[m+h]+;esi-hrmsm/z(%):calcdforc26h33n2o7[m+h]+485.2282,found485.2284.。
实施例16(s)-1-(3,4,5-三甲氧基苯基)-4-(3-氨甲酰甲氧基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(16)的合成
20ml茄形瓶中加入丁酮2ml,化合物1(20mg,0.054mmol),碳酸钾(22mg,0.162mmol),碘化钾(1mg,0.0054mmol)和氯乙酰胺(7mg,0.068mmol),回流反应9h后,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料1(rf=0.3)完全消失,有新点生成(rf=0.1),停止反应,加入10mldcm,以水(2x10ml)洗涤,饱和食盐水(10ml)洗,无水硫酸钠干燥3h,拌硅胶柱层析分离纯化,收集对应的洗脱液,蒸干溶剂得无色油状物(16)22mg,收率95%;[α]d20=+20.9(c0.29,chcl3);1hnmr(400mhz,cdcl3)δ7.08(d,j=8.3hz,1h),6.92–6.90(m,3h),6.57(s,2h),6.02(s,1h),5.84(s,1h),5.30(s,1h),5.15(s,1h),4.48(s,2h),3.87(s,3h),3.76(s,3h),3.74(s,6h);13cnmr(150mhz,cdcl3)δ170.5,160.1,153.0,149.7,149.0,147.1,134.2,133.0,128.7,121.2,113.1,111.6,110.3,94.3,68.7,62.8,60.3,55.5,55.3;esi-lrmsm/z(%):429.2[m+h]+;esi-hrmsm/z(%):calcdforc22h24nao7[m+na]+451.1476,found451.1476.。
实施例17(s)-1-(3,4,5-三甲氧基苯基)-4-(3-苄氧酰氨基丙氧基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(17)的合成
20ml茄形瓶中加入无水dmf2ml,化合物1(20mg,0.054mmol),碳酸钾(15mg,0.108mmol),碘化钾(1.5mg,0.0081mmol),搅拌条件下加入n-苄氧羰基-3-溴丙胺(44mg,0.162mmol),升温至55摄氏度过夜反应后,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料1(rf=0.3)完全消失,有新点生成(rf=0.25),停止反应,加入10mldcm,以水(2x10ml)洗涤,饱和食盐水(10ml)洗,无水硫酸钠干燥3h,拌硅胶柱层析分离纯化,收集对应的洗脱液,蒸干溶剂得白色固体(17)22mg,收率73%;1hnmr(400mhz,cdcl3)δ7.39–7.27(m,5h),7.00(d,j=8.0hz,1h),6.83–6.81(m,2h),6.59(s,2h),6.00(s,1h),5.83(s,1h),5.28(s,1h),5.15(s,1h),5.09(s,2h),4.04(t,j=5.5hz,2h),3.75(s,3h),3.72(s,6h),3.71(s,3h),3.43(dd,j=11.2,5.5hz,2h),2.03–1.96(m,2h);esi-lrmsm/z(%):563.2[m+h]+.。
实施例18(s)-1-(3,4,5-三甲氧基苯基)-4-(3-苯甲酰氧基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(18)的合成
化合物1(20mg,0.054mmol)溶于1.5ml无水dcm中,0℃搅拌条件下,依次加入tea(11mg,0.108mmol),苯甲酰氯(12mg,0.081mmol),后升至室温反应0.5h,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料1(rf=0.3)完全消失,有新点生成(rf=0.4),停止反应(1ml水淬灭),加入10mldcm,以水(2x10ml)洗涤,饱和食盐水(10ml)洗,无水硫酸钠干燥3h,拌硅胶柱层析分离纯化,收集对应的洗脱液,蒸干溶剂得白色固体(18)23mg,收率90%;m.p.151~152℃;[α]d20=+22.2(c0.34,chcl3);1hnmr(400mhz,cdcl3)δ8.18(d,j=7.8hz,2h),7.66–7.62(m,1h),7.53–7.49(m,2h),7.30(dd,j=8.4,2.0hz,1h),7.24(d,j=2.0hz,1h),7.02(d,j=8.4hz,1h),6.61(s,2h),5.86(s,1h),5.34(s,1h),5.22(s,1h),3.82(s,3h),3.78(s,3h),3.76(s,6h);13cnmr(100mhz,cdcl3)δ164.5,160.9,153.6,151.9,149.6,140.5,134.8,133.8,133.7,130.3,129.1,129.0,128.6,125.3,121.9,113.0,111.1,94.9,63.4,61.0,56.1,56.0;esi-lrmsm/z(%):476.2[m+h]+;esi-hrmsm/z(%):calcdforc27h26no7[m+h]+476.1704,found476.1705.。
实施例19(s)-1-(3,4,5-三甲氧基苯基)-4-(3-对硝基苯甲酰氧基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(19)的合成
化合物1(20mg,0.054mmol)溶于1.5ml无水dcm中,0℃搅拌条件下,依次加入tea(11mg,0.108mmol),对硝基苯甲酰氯(15mg,0.081mmol),后升至室温反应0.5h,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料1(rf=0.3)完全消失,有新点生成(rf=0.4),停止反应(1ml水淬灭),加入10mldcm,以水(2x10ml)洗涤,饱和食盐水(10ml)洗,无水硫酸钠干燥3h,拌硅胶柱层析分离纯化,收集对应的洗脱液,蒸干溶剂得淡黄色固体(19)27mg,收率96%;m.p.88~89℃;[α]d20=+45.2(c0.31,chcl3);1hnmr(400mhz,cdcl3)δ8.35(s,4h),7.34(dd,j=8.5,2.1hz,1h),7.24(d,j=2.1hz,1h),7.05(d,j=8.5hz,1h),6.60(s,2h),5.87(s,1h),5.35(s,1h),5.22(s,1h),3.83(s,3h),3.77(s,3h),3.76(s,6h);13cnmr(100mhz,cdcl3)δ162.6,160.7,153.6,151.6,151.0,149.6,140.0,134.9,134.5,133.7,131.4,129.2,125.9,123.7,121.4,113.0,111.2,94.9,63.3,61.0,56.2,56.1;esi-lrmsm/z(%):521.1[m+h]+;esi-hrmsm/z(%):calcdforc27h25n2o9[m+h]+521.1555,found521.1554.。
实施例20(s)-1-(3,4,5-三甲氧基苯基)-4-(3-对氟苯甲酰氧基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(20)的合成
化合物1(29mg,0.078mmol)溶于1.5ml无水dcm中,0℃搅拌条件下,依次加入无水吡啶(130mg,1.6mmol),对氟苯甲酰氯(109mg,0.78mmol),升至室温反应0.5h后,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料1(rf=0.3)完全消失,有新点生成(rf=0.4),停止反应(1ml水淬灭),加入10mldcm,以水(2x10ml)洗涤,饱和食盐水(10ml)洗,无水硫酸钠干燥3h,拌硅胶柱层析分离纯化,收集对应的洗脱液,蒸干溶剂得白色固体(20)35mg,收率91%;m.p.185~186℃;[α]d20=+38.5(c0.15,chcl3);1hnmr(400mhz,cdcl3)δ8.22–8.14(m,2h),7.30(dd,j=8.5,2.2hz,1h),7.22(d,j=2.2hz,1h),7.19–7.15(m,2h),7.02(d,j=8.5hz,1h),6.60(s,2h),5.86(t,j=1.7hz,1h),5.34(s,1h),5.21(s,1h),3.81(s,3h),3.77(s,3h),3.75(s,6h);13cnmr(100mhz,cdcl3)δ167.6,165.1,163.6,161.0,153.7,152.0,149.7,140.4,134.8,133.9,133.1,133.0,129.1,125.6,125.5,125.4,122.0,116.1,116.0,113.0,111.4,95.0,63.5,61.1,56.3,56.2;esi-lrmsm/z(%):494.2[m+h]+;esi-hrmsm/z(%):calcdforc27h25fno8[m+h]+494.1610,found494.1611.。
实施例21(s)-1-(3,4,5-三甲氧基苯基)-4-[3-(2-甲氧基)苯甲酰氧基-4-甲氧基苯基]-3-亚甲基氮杂环丁烷-2-酮(21)的合成
化合物1(20mg,0.054mmol)溶于1.5ml无水dcm中,0℃搅拌条件下,依次加入tea(11mg,0.108mmol),邻甲氧基苯甲酰氯(14mg,0.081mmol),后升至室温反应0.5h,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料1(rf=0.3)完全消失,有新点生成(rf=0.4),停止反应(1ml水淬灭),加入10mldcm,以水(2x10ml)洗涤,饱和食盐水(10ml)洗,无水硫酸钠干燥3h,拌硅胶柱层析分离纯化,收集对应的洗脱液,蒸干溶剂得无色油状物(21)23mg,收率84%;[α]d20=+22.2(c0.11,chcl3);1hnmr(400mhz,cdcl3)δ8.15–8.11(m,2h),7.35–7.32(m,1h),7.28(dd,j=8.7,2.0hz,1h),7.22(d,j=2.0hz,1h),7.02–6.95(m,3h),6.91–6.87(m,1h),6.61(s,2h),5.85(s,1h),5.33(s,1h),5.21(s,1h),3.88(s,3h),3.81(s,3h),3.77(s,3h),3.76(s,6h);13cnmr(151mhz,cdcl3)δ171.3,164.2,164.0,160.9,160.3,153.6,152.0,149.6,140.6,134.8,133.8,132.4,129.5,128.9,128.2,127.2,125.2,122.0,121.4,113.9,113.7,112.9,111.1,94.9,92.8,77.3,77.1,76.8,69.9,66.9,63.4,60.9,56.1,56.1,55.5,55.3,10.6;esi-lrmsm/z(%):506.1[m+h]+;esi-hrmsm/z(%):calcdforc28h27nnao8[m+na]+528.1629,found528.1630.。
实施例22(s)-1-(3,4,5-三甲氧基苯基)-4-[3-(3-吡啶甲酰氧基-4-甲氧基苯基)]-3-亚甲基氮杂环丁烷-2-酮(22)的合成
化合物1(20mg,0.054mmol)溶于1.5ml无水dcm中,0℃搅拌条件下,依次加入tea(17mg,0.216mmol),2-吡啶甲酰氯(15mg,0.081mmol),升至室温反应0.5h后,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料1(rf=0.3)完全消失,有新点生成(rf=0.2),停止反应(1ml水淬灭),加入10mldcm,以水(2x10ml)洗涤,饱和食盐水(10ml)洗,无水硫酸钠干燥3h,拌硅胶柱层析分离纯化,收集对应的洗脱液,蒸干溶剂得白色固体(22)16mg,收率62%;m.p.113~114℃;[α]d20=+44.9(c0.35,chcl3);1hnmr(400mhz,cdcl3)δ9.37(s,1h),8.85(d,j=4.4hz,1h),8.43(d,j=8.0hz,1h),7.46(dd,j=8.0,4.4hz,1h),7.32(dd,j=8.4,1.7hz,1h),7.24(d,j=1.7hz,1h),7.03(d,j=8.5hz,1h),6.60(s,2h),5.86(s,1h),5.34(s,1h),5.22(s,1h),3.82(s,3h),3.77(s,3h),3.76(s,6h);13cnmr(150mhz,cdcl3)δ163.2,160.8,154.0,153.6,151.7,151.5,149.6,140.0,137.7,134.9,133.7,129.1,125.7,125.3,123.5,121.7,113.0,111.2,94.9,63.3,61.0,56.1;esi-lrmsm/z(%):477.2[m+h]+;esi-hrmsm/z(%):calcdforc26h25n2o7[m+h]+477.1656,found477.1659.。
实施例23(s)-1-(3,4,5-三甲氧基苯基)-4-(3-环丙酰甲氧基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(23)的合成
化合物1(20mg,0.054mmol)溶于1.5ml无水dcm中,0℃搅拌条件下,依次加入无水吡啶(90mg,1.2mmol),环丙甲酰氯(40mg,0.54mmol),升至室温反应0.5h后,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料1(rf=0.3)完全消失,有新点生成(rf=0.4),停止反应(1ml水淬灭),加入10mldcm,以水(2x10ml)洗涤,饱和食盐水(10ml)洗,无水硫酸钠干燥3h,拌硅胶柱层析分离纯化,收集对应的洗脱液,蒸干溶剂得白色固体(23)20mg,收率84%;m.p.68~69℃;[α]d20=+12.4(c0.44,chcl3);1hnmr(400mhz,cdcl3)δ7.23(dd,j=8.4,2.1hz,1h),7.11(d,j=2.1hz,1h),6.95(d,j=8.5hz,1h),6.58(s,2h),5.84(s,1h),5.30(s,1h),5.18(s,1h),3.83(s,3h),3.76(s,3h),3.73(s,6h),1.91–1.78(m,1h),1.20–1.12(m,2h),1.07–0.98(m,2h);13cnmr(100mhz,cdcl3)δ161.0,153.6,150.0,149.8,148.9,133.8,128.9,120.4,111.4,110.9,110.8,94.9,68.3,64.0,61.3,61.0,56.1,56.0,31.7;esi-lrmsm/z(%):440.2[m+h]+;esi-hrmsm/z(%):calcdforc24h25nnao7[m+na]+462.1523,found462.1526.。
实施例24(s)-1-(3,4,5-三甲氧基苯基)-4-(3-丙烯酰氧基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(24)的合成
化合物1(20mg,0.054mmol)溶于1.5ml无水dcm中,0℃搅拌条件下,依次加入tea(8.2mg,0.108mmol),丙烯酰氯(10mg,0.081mmol),升至室温反应0.5h后,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料1(rf=0.3)完全消失,有新点生成(rf=0.4),停止反应(1ml水淬灭),加入10mldcm,以水(2x10ml)洗涤,饱和食盐水(10ml)洗,无水硫酸钠干燥3h,拌硅胶柱层析分离纯化,收集对应的洗脱液,蒸干溶剂得白色固体(24)17mg,收率74%;m.p.109~110℃;[α]d20=+52.1(c0.26,chcl3);1hnmr(400mhz,cdcl3)δ7.26(dd,,j=8.4hz,2.0hz,1h),7.15(d,j=2.0hz,1h),6.98(d,j=8.4hz,1h),6.61–6.57(m,3h),6.32(dd,j=17.6,10.4hz,1h),6.02(d,j=10.4hz,1h),5.85(s,1h),5.31(s,1h),5.19(s,1h),3.82(s,3h),3.76(s,3h),3.74(s,6h);13cnmr(100mhz,cdcl3)δ163.8,160.8,153.6,151.7,149.6,140.0,134.8,133.8,132.9,128.9,127.4,125.3,121.8,112.9,111.1,94.8,63.3,61.0,56.1,56.0;esi-lrmsm/z(%):426.1[m+h]+;esi-hrmsm/z(%):calcdforc23h23nnao7[m+na]+448.1367,found448.1369.。
实施例25(s)-1-(3,4,5-三甲氧基苯基)-4-[3-(4-吗啉碳酰氧基)-4-甲氧基苯基]-3-亚甲基氮杂环丁烷-2-酮(25)的合成
化合物1(20mg,0.054mmol)溶于1.5ml无水dcm中,0℃搅拌条件下,依次加入无水吡啶(90mg,1.2mmol),n-吗啉酰氯(81mg,0.54mmol),升至室温反应9h后,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料1(rf=0.3)基本消失,有新点生成(rf=0.2),停止反应(1ml水淬灭),加入10mldcm,以水(2x10ml)洗涤,饱和食盐水(10ml)洗,无水硫酸钠干燥3h,拌硅胶柱层析分离纯化,收集对应的洗脱液,蒸干溶剂得白色固体(25)20mg,收率84%;m.p.82~83℃;[α]d20=+30.0(c0.16,chcl3);1hnmr(400mhz,cdcl3)δ7.23(dd,j=8.4,2.1hz,1h),7.15(d,j=2.1hz,1h),6.95(d,j=8.4hz,1h),6.59(s,2h),5.84(s,1h),5.31(s,1h),5.19(s,1h),3.84(s,3h),3.76(s,3h),3.76(s,3h),3.75–3.73(m,10h),3.67(s,2h),3.54(s,2h);13cnmr(150mhz,cdcl3)δ160.2,153.0,152.5,151.5,149.0,140.2,134.1,133.1,128.2,124.3,121.3,112.0,110.5,94.3,66.0,62.8,60.3,55.5,55.4,44.5,43.7;esi-lrmsm/z(%):485.2[m+h]+;esi-hrmsm/z(%):calcdforc25h28n2nao8[m+na]+507.1738,found507.1739.。
实施例26(s)-1-(3,4,5-三甲氧基苯基)-4-(3-氨基磺酰氧基-4-甲氧基苯基)-3-乙氧酰基甲基丁胺-2-酮(26)的合成
氮气保护条件下,化合物1(40mg,0.108mmol)和氨基磺酰氯(62mg,0.54mmol)置于schlenk中,加入2.5ml经分子筛除水的dma,室温搅拌反应4.5h,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料1(rf=0.3)完全消失,有新点生成(rf=0.2),停止反应(1ml水淬灭),加入10ml乙酸乙酯,以水洗涤(3x10ml),饱和食盐(10ml)洗,无水硫酸钠干燥3h,直接拌硅胶柱层析分离纯化,收集对应的洗脱液并蒸干溶剂,得白色固体(26)46mg,收率95%;mp199-200℃;[α]d20=-14.3(c0.10,dmso);1hnmr(400mhz,dmso)δ7.98(d,j=2.2hz,1h),7.83(dd,j=8.5,2.2hz,1h),7.62(d,j=8.5hz,1h),7.12(s,2h),6.23(t,j=1.7hz,1h),6.09(s,1h),5.70(s,1h),4.30(s,3h),4.15(s,6h),4.07(s,3h);13cnmr(100mhz,cdcl3)δ160.1,153.1,151.8,149.2,138.6,133.9,132.9,128.6,125.9,122.7,113.7,111.6,94.6,61.6,60.0,55.7,55.6;esi-lrmsm/z(%):451.1[m+h]+;esi-hrmsm/z(%):calcdforc20h26n3o8s[m+nh4]+468.1435,found468.1435.。
实施例27(s)-1-(3,4,5-三甲氧基苯基)-4-(3-对甲苯磺酰氧基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(27)的合成
化合物1(20mg,0.054mmol)溶于1.5ml无水dcm中,0℃搅拌条件下,依次加入tea(15mg,0.162mmol),对甲基苯磺酰氯(16mg,0.081mmol),升至室温反应0.5h后,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料1(rf=0.3)完全消失,有新点生成(rf=0.2),停止反应(1ml水淬灭),加入10mldcm,以水(2x10ml)洗涤,饱和食盐水(10ml)洗,无水硫酸钠干燥3h,拌硅胶柱层析分离纯化,收集对应的洗脱液,蒸干溶剂得白色固体(27)27mg,收率95%;mp162-163℃;[α]d20=+53.1(c0.16,chcl3);1hnmr(400mhz,cdcl3)δ7.66(d,j=8.3hz,2h),7.30–7.20(m,3h),7.14(d,j=2.1hz,1h),6.88(d,j=8.5hz,1h),6.56(s,2h),5.84(t,j=1.5hz,1h),5.29(s,1h),5.16(s,1h),3.79(s,3h),3.77(s,6h),3.61(s,3h),2.44(s,3h);13cnmr(150mhz,cdcl3)δ160.0,153.0,151.80,148.9,144.7,138.1,134.2,133.0,132.4,128.8,128.3,127.8,125.6,122.2,112.7,110.5,94.2,62.3,60.3,55.5,55.2,21.0;esi-lrmsm/z(%):548.1[m+na]+;esi-hrmsm/z(%):calcdforc27h31n2o8s[m+nh4]+543.1796,found543.1794.。
实施例28(s)-1-(3,4,5-三甲氧基苯基)-4-[3-(2-邻苯二甲酰亚氨基乙磺酰氧基)]-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(28)的合成
化合物1(20mg,0.054mmol)溶于1.5ml无水dcm中,0℃搅拌条件下,依次加入tea(14mg,0.162mmol),2-邻苯二甲酰亚氨基乙烷磺酰氯(16mg,0.081mmol),升至室温反应2d后,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示有新点生成(rf=0.2),原料1(rf=0.3)不再有减少趋势,停止反应(1ml水淬灭),加入10mldcm,以水(2x10ml)洗涤,饱和食盐水(10ml)洗,无水硫酸钠干燥3h,拌硅胶柱层析分离纯化,收集对应的洗脱液,蒸干溶剂得白色固体(28)15mg,收率46%;mp69-70℃;[α]d20=+12.1(c0.45,chcl3);1hnmr(400mhz,cdcl3)δ7.88–7.85(m,2h),7.76–7.73(m,2h),7.43(s,1h),7.27(d,j=8.6hz,1h),7.00(d,j=8.6hz,1h),6.55(s,2h),5.85(s,1h),5.31(s,1h),5.19(s,1h),4.34(t,j=6.9hz,2h),3.91(s,3h),3.80–3.64(m,11h);13cnmr(150mhz,cdcl3)δ166.9,160.0,153.0,151.2,148.8,137.4,134.2,133.7,132.9,131.2,128.8,125.6,123.4,123.0,113.0,110.7,94.1,62.2,60.3,55.6,55.5,48.4,31.9;esi-lrmsm/z(%):609.1[m+h]+;esi-hrmsm/z(%):calcdforc30h28n2nao10s[m+na]+631.1357,found631.1357.。
实施例29(s)-1-(3,4,5-三甲氧基苯基)-4-(3-吗啉磺酰氧基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(29)的合成
化合物1(20mg,0.054mmol)溶于1.5ml无水dcm中,0℃搅拌条件下,依次加入tea(14mg,0.162mmol),4-吗啉磺酰氯(15mg,0.081mmol),升至室温反应4d后,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料1(rf=0.3)完全消失,有新点生成(rf=0.25),停止反应(1ml水淬灭),加入10mldcm,以水(2x10ml)洗涤,饱和食盐水(10ml)洗,无水硫酸钠干燥3h,拌硅胶柱层析分离纯化,收集对应的洗脱液,蒸干溶剂得白色固体(29)20mg,收率71%;m.p.134~135℃;[α]d20=+24.7(c0.11,chcl3);1hnmr(400mhz,cdcl3)δ7.43(d,j=2.0hz,1h),7.26(dd,j=8.5,2.0hz,1h),6.99(d,j=8.5hz,1h),6.57(s,2h),5.86(t,j=1.6hz,1h),5.31(s,1h),5.19(s,1h),3.88(s,3h),3.76–3.73(m,13h),3.38(t,j=4.8hz,4h);13cnmr(150mhz,cdcl3)δ160.0,153.0,151.3,148.9,138.5,134.2,132.9,128.6,125.1,122.1,113.0,110.6,94.2,65.3,62.4,60.3,55.6,55.5,46.3;esi-lrmsm/z(%):521.2[m+h]+;esi-hrmsm/z(%):calcdforc24h29n2o9s[m+h]+521.1588,found521.1590.。
实施例30(s)-1-(3,4,5-三甲氧基苯基)-4-(3-对乙酰氨基苯磺酰氧基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(30)的合成
化合物1(20mg,0.054mmol)溶于1.5ml无水dcm中,0℃搅拌条件下,依次加入tea(15mg,0.162mmol),对乙酰氨基苯磺酰氯(21mg,0.081mmol),升至室温反应0.5h后,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料1(rf=0.3)完全消失,有新点生成(rf=0.1),停止反应(1ml水淬灭),加入10mldcm,以水(2x10ml)洗涤,饱和食盐水(10ml)洗,无水硫酸钠干燥3h,拌硅胶柱层析分离纯化,收集对应的洗脱液,蒸干溶剂得淡黄色固体(30)22mg,收率72%;mp87-88℃;[α]d20=+64.6(c0.13,chcl3);1hnmr(400mhz,cdcl3)δ8.01(s,1h),7.63(d,j=8.8hz,2h),7.55(d,j=8.8hz,2h),7.23(dd,j=8.5,1.8hz,1h),6.98(d,j=1.8hz,1h),6.89(d,j=8.5hz,1h),6.49(s,2h),5.81(s,1h),5.28(s,1h),5.15(s,1h),3.79(s,3h),3.73(s,6h),3.66(s,3h),2.22(s,3h);13cnmr(150mhz,cdcl3)δ168.2,159.9,153.1,151.9,148.6,142.8,138.3,133.9,133.0,129.2,129.0,128.1,125.6,121.2,118.2,112.8,110.6,94.1,62.1,60.4,55.5,55.3,24.1;esi-lrmsm/z(%):569.1[m+h]+;esi-hrmsm/z(%):calcdforc28h28n2nao9s[m+na]+591.1408,found591.1409.。
实施例31(s)-1-(3,4,5-三甲氧基苯基)-4-(3-丁磺酰氧基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(31)的合成
化合物1(20mg,0.054mmol)溶于1.5ml无水dcm中,0℃搅拌条件下,依次加入tea(15mg,0.162mmol),1-丁基磺酰氯(21mg,0.081mmol),升至室温反应0.5h后,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料1(rf=0.3)完全消失,有新点生成(rf=0.25),停止反应(1ml水淬灭),加入10mldcm,以水(2x10ml)洗涤,饱和食盐水(10ml)洗,无水硫酸钠干燥3h,拌硅胶柱层析分离纯化,收集对应的洗脱液,蒸干溶剂得白色固体(31)24mg,收率82%;m.p.99~100℃;[α]d20=+18.6(c0.35,chcl3);1hnmr(400mhz,cdcl3)δ7.39(d,j=2.1hz,1h),7.27(dd,j=8.5,2.1hz,1h),6.99(d,j=8.5hz,1h),6.56(s,2h),5.85(s,1h),5.31(s,1h),5.19(s,1h),3.87(s,3h),3.76(s,3h),3.74(s,6h),3.29(td,j=6.9,1.6hz,2h),2.00–1.92(m,2h),1.54–1.45(m,2h),0.97(t,j=7.4hz,3h);13cnmr(100mhz,cdcl3)δ160.7,153.6,151.9,149.5,138.2,134.8,133.6,129.4,126.0,124.0,113.6,111.2,94.8,62.9,61.0,56.1,51.6,29.7,25.5,21.5,13.5;esi-lrmsm/z(%):492.2[m+h]+;esi-hrmsm/z(%):calcdforc24h29nnao8s[m+na]+514.1506,found514.1508.。
实施例32(s)-1-(3,4,5-三甲氧基苯基)-4-(3-苄基磺酰氧基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(32)的合成
化合物1(20mg,0.054mmol)溶于1.5ml无水dcm中,0℃搅拌条件下,依次加入tea(15mg,0.162mmol),苄基磺酰氯(16mg,0.081mmol),升至室温过夜反应后,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料1(rf=0.3)完全消失,有新点生成(rf=0.25),停止反应(1ml水淬灭),加入10mldcm,以水(2x10ml)洗涤,饱和食盐水(10ml)洗,无水硫酸钠干燥3h,拌硅胶柱层析分离纯化,收集对应的洗脱液,蒸干溶剂得白色固体(32)25mg,收率88%;m.p.58~59℃;[α]d20=-0.7(c0.14,chcl3);1hnmr(400mhz,cdcl3)δ7.46–7.36(m,5h),7.26(dd,j=8.4,2.0hz,1h),7.05(d,j=2.0hz,1h),6.99(d,j=8.4hz,1h),6.54(s,2h),5.84(s,1h),5.23(s,1h),5.15(s,1h),4.58(q,j=14.0hz,2h),3.89(s,3h),3.76(s,3h),3.74(s,6h);13cnmr(150mhz,cdcl3)δ160.0,153.0,151.4,148.8,137.9,134.2,132.9,130.3,128.7,128.3,126.8,125.5,122.9,112.9,110.6,94.2,62.4,60.3,57.3,55.6,55.5;esi-lrmsm/z(%):526.1[m+h]+;esi-hrmsm/z(%):calcdforc27h31n2o8s[m+nh4]+543.1796,found543.1797.。
实施例33(s)-1-(3,4,5-三甲氧基苯基)-4-[3-(3,4-二甲氧基)苯磺酰氧基-4-甲氧基苯基]-3-亚甲基氮杂环丁烷-2-酮(33)的合成
化合物1(20mg,0.054mmol)溶于1.5ml无水dcm中,0℃搅拌条件下,依次加入tea(15mg,0.162mmol),3,4-二甲氧基苯磺酰氯(19mg,0.081mmol),升至室温过夜反应后,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料1(rf=0.3)完全消失,有新点生成(rf=0.15),停止反应(1ml水淬灭),加入10mldcm,以水(2x10ml)洗涤,饱和食盐水(10ml)洗,无水硫酸钠干燥。拌硅胶柱层析分离纯化,收集对应的洗脱液,蒸干溶剂得白色固体(33)27mg,收率87%;[α]d20=+29.3(c0.14,chcl3);m.p.70~71℃;1hnmr(400mhz,cdcl3)δ7.30–7.26(m,2h),7.24(dd,j=8.5,2.1hz,1h),7.18(d,j=2.1hz,1h),6.87(d,j=8.5hz,1h),6.81(d,j=8.2hz,1h),6.55(s,2h),5.83(s,1h),5.29(s,1h),5.15(s,1h),3.94(s,3h),3.83(s,3h),3.77(s,3h),3.76(s,6h),3.63(s,3h);13cnmr(150mhz,cdcl3)δ159.9,153.3,153.0,151.8,148.9,148.4,138.1,134.2,132.9,128.4,126.6,125.5,122.2,122.2,112.7,110.4,110.0,109.5,94.2,62.3,60.3,55.7,55.5,55.3;esi-lrmsm/z(%):572.2[m+h]+;esi-hrmsm/z(%):calcdforc28h29nnao10s[m+na]+594.1404,found594.1405.。
实施例34(s)-1-(3,4,5-三甲氧基苯基)-4-(3-叔丁氧酰氨基乙氧基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(34)的合成
化合物1(20mg,0.054mmol),dmap(3.6mg,0.003mmol),boc-甘氨酸(38mg,0.216mmol)和2mldcm置于schlenk中,最后加入edc盐酸盐(41mg,0.216mmol),室温搅拌过夜反应,tlc(展开剂:二氯甲烷/甲醇=70:1)只显示原料1(rf=0.3),无新点生成,lc-ms监测原料完全消失,停止反应,直接拌硅胶柱层析分离纯化,收集对应的洗脱液,蒸干溶剂得白色固体(34)26mg,收率91%;1hnmr(400mhz,cdcl3)δ7.25(dd,j=8.5,2.0hz,1h),7.14(s,1h),6.97(d,j=8.5hz,1h),6.57(s,2h),5.84(t,j=1.4hz,1h),5.30(s,1h),5.18(t,j=1.4hz,1h),5.07(s,1h),4.19(d,j=5.5hz,2h),3.81(s,3h),3.76(s,3h),3.73(s,6h),1.45(s,9h);esi-lrmsm/z(%):551.0[m+na]+.。
实施例352-甲氧基-5-[(s)-3-亚甲基-4-氧代-1-(3,4,5-三甲氧基苯基)氮杂环丁烷-2-基]-n-(叔丁氧羰基)-l-丙氨酸苯酯(35)的合成
化合物1(20mg,0.054mmol),dmap(3.6mg,0.0324mmol),boc-l-丙氨酸(80mg,0.432mmol)和2ml无水dcm置于schlenk中,最后加入edc盐酸盐(41mg,0.216mmol),室温搅拌过夜反应,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料1(rf=0.5)完全消失,有新点生成(rf=0.6),停止反应直接拌硅胶柱层析分离纯化,收集对应的洗脱液,蒸干溶剂得白色固体(35)25mg,收率85%;1hnmr(400mhz,cdcl3)δ7.24(d,j=8.5hz,1h),7.14(s,1h),6.95(d,j=8.5hz,1h),6.56(s,2h),5.84(s,1h),5.29(s,1h),5.18(s,1h),5.08(d,j=7.5hz,1h),4.61–4.49(m,1h),3.80(s,3h),3.75(s,3h),3.72(s,3h),1.55(d,j=7.2hz,3h),1.44(s,9h);esi-lrmsm/z(%):565.1[m+na]+.。
实施例362-甲氧基-5-[(s)-3-亚甲基-4-氧代-1-(3,4,5-三甲氧基苯基)杂氮环丁烷-2-基]-n-(叔丁氧羰基)-l-缬氨酸苯酯(36)的合成
化合物1(20mg,0.054mmol),dmap(3.6mg,0.0324mmol),boc-l-缬氨酸(94mg,0.432mmol)和2ml无水dcm置于schlenk中,最后加入edc盐酸盐(41mg,0.216mmol),室温搅拌过夜反应,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料1(rf=0.5)完全消失,有新点生成(rf=0.6),停止反应直接拌硅胶柱层析分离纯化,收集对应的洗脱液,蒸干溶剂得白色固体(36)19mg,收率62%;1hnmr(400mhz,cdcl3)δ7.25(d,j=8.5hz,1h),7.12(s,1h),6.96(d,j=8.5hz,1h),6.57(s,2h),5.85(s,1h),5.30(s,1h),5.18(s,1h),5.06(d,j=9.0hz,1h),4.49(dd,j=9.0,6.4hz,1h),3.80(s,3h),3.76(s,3h),3.74(s,6h),2.40–2.30(m,1h),1.45(s,9h),1.08(d,j=6.8hz,3h),1.02(d,j=6.8hz,3h);esi-lrmsm/z(%):593.1[m+na]+.。
实施例37(s)-二苄基[2-甲氧基-5-(3-亚甲基-4-氧代-1-(3,4,5-三甲氧基苯基)氮杂环丁烷-2-基)苯基]磷酸酯(37)的合成
化合物1(80mg,0.216mmol)2ml无水dcm置于50ml茄型瓶中,冷制0℃,搅拌条件下依次加入60%氢化钠(10.5mg,0.26mmol)(溶液由无色变为黄色),tbpo(173mg,0.32mmol),升至室温,15min后反应液变为白色膏状,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料1(rf=0.3)完全消失,有新点生成(rf=0.25),停止反应(1ml水淬灭),加入10ml乙酸乙酯,以水洗涤(3x10ml),饱和食盐水(10ml)洗,无水硫酸钠干燥。直接拌硅胶柱层析分离纯化,收集对应的洗脱液并蒸干溶剂,得白色固体(37)120mg,收率96%;1hnmr(400mhz,cdcl3)δ7.37–7.23(m,11h),7.15(dd,j=8.5,1.0hz,1h),6.91(d,j=8.5hz,1h),6.56(s,2h),5.80(t,j=1.7hz,1h),5.23(s,1h),5.18–5.07(m,5h),3.78(s,3h),3.73(s,3h),3.70(s,6h);esi-lrmsm/z(%):632.2[m+h]+.。
实施例38(s)-1-(3,4,5-三甲氧基苯基)-4-(3-氟-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(38)的合成
化合物38的合成方法与化合物1的合成方法类似:
38.12-[1-(3-氟-4-甲氧基苯基)-1-羟基甲基]丙烯酸乙酯(38b)的合成
在50ml茄形瓶中加入化合物3-氟-4-甲氧基苯甲醛38a(175mg,1.14mmol),丙烯酸乙酯(114mg,1.14mmol),dabco(128mg,1.14mmol),室温搅拌反应5d后,tlc(展开剂:石油醚/乙酸乙酯=9:1)显示原料(rf=0.5)明显减少,有大量新点生成(rf=0.2),停止反应,用较粗的柱子,直接将反应液倒到硅胶柱上,用少量甲苯洗涤反应瓶再倒到柱子上,然后用pe及pe/ea10/1先除去丙烯酸乙酯,继而进行梯度洗脱,收集对应的洗脱液,蒸干溶剂得无色油状物质(38b)207mg,收率71%.1hnmr(400mhz,cdcl3)δ7.13–7.02(m,2h),6.90(t,j=8.4,1h),6.32(s,1h),5.82(s,1h),5.46(d,j=5.5hz,1h),4.16(q,j=7.1hz,2h),3.86(s,3h),3.17(d,j=5.6hz,1h),1.24(t,j=7.1hz,3h);esi-lrmsm/z(%):255.1[m+h]+.
38.22-[1-(3-氟-4-甲氧基苯基)-1-乙酰氧基甲基]丙烯酸乙酯(38c)的合成
化合物38b(166mg,0.65mmol),dmap(8mg,0.065mmol)和tea(132mg,1.3mmol)溶于5ml无水dcm中,0℃搅拌条件下,缓慢滴加乙酸酐(133mg,1.3mmol),加完后在冰浴中继续搅拌;10min后,tlc(展开剂:石油醚/乙酸乙酯=7:1)显示原料(rf=0.3)完全消失,有新点(rf=0.5)生成,缓慢加入饱和碳酸氢钠水溶液3ml淬灭反应;分液,水层用dcm(3x5ml)萃取,合并有机相,无水硫酸钠干燥。拌硅胶柱层析分离纯化,收集对应的洗脱液,减压蒸干溶剂得无色油状化合物(38c)164mg,收率85%;1hnmr(400mhz,cdcl3)δ7.12–7.07(m,2h),6.91(t,j=8.6hz,1h),6.59(s,1h),6.39(s,1h),5.85(s,1h),4.20–4.10(m,2h),3.87(s,3h),2.09(s,2h),1.22(t,j=7.1hz,3h);13cnmr(100mhz,cdcl3)δ169.3,164.8,153.2,150.8,147.7,147.5,139.4,130.7,130.7,125.3,124.0,123.9,115.5,115.3,112.9,72.3,61.0,56.1,21.1,14.0;esi-lrmsm/z(%):319.1[m+na]+;esi-hrmsm/z(%):calcdforc15h17fnao5[m+na]+319.0952,found319.0954.
38.3(s)-2-[1-(3-氟-4-甲氧基苯基)-1-(3,4,5-三甲氧基苯基氨基)甲基]丙烯酸乙酯(38d)的合成
氮气氛围下,三(二亚苄基丙酮)二钯(4.3mg,0.0047mmol)和1e(8.5mg,0.0118mmol)分别加入一schlenk管中,加入无水ch2cl2(5ml),室温下搅拌10分钟后,先后加入化合物38c(140mg,0.47mmol),k2co3(1.0m水溶液,1.5ml,1.5mmol)和3,4,5-三甲氧基苯胺(134mg,1.41mmol);室温下搅拌8小时后,tlc(展开剂:石油醚/乙酸乙酯=2:1)显示原料(rf=0.5)完全消失,有新点(rf=0.2)生成,用二氯甲烷萃取(3×10ml),无水硫酸钠干燥,过滤浓缩后,柱层析纯化,得淡黄色油状物(38d)137mg,收率69%;[α]d20=+63.7(c0.16,chcl3);1hnmr(400mhz,cdcl3)δ7.11(t,j=2.3hz,1h),7.08(s,1h),6.91(t,j=8.3hz,1h),6.38(s,1h),5.92(s,1h),5.81(s,1h),5.31(s,1h),4.21–4.13(m,2h),3.87(s,3h),3.76(s,6h),3.74(s,3h),1.24(t,j=7.1hz,3h);13cnmr(100mhz,cdcl3)δ166.0,153.8,153.5,151.0,147.1,147.0,143.3,140.3,133.6,133.6,130.4,126.1,123.1,123.1,115.1,114.9,113.3,91.1,61.1,60.9,58.5,56.2,55.8,14.0;esi-lrmsm/z(%):420.2[m+h]+;esi-hrmsm/z(%):calcdforc22h27fno6[m+h]+420.1817,found420.1817.
以0.005当量的三苯基膦替换1e做配体,其他操作完全相同,可得相应外消旋体。
38.4(s)-1-(3,4,5-三甲氧基苯基)-4-(3-氟-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(38)的合成
化合物38d(137mg,0.33mmol)和sn[n(tms)2]2(183mg,0.40mmol)加入一schlenk管中,加入无水甲苯(5ml),加热回流6小时后,tlc(展开剂:石油醚/乙酸乙酯=2:1)显示原料(rf=0.6)完全消失,有新点(rf=0.5)生成,反应液冷却至室温后,浓缩,柱层析纯化,得无色油状物(38)60mg,收率58%;[α]d20=+107.0(c0.17,chcl3),97%ee[determinedbyhplcanalysisusingachiralcelad-hcolumn;n-hex/i-proh=85:15,1.0ml/min,254nm;tr(minor)=14.68min;tr(major)=21.68min];1hnmr(400mhz,cdcl3)δ7.15–7.10(m,2h),6.98–6.94(m,1h),6.57(s,2h),5.84(s,1h),5.30(s,1h),5.16(s,1h),3.88(s,3h),3.76(s,3h),3.74(s,6h);13cnmr(150mhz,cdcl3)δ160.0,153.0,152.9,151.2,148.9,147.6,147.5,134.2,133.0,128.7,128.7,122.3,122.2,113.9,113.8,113.07,110.4,94.2,62.5,60.3,55.7,55.5;esi-lrmsm/z(%):374.1[m+h]+;esi-hrmsm/z(%):calcdforc20h21eno5[m+h]+374.1398,found374.1400.。
实施例39(s)-1-(3,4,5-三甲氧基苯基)-4-(3-氯-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(39)的合成
化合物39的合成方法与化合物1的合成方法类似:
39.12-[1-(3-氯-4-甲氧基苯基)-1-羟基甲基]丙烯酸乙酯(39b)的合成
在50ml茄形瓶中加入化合物39a(1.15g,6.8mmol),丙烯酸乙酯(681mg,6.8mmol),dabco(763mg,6.8mmol),室温搅拌反应4d后,tlc(展开剂:石油醚/乙酸乙酯=9:1)显示原料(rf=0.5)明显减少,有大量新点生成(rf=0.2),停止反应,用较粗的柱子,直接将反应液倒到硅胶柱上,用少量甲苯洗涤反应瓶再倒到柱子上,然后用pe及pe/ea10/1先除去丙烯酸乙酯,继而进行梯度洗脱,收集对应的洗脱液,回收原料440mg,回收率38%,得无色油状产物(39b)700mg,扣除回收原料,收率62%;1hnmr(400mhz,cdcl3)δ7.37(d,j=2.1hz,1h),7.23(dd,j=8.5,2.1hz,1h),6.89(d,j=8.5hz,1h),6.34(s,1h),5.83(s,1h),5.47(d,j=5.5hz,1h),4.17(q,j=7.1hz,2h),3.88(s,3h),3.10(d,j=5.6hz,1h),1.25(t,j=7.1hz,3h);13cnmr(150mhz,cdcl3)δ165.6,153.9,141.2,134.0,127.9,125.5,125.3,121.7,111.2,71.8,60.4,55.5,13.4;esi-lrmsm/z(%):293.1[m+na]+;esi-hrmsm/z(%):calcdforc13h15clnao4[m+na]+293.0551,found293.0553.
39.22-[1-(3-氯-4-甲氧基苯基)-1-乙酰氧基甲基]丙烯酸乙酯(39c)的合成
化合物39b(700mg,2.6mmol),dmap(32mg,0.26mmol)和tea(523mg,5.2mmol)溶于5ml无水dcm中,0℃搅拌条件下,缓慢滴加乙酸酐(530mg,5.2mmol),加完后在冰浴中继续搅拌;10min后,tlc(展开剂:石油醚/乙酸乙酯=7:1)显示原料(rf=0.3)完全消失,有新点(rf=0.5)生成,缓慢加入饱和碳酸氢钠水溶液3ml淬灭反应;分液,水层用dcm(3x5ml)萃取,合并有机相,无水硫酸钠干燥3h,拌硅胶柱层析分离纯化,收集对应的洗脱液,减压蒸干溶剂得无色油状化合物(39c)640mg,收率79%;1hnmr(400mhz,cdcl3)δ7.36(d,j=2.1hz,1h),7.27(dd,j=8.4,2.0hz,1h),6.87(d,j=8.5hz,1h),6.57(s,1h),6.39(s,1h),5.87(s,1h),4.20–4.08(m,2h),3.87(s,3h),2.09(s,3h),1.22(t,j=7.1hz,3h);13cnmr(150mhz,cdcl3)δ168.8,164.2,154.3,138.8,130.4,128.9,127.1,124.7,121.7,111.1,71.7,60.4,55.5,20.5,13.4;esi-lrmsm/z(%):335.1[m+na]+;esi-hrmsm/z(%):calcdforc15h17clnao5[m+na]+335.0657,found335.0659.
39.3(s)-2-[1-(3-氯-4-甲氧基苯基)-1-(3,4,5-三甲氧基苯基氨基)甲基]丙烯酸乙酯(39d)的合成
氮气氛围下,三(二亚苄基丙酮)二钯(4.3mg,0.0047mmol)和1e(8.5mg,0.0118mmol)分别加入一schlenk管中,加入无水ch2cl2(5ml),室温下搅拌10分钟后,先后加入底物39c(200mg,0.64mmol),k2co3(1m水溶液,2ml,2mmol)和3,4,5-三甲氧基苯胺(176mg,0.96mmol);室温下搅拌4h后,tlc(展开剂:石油醚/乙酸乙酯=2:1)显示原料(rf=0.8)完全消失,有新点(rf=0.5)生成,用二氯甲烷萃取(3×10ml),无水硫酸钠干燥,过滤浓缩后,柱层析纯化,得淡黄色油状物(39d)182mg,收率65%;[α]d20=+70.5(c0.17,chcl3);1hnmr(400mhz,cdcl3)δ7.37(d,j=2.1hz,1h),7.24(dd,j=8.8,2.1hz,1h),6.89(d,j=8.8hz,1h),6.39(s,1h),5.94(s,1h),5.81(s,2h),5.30(s,1h),4.21–4.13(m,2h),3.89(s,3h),3.77(s,6h),3.75(s,3h),1.24(t,j=7.1hz,3h);13cnmr(150mhz,cdcl3)δ165.5,153.9,153.2,142.7,139.6,133.2,129.8,128.5,126.3,125.6,122.0,111.4,90.5,60.5,60.4,57.8,55.6,55.3,13.5;esi-lrmsm/z(%):436.1[m+h]+;esi-hrmsm/z(%):calcdforc22h27clno6[m+h]+436.1521,found436.1523.
以0.005当量的三苯基膦替换1e做配体,其他操作完全相同,可得相应外消旋体。
39.4(s)-1-(3,4,5-三甲氧基苯基)-4-(3-氯-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(39)的合成
化合物39d(182mg,0.42mmol)和sn[n(tms)2]2(220mg,0.50mmol)加入一schlenk管中,加入无水甲苯(5ml),加热回流3小时后,tlc(展开剂:石油醚/乙酸乙酯=2:1)显示原料(rf=0.5)基本消失,有新点(rf=0.4)生成,反应液冷却至室温后,浓缩,柱层析纯化,得白色固体(39)110mg,收率68%;mp119-120℃;[α]d20=+35.1(c0.29,chcl3),97%ee[determinedbyhplcanalysisusingachiralcelad-hcolumn;n-hex/i-proh=80:20,1.0ml/min,254nm;tr(minor)=10.62min;tr(major)=13.97min]1hnmr(400mhz,cdcl3)δ7.41(d,j=2.0hz,1h),7.25(dd,j=8.5,2.0hz,1h),6.92(d,j=8.5hz,1h),6.56(s,2h),5.83(s,1h),5.28(s,1h),5.16(s,1h),3.88(s,3h),3.75(s,3h),3.73(s,6h);13cnmr(150mhz,cdcl3)δ160.1,154.8,153.0,148.8,134.2,133.0,128.9,128.2,125.7,122.6,111.8,110.5,94.2,62.3,60.3,55.6,55.5;esi-lrmsm/z(%):390.1[m+h]+;esi-hrmsm/z(%):calcdforc20h21clno5[m+h]+390.1103,found390.1103.。
实施例40(s)-1-(3,4,5-三甲氧基苯基)-4-(3-甲氧基羰基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(40)的合成
化合物40的合成路线与化合物1类似。
40.12-[1-(3-甲氧羰基-4-甲氧基苯基)-1-羟基甲基]丙烯酸乙酯(40b)的合成
在50ml茄形瓶中加入化合物40a(2.2g,11.3mmol),丙烯酸乙酯(1.13g,11.3mmol),dabco(1.27g,11.3mmol),室温搅拌反应8d后,tlc(展开剂:石油醚/乙酸乙酯=5:1)显示原料(rf=0.5)明显减少,有新点生成(rf=0.3),停止反应,用较粗的柱子,直接将反应液倒到硅胶柱上,用少量甲苯洗涤反应瓶再倒到柱子上,然后用pe及pe/ea10/1先除去丙烯酸乙酯,继而进行梯度洗脱,收集对应的洗脱液,回收原料800mg,回收率36%,得无色油状产物(40b)1.6g,扣除回收原料,收率75%;1hnmr(400mhz,cdcl3)δ7.74(d,j=2.0hz,1h),7.45(dd,j=8.6,2.0hz,1h),6.91(d,j=8.6hz,1h),6.30(s,1h),5.84(s,1h),5.49(s,1h),4.18–4.07(m,2h),3.85(s,3h),3.83(s,3h),1.21(t,j=7.1hz,3h);13cnmr(150mhz,cdcl3)δ165.8,165.6,158.1,141.3,132.6,131.2,129.4,125.3,119.24,111.4,72.0,60.4,55.5,51.4,13.4;esi-lrmsm/z(%):317.0[m+na]+.
40.22-[1-(3-甲氧羰基-4-甲氧基苯基)-1-乙酰氧基甲基]丙烯酸乙酯(40c)的合成
化合物40b(438mg,1.49mmol),dmap(18mg,0.149mol)和tea(300mg,2.98mmol)溶于5ml无水dcm中,0℃搅拌条件下,缓慢滴加乙酸酐(304mg,2.98mmol),加完后在冰浴中继续搅拌;10min后,tlc(展开剂:石油醚/乙酸乙酯=5:1)显示原料(rf=0.3)完全消失,有新点(rf=0.5)生成,缓慢加入饱和碳酸氢钠水溶液淬灭反应;分液,水层用dcm(3x5ml)萃取,合并有机相,无水硫酸钠干燥3h,拌硅胶柱层析分离纯化,收集对应的洗脱液,减压蒸干溶剂得无色油状化合物(40c)350mg,收率70%;1hnmr(400mhz,cdcl3)δ7.79(d,j=2.4hz,1h),7.50(dd,j=8.6,2.4hz,1h),7.26(s,1h),6.94(d,j=8.6hz,1h),6.62(s,1h),6.40(s,1h),5.89(s,1h),4.14(dtt,j=10.8,7.4,3.7hz,2h),3.89(s,3h),3.88(s,3h),2.10(s,3h),1.21(t,j=7.1hz,3h);13cnmr(150mhz,cdcl3)δ168.8,165.7,164.2,158.5,138.9,132.7,130.6,129.1,124.6,119.4,111.3,71.8,60.4,55.5,51.4,20.5,13.4;esi-lrmsm/z(%):359.0[m+na]+.
40.3(s)-2-[1-(3-甲氧羰基-4-甲氧基苯基)-1-(3,4,5-三甲氧基苯基氨基)甲基]丙烯酸乙酯(40d)的合成
氮气氛围下,三(二亚苄基丙酮)二钯(9.2mg,0.01mmol)和1e(18mg,0.025mmol)分别加入一schlenk管中,加入无水ch2cl2(5ml),室温下搅拌10分钟后,先后加入底物40c(350mg,1.04mmol),k2co3(1.0m水溶液,3ml,3mmol)和3,4,5-三甲氧基苯胺(286mg,1.56mmol);室温搅拌1h后,tlc(展开剂:石油醚/乙酸乙酯=2:1)显示原料(rf=0.9)完全消失,有新点(rf=0.4)生成,用二氯甲烷萃取(3×5ml),无水硫酸钠干燥,过滤浓缩后,柱层析纯化,得淡黄色油状化合物(40d)280mg,收率59%;[α]d20=+65.2(c0.34,chcl3);1hnmr(400mhz,cdcl3)δ7.77(t,j=4.9hz,1h),7.47(dd,j=8.6,2.1hz,1h),6.92(d,j=8.6hz,1h),6.38(s,1h),5.94(s,1h),5.80(s,2h),5.33(s,1h),4.20–4.03(m,3h),3.87(s,3h),3.85(s,3h),3.74(s,6h),3.72(s,3h),1.21(t,j=7.1hz,2h);13cnmr(150mhz,cdcl3)δ165.8,165.5,158.0,153.2,142.8,139.8,131.9,131.8,130.0,129.9,125.5,119.6,111.7,90.7,60.4,60.3,57.9,55.5,55.3,51.4,13.4;esi-lrmsm/z(%):460.1[m+h]+.
以0.005当量的三苯基膦替换1e做配体,其他操作完全相同,可得相应外消旋体。
40.4(s)-1-(3,4,5-三甲氧基苯基)-4-(3-甲氧基羰基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(40)的合成
化合物40d(280mg,0.61mmol)和sn[n(tms)2]2(335mg,0.73mmol)加入一schlenk管中,加入无水甲苯(5ml),加热回流3.5h后,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料(rf=0.7)少量剩余,有新点(rf=0.6生成),反应液冷却至室温后,浓缩,柱层析纯化,得白色固体(40)100mg,收率40%;mp112-113℃;[α]d20=+5.5(c0.34,chcl3);97%ee[determinedbyhplcanalysisusingachiralcelad-hcolumn;n-hex/i-proh=75:25,1.0ml/min,254nm;tr(minor)=10.18min;tr(major)=15.93min];1hnmr(400mhz,cdcl3)δ7.89(d,j=2.1hz,1h),7.51(dd,j=8.7,2.1hz,1h),7.01(d,j=8.7hz,1h),6.59(s,2h),5.88(s,1h),5.37(s,1h),5.20(s,1h),3.92(s,3h),3.91(s,3h),3.78(s,3h),3.75(s,6h);13cnmr(150mhz,cdcl3)δ166.1,160.8,159.6,153.6,149.5,134.8,133.6,131.7,130.8,128.1,120.5,112.9,111.2,94.8,63.1,61.0,56.2,56.1,52.3;esi-lrmsm/z(%):414.0[m+h]+;esi-hrmsm/z(%):calcdforc22h24no7[m+h]+414.1547,found414.1550.。
实施例41(s)-1-(3,4,5-三甲氧基苯基)-4-(3-氨基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(41)的合成
化合物41的合成路线与化合物1类似:
41.12-[1-(3-硝基-4-甲氧基苯基)-1-羟基甲基]丙烯酸乙酯(41b)的合成
在50ml茄形瓶中加入化合物4-甲氧基-3-硝基苯甲醛(41a)(1.38g,7.62mmol),丙烯酸乙酯(762mg,7.62mmol),dabco(855mg,7.62mmol),室温搅拌反应3d后,tlc(展开剂:石油醚/乙酸乙酯=4:1,3次)显示原料(rf=0.6)明显减少,有大量新点生成(rf=0.5),停止反应,用较粗的柱子,直接将反应液倒到硅胶柱上,用少量甲苯洗涤反应瓶再倒到柱子上,然后用pe及pe/ea10/1先除去丙烯酸乙酯,继而进行梯度洗脱,收集对应的洗脱液,蒸干溶剂得黄色油状物质(41b)5.9g,收率99%;1hnmr(400mhz,cdcl3)δ7.85(d,j=2.1hz,1h),7.57(dd,j=8.7,2.2hz,1h),7.07(d,j=8.7hz,1h),6.37(s,1h),5.90(s,1h),5.53(d,j=5.6hz,1h),4.18(q,j=7.1hz,2h),3.95(s,3h),3.43(d,j=5.6hz,1h),1.27(t,j=7.1hz,3h);13cnmr(100mhz,dmso)δ166.1,152.5,141.6,139.4,134.3,132.7,126.5,124.1,113.6,72.1,72.0,61.4,56.8,14.2;esi-lrmsm/z(%):304.1[m+na]+;esi-hrmsm/z(%):calcdforc13h19n2o6[m+nh4]+299.1238,found299.1238.
41.22-[1-(3-硝基-4-甲氧基苯基)-1-乙酰氧基甲基]丙烯酸乙酯(41c)的合成
化合物41b(1.94g,6.9mmol),dmap(100mg,0.69mmol)和tea(1.4g,13.8mmol)溶于20ml无水dcm中,0℃搅拌条件下,缓慢滴加乙酸酐(1.4g,13.8mmol),加完后在冰浴中继续搅拌;5min后,tlc(展开剂:石油醚/乙酸乙酯=4:1)显示原料(rf=0.1)完全消失,有新点(rf=0.2)生成,缓慢加入饱和碳酸氢钠水溶液10ml淬灭反应;分液,水层用dcm(3x10ml)萃取,合并有机相,无水硫酸钠干燥3h,拌硅胶柱层析分离纯化,收集对应的洗脱液,减压蒸干溶剂得无色油状化合物(41c)1.7g,收率76%;1hnmr(400mhz,cdcl3)δ7.84(d,j=2.2hz,1h),7.59(dd,j=8.7,2.2hz,1h),7.05(d,j=8.7hz,1h),6.60(s,1h),6.43(s,1h),5.95(s,1h),4.19–4.10(m,2h),3.94(s,3h),2.10(s,3h),1.23(t,j=7.1hz,3h);esi-lrmsm/z(%):346.1[m+na]+;esi-hrmsm/z(%):calcdforc15h21n2o7[m+nh4]+341.1343,found341.1344.
41.32-[1-(3-叔丁氧基甲酰氨基-4-甲氧基苯基)-1-乙酰氧基甲基]丙烯酸乙酯(41e)的合成
50ml茄形瓶中加入化合物41c(1.7g,5.2mmol),醋酸(3.2g,52mmol),和20ml甲醇,搅拌条件下分批加入锌粉(1.03g),加完后回流反应2h,tlc(展开剂:石油醚/乙酸乙酯=3:1)显示原料(rf=0.6)完全消失,有新点(rf=0.5)生成,反应液用硅藻土过滤以除去锌粉,加入饱和碳酸钠水溶液中和多余醋酸,再以乙酸乙酯萃取,无水硫酸钠干燥有机相。加入无水thf8ml,碳酸钾(45mg,0.323mmol),回流反应16h后,tlc(展开剂:石油醚/乙酸乙酯=3:1)显示原料(rf=0.5)完全消失,有新点生成(rf=0.6),停止反应,减压旋干,10ml乙酸乙酯溶解,水洗(5mlx2),饱和食盐水洗(5ml),无水硫酸钠干燥。过滤浓缩后,柱层析纯化,得无色油状物(41e)858mg,两步收率40%;1hnmr(400mhz,cdcl3)δ8.10(s,1h),7.07(s,1h),7.01(dd,j=8.3,2.0hz,1h),6.79(d,j=8.3hz,1h),6.62(s,1h),6.39(s,1h),5.85(s,1h),4.20–4.11(m,2h),3.85(s,3h),2.10(s,3h),1.52(s,9h),1.24(t,j=7.1hz,3h);13cnmr(150mhz,cdcl3)δ168.9,164.5,151.9,146.8,139.2,129.8,127.6,124.9,121.4,116.6,108.9,72.7,60.3,55.1,27.7,20.6,13.4;esi-lrmsm/z(%):416.1[m+na]+;esi-hrmsm/z(%):calcdforc20h31n2o7[m+nh4]+411.2126,found411.2128.
41.4(s)-2-[1-(3-叔丁氧基甲酰氨基-4-甲氧基苯基)-1-(3,4,5-三甲氧基苯基氨基)甲基]丙烯酸乙酯(41e)的合成
氮气氛围下,三(二亚苄基丙酮)二钯(3.8mg,0.0041mmol)和1e(7.2mg,0.01mmol)分别加入一schlenk管中,加入无水ch2cl2(5ml),室温下搅拌10分钟后,先后加入底物41e(163mg,0.41mmol),k2co3(1.0m水溶液,1.5ml,1.5mmol)和3,4,5-,三氧基苯胺(114mg,0.62mmol);室温下搅拌2h后,tlc(展开剂:石油醚/乙酸乙酯=2:1)显示原料(rf=0.7)完全消失,有新点(rf=0.4)生成,用二氯甲烷萃取(3×5ml),无水硫酸钠干燥,过滤浓缩后,柱层析纯化,得黄色油状物(41f)173mg,收率81%;[α]d20=+56.4(c0.15,chcl3);1hnmr(400mhz,cdcl3)δ8.03(s,1h),7.02(s,1h),6.91(dd,j=8.4,2.2hz,1h),6.72(d,j=8.4hz,1h),6.30(s,1h),5.89(s,1h),5.75(s,2h),5.27(s,1h),4.16–4.02(m,2h),3.78(s,3h),3.70(s,6h),3.68(s,3h),1.45(s,9h),1.16(t,j=7.1hz,3h);13cnmr(150mhz,cdcl3)δ165.8,153.1,152.0,146.4,143.0,140.1,132.7,129.7,127.8,125.1,120.6,116.5,109.2,90.6,79.8,60.4,60.1,58.4,55.3,55.1,27.7,13.5;esi-lrmsm/z(%):517.3[m+h]+;esi-hrmsm/z(%):calcdforc27h37n2o8[m+h]+517.2544,found517.2544.
以0.005当量的三苯基膦替换1e做配体,其他操作完全相同,可得相应外消旋体。
41.5(s)-1-(3,4,5-三甲氧基苯基)-4-(3-氨基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(41)的合成
化合物41f(146mg)和sn[n(tms)2]2(149mg,0.33mmol)加入一schlenk管中,加入无水甲苯(5ml),加热回流6小时后,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料(rf=0.6)基本消失,有新点(rf=0.3)生成,反应液冷却至室温后,浓缩,柱层析纯化,得淡黄色油状物(41)37mg,收率35%;[α]d20=+50.0(c0.14,chcl3),97%ee[determinedbyhplcanalysisusingachiralcelad-hcolumn;n-hex/i-proh=80:20,1.0ml/min,254nm;tr(minor)=19.58min;tr(major)=17.42min];1hnmr(400mhz,cdcl3)δ6.79(dd,j=8.1,1.9hz,1h),6.75(d,j=8.1hz,1h),6.71(d,j=1.9hz,1h),6.62(s,2h),5.80(t,j=1.6hz,1h),5.24(s,1h),5.15(t,j=1.6hz,1h),3.84(s,3h),3.76(s,3h),3.74(s,6h);13cnmr(150mhz,cdcl3)δ161.1,153.5,150.0,147.8,136.8,134.6,134.0,129.0,117.5,112.5,110.5,110.3,94.9,64.0,60.9,56.1,55.5;esi-lrmsm/z(%):371.1[m+h]+;esi-hrmsm/z(%):calcdforc20h23n2o5[m+h]+371.1601,found371.1605.。
实施例42(s)-1-(3,4,5-三甲氧基苯基)-4-(3-丙烯酰氨基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(42)的合成
化合物41(20mg,0.054mmol)溶于1.5ml无水dcm中,0℃搅拌条件下,依次加入tea(11mg,0.108mmol),丙烯酰氯(10mg,0.081mmol),升至室温反应0.5h后,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料(rf=0.3)完全消失,有新点生成(rf=0.25),停止反应(1ml水淬灭),加入10mldcm,以水(2x10ml)洗涤,饱和食盐水(10ml)洗,无水硫酸钠干燥。拌硅胶柱层析分离纯化,收集对应的洗脱液,蒸干溶剂得白色固体(42)16mg,收率66%;mp143-144℃;[α]d20=-96.4(c0.11,chcl3);1hnmr(400mhz,cdcl3)δ8.70(s,1h),7.93(s,1h),7.05(dd,j=8.5,1.9hz,1h),6.87(d,j=8.5hz,1h),6.62(s,2h),6.43(d,j=16.8hz,1h),6.29(dd,j=16.8,10.1hz,1h),5.83(s,1h),5.79(d,j=10.1hz,1h),5.36(s,1h),5.19(s,1h),3.89(s,3h),3.75(s,3h),3.74(s,6h);13cnmr(150mhz,cdcl3)δ162.8,160.3,152.9,149.1,147.4,133.9,133.2,130.6,128.4,127.3,127.2,121.0,118.7,110.2,110.0,94.2,63.1,60.3,55.5,55.3;esi-lrmsm/z(%):425.2[m+h]+;esi-hrmsm/z(%):calcdforc23h28n3o6[m+nh4]+442.1973,found442.1975.。
实施例43(s)-1-(3,4,5-三甲氧基苯基)-4-(3-对硝基苯甲酰氨基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(43)的合成
化合物41(20mg,0.054mmol)溶于1.5ml无水dcm中,0℃搅拌条件下,依次加入tea(11mg,0.108mmol),对硝基苯甲酰氯(15mg,0.081mmol),后升至室温反应20h,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示无新点生成,lc-ms显示原料阳离子峰(m+1)消失,大量目标化合物阳离子峰存在(m+1),停止反应(1ml水淬灭),加入10mldcm,以水(2x10ml)洗涤,饱和食盐水(10ml)洗,无水硫酸钠干燥3h,拌硅胶柱层析分离纯化,收集对应的洗脱液,蒸干溶剂得黄色固体(43)25mg,收率89%;mp234-235℃;[α]d20=-81.6(c0.20,chcl3);1hnmr(400mhz,cdcl3)δ8.70(s,1h,nh),8.61(s,1h),8.36(d,j=8.7hz,2h),8.05(d,j=8.7hz,2h),7.14(dd,j=8.4,1.8hz,1h),6.94(d,j=8.4hz,1h),6.64(s,2h),5.86(s,1h),5.41(s,1h),5.22(s,1h),3.95(s,3h),3.76(s,9h);13cnmr(150mhz,cdcl3)δ162.6,160.3,152.9,149.2,149.1,147.7,139.7,134.1,133.1,128.7,127.6,126.9,123.51,121.9,118.6,110.4,110.1,94.3,63.0,60.3,55.5;esi-lrmsm/z(%):520.2[m+h]+;esi-hrmsm/z(%):calcdforc27h25n3nao8[m+na]+542.1534,found542.1534.。
实施例44(s)-1-(3,4,5-三甲氧基苯基)-4-(3-氨基磺酰氨基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(44)的合成
氮气保护条件下,化合物41(20mg,0.054mmol)和氨基磺酰氯(62mg,0.27mmol)置于schlenk中,加入2.5ml无水二氯甲烷,室温搅拌反应1h,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料1(rf=0.3)完全消失,有新点生成(rf=0.2),停止反应(1ml水淬灭),加入10ml乙酸乙酯,以水洗涤(3x10ml),无水硫酸钠干燥。直接拌硅胶柱层析分离纯化,收集对应的洗脱液并蒸干溶剂,得白色固体(44)11mg,收率42%;mp101-102℃;[α]d20=-20.3(c0.35,chcl3);1hnmr(400mhz,cdcl3)δ7.60(d,j=1.9hz,1h),7.12(dd,j=8.5,1.9hz,1h),7.03(s,1h,nh),6.89(d,j=8.5hz,1h),6.59(s,2h),5.85(s,1h),5.34(s,1h),5.19(s,1h),4.84(s,2h,nh2),3.86(s,3h),3.75(m,9h);13cnmr(150mhz,cdcl3)δ160.3,152.9,148.9,148.7,134.2,133.0,128.7,126.4,122.3,118.4,110.7,110.5,94.5,62.8,60.3,55.6,55.4;esi-lrmsm/z(%):450.1[m+h]+;esi-hrmsm/z(%):calcdforc20h24n3o7s[m+h]+450.1329,found450.1346.。
实施例45(s)-1-(3,4,5-三甲氧基苯基)-4-(3-环丙甲酰氨基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(45)的合成
化合物41(20mg,0.054mmol)溶于1.5ml无水dcm中,0℃搅拌条件下,依次加入tea(11mg,0.108mmol),环丙甲酰氯(8.5mg,0.081mmol),升至室温反应0.5h后,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料1(rf=0.3)完全消失,有新点生成(rf=0.25),1ml水淬灭反应,加入10mldcm,以水(2x10ml)洗涤,饱和食盐水(10ml)洗,无水硫酸钠干燥。拌硅胶柱层析分离纯化,收集对应的洗脱液,蒸干溶剂得白色固体(45)11mg,收率54%;mp181-182℃;[α]d20=-77.3(c0.24,chcl3);1hnmr(400mhz,cdcl3)δ8.59(s,1h,nh),8.05(s,1h),7.01(dd,j=8.5,1.8hz,1h),6.85(d,j=8.5hz,1h),6.61(s,2h),5.81(s,1h),5.32(s,1h),5.18(s,1h),3.89(s,3h),3.75(s,3h),3.73(s,6h),1.63–1.53(m,1h),1.08(s,2h),0.87(dd,j=7.6,3.1hz,2h);13cnmr(150mhz,cdcl3)δ171.4,160.3,152.9,149.1,147.0,133.9,133.2,128.3,127.7,120.4,118.5,110.2,109.9,94.2,63.1,60.3,55.5,55.2,15.5,7.5,7.4;esi-lrmsm/z(%):439.2[m+h]+;esi-hrmsm/z(%):calcdforc24h26n2nao6[m+na]+461.1683,found461.1685.。
实施例46(s)-1-(3,4,5-三甲氧基苯基)-4-(3-乙酰氧基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(46)的合成
在250ml茄形瓶中加入化合物1(0.25g,0.673mmol),4-二甲氨基吡啶(8mg,0.067mmol),三乙胺(0.13ml,0.94mmol)与二氯甲烷10ml,醋酸酐(0.09ml,0.094mmol),室温反应5分钟;tlc(展开剂:pe/ea=2:1)显示原料点(rf=0.4)消失,生成新点(rf=0.6);反应液加饱和nahco3,用ea萃取三次,有机相合并后用饱和食盐水洗一次,na2so4干燥;柱层析分离(展开剂:pe/ea=2:1),得到白色固体(46)0.23g,收率83%;1hnmr(400mhz,cdcl3)δ7.25(brd,j=8.4hz,1h),7.10(brs,1h),6.97(d,j=8.4hz,1h),6.57(s,1h),5.84(s,1h),5.30(s,1h),5.18(s,1h),3.83(s,3h),3.76(s,3h),3.73(s,6h),2.29(s,3h).13cnmr(151mhz,cdcl3)δ168.0,160.2,153.0,151.0,149.0,139.6,134.1,133.1,128.3,124.6,121.1,112.2,110.4,94.2,62.7,60.3,55.5,55.4,20.0;esi-lrmsm/z(%):414.1[m+h]+.。
实施例47(s)-1-(3,4,5-三甲氧基苯基)-4-(3-苄氧基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(47)的合成
在100ml茄形瓶中加入化合物1(0.15g,0.4mmol),溴化苄(58μl,0.5mmol),碳酸钾(72mg,0.5mmol)与dmf5ml,100℃反应8小时;tlc(展开剂:pe/ea=2:1)显示原料点(rf=0.5)消失,生成新点(rf=0.65);反应液加水,用ea萃取三次,有机相合并后用饱和食盐水洗一次,na2so4干燥;柱层析分离(展开剂:pe/ea=3:1),得到白色固体(47)0.146g,收率78%;1hnmr(400mhz,cdcl3)δ7.40–7.23(m,5h),6.98(dd,j=8.2,1.6hz,1h),6.92–6.82(m,2h),6.54(s,2h),5.78(s,1h),5.24(s,1h),5.11(s,2h),5.07(s,1h),3.89(s,3h),3.77(s,3h),3.70(s,6h).13cnmr(151mhz,cdcl3)δ160.3,152.9,149.7,149.1,147.9,135.9,134.0,133.2,128.1,127.9,127.3,126.7,119.6,111.5,111.2,110.1,94.1,70.4,63.2,60.3,55.4;esi-lrmsm/z(%):462.2[m+h]+.。
实施例48(s)-1-(3,4-二甲氧基苯基)-4-(3-羟基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮的(48)合成
化合物48的合成路线与化合物1类似:
48.1(s)-2-[1-(3-叔丁基二甲基硅氧基-4-甲氧基苯基)-1-(3,4-二甲氧基苯基氨基)甲基]丙烯酸乙酯(48a)的合成
氮气氛围下,三(二亚苄基丙酮)二钯(5.7mg,0.0062mmol)和1e(11.1mg,0.016mmol)分别加入一schlenk管中,加入无水ch2cl2(5ml),室温下搅拌10分钟后,先后加入1d(250mg,0.62mmol),k2co3(1.0m水溶液,3ml,3mmol)和3,5-二氧基苯胺(142mg,0.93mmol);室温下搅拌1.5小时后,tlc(展开剂:石油醚/乙酸乙酯=5:1)显示原料(rf=0.6)完全消失,有新点(rf=0.2)生成,用二氯甲烷萃取(3×5ml),无水硫酸钠干燥,过滤浓缩后,柱层析纯化,得淡黄色油状物(48a)220mg,收率72%;[α]d20=-5.1(c0.26,chcl3);1hnmr(400mhz,cdcl3)δ6.91(dd,j=8.3,1.9hz,1h),6.84(d,j=2.0hz,1h),6.79(d,j=8.3hz,1h),6.70(d,j=8.6hz,1h),6.33(s,1h),6.22(d,j=2.5hz,1h),6.08(dd,j=8.6,2.5hz,1h),5.88(s,1h),5.24(s,1h),4.21–4.08(m,2h),3.88–3.71(m,9h),1.28–1.16(m,3h),0.97(s,9h),0.11(d,j=9.2hz,6h);13cnmr(150mhz,cdcl3)δ165.8,149.8,149.3,144.4,141.1,140.3,132.8,124.7,120.1,119.6,112.5,111.5,103.8,99.0,60.1,58.5,56.1,55.1,54.9,25.1,17.8,13.5,-5.2;esi-lrmsm/z(%):502.2[m+h]+.
以0.005当量的三苯基膦替换1e做配体,其他操作完全相同,可得相应外消旋体。
48.2(s)-1-(3,4-二甲氧基苯基)-4-(3-叔丁基二甲基硅氧基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(48b)的合成
化合物48a(220mg)和sn[n(tms)2]2(231mg,0.53mmol)加入一schlenk管中,加入无水甲苯(5ml),加热回流4小时后,tlc(展开剂:石油醚/乙酸乙酯=3:1)显示原料(rf=0.7)基本消失,有新点(rf=0.5)生成,反应液冷却至室温后,浓缩,柱层析纯化,得无色油状化合物(48b)147mg,收率74%;[α]d20=+29.9(c0.32,chcl3);1hnmr(400mhz,cdcl3)δ7.23(s,1h),6.94(dd,j=8.2,1.7hz,1h),6.82(d,j=8.4hz,2h),6.68(d,j=8.6hz,1h),6.57(dd,j=8.6,2.0hz,1h),5.78(s,1h),5.25(s,1h),5.11(s,1h),3.86–3.72(m,9h),0.94(s,6h),0.08(s,3h),0.07(s,3h);13cnmr(150mhz,cdcl3)δ160.1,150.8,149.5,148.7,145.2,144.9,130.9,128.2,119.6,118.8,111.7,110.8,109.4,108.0,101.5,62.9,55.5,55.3,54.9,25.0,17.8,-5.2,-5.3;esi-lrmsm/z(%):456.2[m+h]+.
48.3(s)-1-(3,4-二甲氧基苯基)-4-(3-羟基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮的(48)合成
化合物48b(1117mg,0.26mmol)溶于5mlthf,冷至冰浴下,tbaf(136mg,0.52mmol)溶于1mlthf,由分液漏斗缓慢滴加,滴完后继续搅拌15min后,tlc(展开剂:石油醚/乙酸乙酯=3:1)显示原料(rf=0.5)完全消失,有新点(rf=0.1)生成,减压旋干,10ml乙酸乙酯溶解,水洗(5mlx2),饱和食盐水洗(5ml),无水硫酸钠干燥3h,过滤浓缩后,柱层析纯化,得白色固体(48)72mg,收率82%;mp74-75℃;[α]d20=+1.9(c0.32,chcl3);99%ee[determinedbyhplcanalysisusingachiralcelad-hcolumn;n-hex/i-proh=75:25,1.0ml/min,254nm;tr(minor)=11.78min;tr(major)=16.22min];1hnmr(400mhz,cdcl3)δ7.28(d,j=2.1hz,1h),6.94(d,j=1.7hz,1h),6.87(dd,j=8.2,1.7hz,1h),6.81(d,j=8.2hz,1h),6.67(d,j=8.6hz,1h),6.54(dd,j=8.6,2.1hz,1h),5.91(s,1h),5.76(s,1h),5.26(s,1h),5.11(s,1h),3.85(s,3h),3.81(s,3h),3.78(s,3h);13cnmr(150mhz,cdcl3)δ160.1,149.4,148.8,146.4,145.6,145.2,130.9,129.0,118.0,112.3,110.8,110.3,109.5,107.8,101.6,62.9,55.5,55.4,55.3;esi-lrmsm/z(%):342.1[m+h]+;esi-hrmsm/z(%):calcdforc20h26no4[m+h]+342.1336,found342.1339.。
实施例49(s)-1-(3,5-二甲氧基苯基)-4-(3-羟基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(49)的合成
化合物49和合成路线与化合物1类似:
49.1(s)-2-[1-(3-叔丁基二甲基硅氧基-4-甲氧基苯基)-1-(3,5-二甲氧基苯基氨基)甲基]丙烯酸乙酯(49a)的合成
氮气氛围下,三(二亚苄基丙酮)二钯(5.7mg,0.0062mmol)和1e(11.1mg,0.016mmol)分别加入一schlenk管中,加入无水ch2cl2(5ml),室温下搅拌10分钟后,先后加入1d(250mg,0.62mmol),k2co3(1.0m水溶液,3ml,3mmol)和3,5-二氧基苯胺(142mg,0.93mmol);室温下搅拌3.5小时后,tlc(展开剂:石油醚/乙酸乙酯=8:1)显示原料(rf=0.5)完全消失,有新点(rf=0.4)生成,用二氯甲烷萃取(3×5ml),无水硫酸钠干燥,过滤浓缩后,柱层析纯化,得淡黄色油状物(49a)270mg,收率88%;[α]d20=+27.7(c0.27,chcl3);1hnmr(400mhz,cdcl3)δ6.89(dd,j=8.3,2.1hz,1h),6.82–6.78(m,2h),6.35(s,1h),5.88(d,j=2.1hz,2h),5.77(d,j=2.1hz,2h),5.28(s,1h),4.21–4.05(m,2h),3.78(s,3h),3.72(s,6h),1.20(t,j=7.1hz,3h),0.97(s,9h),0.12(s,6h);13cnmr(150mhz,cdcl3)δ166.2,161.6,150.5,148.7,145.1,140.5,133.2,125.4,120.7,120.3,112.2,92.4,90.1,60.7,58.5,55.5,55.1,25.7,18.5,14.,-4.6;esi-lrmsm/z(%):502.2[m+h]+;esi-hrmsm/z(%):calcdforc27h40no6si[m+h]+502.2619,found502.2619.
以0.005当量的三苯基膦替换1e做配体,其他操作完全相同,可得相应外消旋体。
49.2(s)-1-(3,5-二甲氧基苯基)-4-(3-叔丁基二甲基硅氧基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(49b)的合成
化合物49a(270mg)和sn[n(tms)2]2(284mg,0.65mmol)加入一schlenk管中,加入无水甲苯(5ml),加热回流6小时后,tlc(展开剂:石油醚/乙酸乙酯=3:1)显示原料(rf=0.7)基本消失,有新点(rf=0.5)生成,反应液冷却至室温后,浓缩,柱层析纯化,得无色油状化合物(49b)213mg,收率76%;[α]d20=+39.5(c0.20,chcl3);1hnmr(400mhz,cdcl3)δ6.94(dd,j=8.3,2.1hz,1h),6.83–6.81(m,2h),6.53(d,j=2.2hz,2h),6.16(t,j=2.2hz,1h),5.81(t,j=1.7hz,1h),5.25(s,1h),5.18–5.10(m,1h),3.79(s,3h),3.70(s,6h),0.94(s,9h),0.09(s,3h),0.08(s,3h);13cnmr(150mhz,cdcl3)δ161.2,161.1,151.4,150.1,145.6,139.2,128.8,120.2,119.3,112.3,110.7,96.7,95.8,63.6,55.5,55.3,25.7,18.4,-4.7;esi-lrmsm/z(%):456.2[m+h]+;esi-hrmsm/z(%):calcdforc25h34no75si[m+h]+456.2201,found456.2202.
49.3(s)-1-(3,5-二甲氧基苯基)-4-(3-羟基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(49)的合成
化合物49b(172mg,0.38mmol)溶于5mlthf,冷至冰浴下,tbaf(198mg,0.76mmol)溶于2mlthf,由分液漏斗缓慢滴加,滴完后继续搅拌15min后,tlc(展开剂:石油醚/乙酸乙酯=2:1)显示原料(rf=0.7)完全消失,有新点(rf=0.3)生成,减压旋干,10ml乙酸乙酯溶解,水洗(5mlx2),饱和食盐水洗(5ml),无水硫酸钠干燥。过滤浓缩后,柱层析纯化,得无色油状物(49)116mg,收率90%;[α]d20=+49.6(c0.12,chcl3),99%ee[determinedbyhplcanalysisusingachiralcelad-hcolumn;n-hex/i-proh=80:20,1.0ml/min,254nm;tr(minor)=13.41min;tr(major)=14.97min];1hnmr(400mhz,cdcl3)δ6.93(d,j=2.0hz,1h),6.87(dd,j=8.2,2.0hz,1h),6.81(d,j=8.2hz,1h),6.53(d,j=2.2hz,2h),6.15(t,j=2.2hz,1h),5.94(brd,1h),5.80(t,j=1.2hz,1h),5.25(s,1h),5.13(s,1h),3.84(s,3h),3.70(s,6h);13cnmr(150mhz,cdcl3)δ160.6,160.5,149.3,146.5,145.6,138.6,128.9,118.0,112.2,110.4,110.3,95.9,95.2,63.0,55.3,54.7;esi-lrmsm/z(%):342.1[m+h]+;esi-hrmsm/z(%):calcdforc19h20no5[m+h]+342.1336,found342.1337.。
实施例50(s)-1-(2,4-二甲氧基苯基)-4-(3-羟基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(50)的合成
化合物50的合成路线与化合物1类似:
50.1(s)-2-[1-(3-叔丁基二甲基硅氧基-4-甲氧基)苯基-1-(2,4-二甲氧基苯基氨基)甲基]丙烯酸乙酯(50a)的合成
氮气氛围下,三(二亚苄基丙酮)二钯(3.8mg,0.0042mmol)和1e(7.56mg,0.0105mmol)分别加入一schlenk管中,加入无水ch2cl2(5ml),室温下搅拌10分钟后,先后加入化合物1d(170mg,0.42mmol),k2co3(1.0m水溶液,1.5ml,1.5mmol)和2,4-二甲氧基苯胺(104mg,0.68mmol);室温下搅拌4小时后,tlc(展开剂:石油醚/乙酸乙酯=10:1)展开5次显示原料(rf=0.8)完全消失,有新点(rf=0.7)生成,用二氯甲烷萃取(3×5ml),无水硫酸钠干燥,过滤浓缩后,柱层析纯化,得淡黄色油状物(50a)180mg,收率86%;1hnmr(400mhz,cdcl3)δ6.92(dd,j=8.3,2.0hz,1h),6.84(d,j=2.0hz,1h),6.79(d,j=8.3hz,1h),6.44(d,j=2.4hz,1h),6.41(d,j=8.5hz,1h),6.37–6.30(m,2h),5.85(s,1h),5.24(s,1h),4.23–4.05(m,2h),3.79(s,3h),3.78(s,3h),3.74(s,3h),1.23(q,j=7.5hz,3h),0.97(s,9h),0.12(s,6h);13cnmr(151mhz,cdcl3)δ166.5,152.1,150.4,147.9,145.0,141.1,133.6,131.2,129.0,128.4,125.1,120.9,120.4,112.1,111.3,103.7,99.1,60.7,58.7,55.8,55.5,55.5,25.7,18.5,14.1,-4.6;esi-lrmsm/z(%):502.2[m+h]+.
以0.005当量的三苯基膦替换1e做配体,其他操作完全相同,可得相应外消旋体。
50.2(s)-1-(2,4-二甲氧基苯基)-4-(3-叔丁基二甲基硅氧基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(50b)的合成
化合物50a(180mg)和sn[n(tms)2]2(189mg,0.43mmol)加入一schlenk管中,加入无水甲苯(5ml),加热回流4.5小时后,tlc(展开剂:石油醚/乙酸乙酯=3:1)显示原料(rf=0.7)基本消失,有新点(rf=0.5)生成,反应液冷却至室温后,浓缩,柱层析纯化,得无色油状化合物(50b)77mg,收率47%;1hnmr(400mhz,cdcl3)δ7.61(d,j=8.7hz,1h),6.84(dd,j=8.2,2.0hz,1h),6.76–6.73(m,2h),6.47–6.38(m,1h),6.36(d,j=2.4hz,1h),5.76(s,1h),5.60(s,1h),5.08(s,1h),6.76–6.73(m,6h),3.67(s,3h),0.94(s,9h),0.09(t,j=24.3hz,6h);esi-lrmsm/z(%):456.2[m+h]+.
50.3(s)-1-(2,4-二甲氧基苯基)-4-(3-羟基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(50)的合成
化合物50b(55mg,0.12mmol)溶于5mlthf,冷至冰浴下,tbaf(63mg,0.24mmol)溶于1mlthf,由分液漏斗缓慢滴加,滴完后继续搅拌15min后,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料(rf=0.8)完全消失,有新点(rf=0.5)生成,减压旋干,10ml乙酸乙酯溶解,水洗(5mlx2),饱和食盐水洗(5ml),无水硫酸钠干燥。过滤浓缩后,柱层析纯化,得棕色油状物(50)37mg,收率90%;1hnmr(400mhz,cdcl3)δ7.64(d,j=8.7hz,1h),6.88(d,j=2.0hz,1h),6.79(dd,j=8.3,2.0hz,1h),6.75(d,j=8.3hz,1h),6.43(dd,j=8.7,2.5hz,1h),6.36(d,j=2.5hz,1h),5.75(t,j=1.5hz,1h),5.66(d,j=8.3hz,1h),5.62(s,1h),5.08(s,1h),3.83(s,3h),3.74(s,3h),3.69(s,3h);13cnmr(150mhz,cdcl3)δ162.3,158.5,152.4,151.4,146.6,145.7,131.0,124.7,118.7,118.4,113.2,110.6,109.4,104.6,99.7,66.3,55.9,55.5;esi-lrmsm/z(%):342.0[m+h]+.。
实施例51(s)-1-(3-甲氧基苯基)-4-(3-羟基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(51)的合成
化合物51的合成路线与化合物1类似:
51.1(s)-2-[1-(3-叔丁基二甲基硅氧基-4-甲氧基苯基)-1-(3-甲氧基苯基氨基)甲基]丙烯酸乙酯(51a)的合成
氮气氛围下,三(二亚苄基丙酮)二钯(3.8mg,0.0042mmol)和1e(7.56mg,0.0105mmol)分别加入一schlenk管中,加入无水ch2cl2(5ml),室温下搅拌10分钟后,先后加入1d(170mg,0.42mmol),k2co3(1.0m水溶液,1.5ml,1.5mmol)和3-甲氧基苯胺(84mg,0.68mmol);室温下搅拌4小时后,tlc(展开剂:石油醚/乙酸乙酯=5:1)显示原料(rf=0.5)完全消失,有新点(rf=0.45)生成,用二氯甲烷萃取(3×5ml),无水硫酸钠干燥,过滤浓缩后,柱层析纯化,得淡黄色油状物(51a)170mg,87%;[α]d20=+118.9(c0.14,chcl3);1hnmr(400mhz,cdcl3)δ7.07(t,j=8.1hz,1h),6.92(dd,j=8.3,2.1hz,1h),6.84–6.80(m,2h),6.36(s,1h),6.30(dd,j=8.1,1.8hz,1h),6.21(d,j=8.0hz,1h),6.14(t,j=2.2hz,1h),5.90(s,1h),5.30(s,1h),4.18–4.12(m,2h),3.80(s,3h),3.76(s,3h),1.23(t,j=7.1hz,3h),0.99(s,9h),0.14(s,6h);13cnmr(150mhz,cdcl3)δ165.7,160.1,149.9,147.6,144.4,139.9,132.6,129.2,124.7,120.1,119.7,111.5,105.9,102.2,98.9,60.1,57.8,54.9,54.4,25.1,17.8,13.5,-5.2;esi-lrmsm/z(%):472.1[m+h]+;esi-hrmsm/z(%):calcdforc26h38no5si[m+h]+472.2514,found472.2514.
以0.005当量的三苯基膦替换1e做配体,其他操作完全相同,可得相应外消旋体。
51.2(s)-1-(3-甲氧基苯基)-4-(3-叔丁基二甲基硅氧基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(51b)的合成
化合物51a(170mg)和sn[n(tms)2]2(190mg,0.43mmol)加入一schlenk管中,加入无水甲苯(5ml),加热回流4.5小时后,tlc(展开剂:石油醚/乙酸乙酯=3:1)显示原料(rf=0.7)基本消失,有新点(rf=0.5)生成,反应液冷却至室温后,浓缩,柱层析纯化,得无色油状化合物(51b)106mg,收率69%;[α]d20=+36.5(c0.12,chcl3);1hnmr(400mhz,cdcl3)δ7.15(t,j=8.2hz,1h),7.04(s,1h),6.97(dd,j=8.3,1.9hz,1h),6.86–6.84(m,3h),6.61(dd,j=8.3,1.7hz,1h),5.84(s,1h),5.30(s,1h),5.17(s,1h),3.81(s,3h),3.76(s,3h),0.96(s,9h),0.11(s,3h),0.10(s,3h);13cnmr(150mhz,cdcl3)δ160.5,159.5,150.8,149.4,144.8,138.1,129.2,128.1,119.6,118.7,111.6,110.1,109.5,108.9,102.4,62.8,54.9,54.6,25.1,17.8,-5.3;esi-lrmsm/z(%):426.2[m+h]+;esi-hrmsm/z(%):calcdforc24h32no4si[m+h]+426.2095,found426.2096.
51.3(s)-1-(3-甲氧基苯基)-4-(3-羟基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(51)的合成
化合物51b(106mg,0.25mmol)溶于5mlthf,冷至冰浴下,tbaf(130mg,0.50mmol)溶于1mlthf,由分液漏斗缓慢滴加,滴完后继续搅拌15min后,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料(rf=0.8)完全消失,有新点(rf=0.5)生成,减压旋干,10ml乙酸乙酯溶解,水洗(5mlx2),饱和食盐水洗(5ml),无水硫酸钠干燥。过滤浓缩后,柱层析纯化,得白色固体(51)63mg,收率77%;mp104-105℃;[α]d20=+61.0(c0.21,chcl3);99%ee[determinedbyhplcanalysisusingachiralcelad-hcolumn;n-hex/i-proh=75:25,1.0ml/min,254nm;tr(minor)=10.45min;tr(major)=12.04min];1hnmr(400mhz,cdcl3)δ7.14(t,j=8.2hz,1h),7.04(s,1h),6.95(d,j=1.9hz,1h),6.89(dd,j=8.2,1.9hz,1h),6.84(s,1h),6.82(s,1h),6.60(dd,j=8.2,1.6hz,1h),5.82(s,1h),5.73(brd,1h),5.29(s,1h),5.15(s,1h),3.88(s,3h),3.75(s,3h);13cnmr(150mhz,cdcl3)δ161.1,160.1,145.0,147.0,146.2,138.7,129.9,129.6,118.6,112.8,110.9,110.8,110.1,109.5,103.1,63.5,56.0,55.3;esi-lrmsm/z(%):334.1[m+na]+;esi-hrmsm/z(%):calcdforc18h18no4[m+h]+312.1230,found312.1232.。
实施例52(s)-1-(4-甲氧基苯基)-4-(3-羟基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(52)的合成
化合物52的合成路线与化合物1类似:
52.1(s)-2-[1-(3-叔丁基二甲基硅氧基-4-甲氧基苯基)-1-(4-甲氧基苯基氨基)甲基]丙烯酸乙酯(52a)的合成
氮气氛围下,三(二亚苄基丙酮)二钯(5mg,0.0054mmol)和1e(10mg,0.0108mmol)分别加入一schlenk管中,加入无水ch2cl2(5ml),室温下搅拌10分钟后,先后加入1d(220mg,0.54mmol),k2co3(1.0m水溶液,1.5ml,1.5mmol)和4-甲氧基苯胺(100mg,0.81mmol);室温下搅拌1h后,tlc(展开剂:石油醚/乙酸乙酯=5:1)显示原料(rf=0.5)完全消失,有新点(rf=0.45)生成,用二氯甲烷萃取(3×5ml),无水硫酸钠干燥,过滤浓缩后,柱层析纯化,淡黄色油状物(52a)186mg,收率73%;[α]d20=-2.3(c0.30,chcl3);1hnmr(400mhz,cdcl3)δ6.91(dd,j=8.2,1.8hz,1h),6.86–6.69(m,4h),6.53(d,j=8.8hz,2h),6.33(s,1h),5.88(s,1h),5.22(s,1h),4.23–4.07(m,2h),3.89(s,1h),3.78(s,3h),3.73(s,3h),1.22(t,j=7.1hz,3h),0.97(s,9h),0.12(s,6h);13cnmr(150mhz,cdcl3)δ167.4,151.8,151.5,144.4,141.9,141.4,127.1,126.8,123.6,121.8,114.2,111.2,60.3,55.2,54.8,41.4,25.1,25.0,17.7,13.7,-5.4;esi-lrmsm/z(%):472.2[m+h]+;esi-hrmsm/z(%):calcdforc26h38no5si[m+h]+472.2514,found472.2515.
以0.005当量的三苯基膦替换1e做配体,其他操作完全相同,可得相应外消旋体。
52.2(s)-1-(4-甲氧基苯基)-4-(3-叔丁基二甲基硅氧基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(52b)的合成
化合物52a(186mg)和sn[n(tms)2]2(208mg,0.47mmol)加入一schlenk管中,加入无水甲苯(5ml),加热回流4.5小时后,tlc(展开剂:石油醚/乙酸乙酯=5:1)显示原料(rf=0.5)基本消失,有新点(rf=0.1)生成,反应液冷却至室温后,浓缩,柱层析纯化,得无色油状化合物(52b)130mg,收率77%;[α]d20=+68.0(c0.10,chcl3);1hnmr(400mhz,cdcl3)δ7.28(d,j=9.1hz,2h),6.93(dd,j=8.3,1.8hz,1h),6.83–6.77(m,4h),5.78(s,1h),5.25(s,1h),5.11(s,1h),3.79(s,3h),3.74(s,3h),0.94(s,9h),0.09(s,3h),0.07(s,3h);13cnmr(150mhz,cdcl3)δ160.1,155.6,150.8,149.5,144.8,130.5,128.2,119.7,118.8,117.9,113.7,111.6,109.3,62.7,54.8,25.1,17.8,-5.2,-5.3;esi-lrmsm/z(%):426.2[m+h]+;esi-hrmsm/z(%):calcdforc24h32no4si[m+h]+426.2095,found426.2096.
52.3(s)-1-(4-甲氧基苯基)-4-(3-羟基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(52)的合成
化合物52b(100mg,0.25mmol)溶于5mlthf,冷至冰浴下,tbaf(130mg,0.5mmol)溶于1mlthf,由分液漏斗缓慢滴加,滴完后继续搅拌15min后,tlc(展开剂:石油醚/乙酸乙酯=3:1)显示原料(rf=0.4)完全消失,有新点(rf=0.1)生成,减压旋干,10ml乙酸乙酯溶解,水洗(5mlx2),饱和食盐水洗(5ml),无水硫酸钠干燥。过滤浓缩后,柱层析纯化,得白色固体(52)63mg,收率86%;mp180-181℃;[α]d20=+113.4(c0.10,chcl3);97%ee[determinedbyhplcanalysisusingachiralcelad-hcolumn;n-hex/i-proh=75:25,1.0ml/min,254nm;tr(minor)=15.28min;tr(major)=19.43min];1hnmr(400mhz,cdcl3)δ7.28(d,j=8.9hz,2h),6.98–6.74(m,5h),5.81–5.73(m,2h),5.26(s,1h),5.10(s,1h),3.87(s,3h),3.73(s,3h);13cnmr(150mhz,cdcl3)δ160.0,155.6,149.5,146.3,145.5,130.5,129.0,117.9,117.9,113.8,112.3,110.3,109.4,62.7,55.3,54.8;esi-lrmsm/z(%):334.1[m+na]+;esi-hrmsm/z(%):calcdforc18h18no4[m+h]+312.1230,found312.1231.。
实施例53(s,z)-1-(3,4,5-三甲氧基苯基)-4-(3-羟基-4-甲氧基苯基)-3-[2-(三甲基硅基)亚乙基]氮杂环丁烷-2-酮(53)和(s,e)-1-(3,4,5-三甲氧基苯基-4-(3-羟基-4-甲氧基苯基-3-[2-(三甲基硅基)亚乙基]氮杂环丁烷-2-酮(54)的合成
向50mlschlenk管中加入化合物1(40mg,0.1mmol),无水甲苯1ml,氮气保护下,加入grubb’s二代催化剂(12mg,0.01mmol),烯丙基三甲基硅烷(86μl,0.5mmol),80℃反应8h;tlc(展开剂:pe/ea=2:1)显示生成新点(rf=0.8);加水,ea萃取,饱和食盐水洗,硫酸钠干燥,柱层析分离(展开剂:pe/ea=5:1),得到白色固体顺式产物(53)24mg,收率49%;1hnmr(400mhz,cdcl3)δ6.96(d,j=1.7hz,1h),6.90(dd,j=8.2hz,1.7hz,1h),6.83(d,j=8.2hz,1h),6.59(s,2h),5.72(brs,1h),5.64(t,j=9.2hz,1h),5.17(s,1h),3.88(s,3h),3.75(s,3h),3.73(s,6h),2.17–2.00(m,2h),0.07(d,j=18.0hz,9h);13cnmr(150mhz,cdcl3)δ161.81,152.82,146.13,145.45,137.81,133.83,133.42,130.22,129.89,118.01,112.33,110.14,93.65,62.29,60.32,55.34,21.24,-2.38;esi-lrmsm/z(%):458.2[m+h]+;白色固体反式产物(54)17mg,收率35%;1hnmr(400mhz,cdcl3)δ7.00(d,j=1.8hz,1h),6.94(d,j=8.2hz,1.8hz,1h),6.84(d,j=8.2hz,1h),6.57(s,2h),6.35(t,j=8.7hz,1h),5.74(s,1h),5.23(s,1h),3.89(s,3h),3.75(s,3h),3.73(s,6h),1.53–1.34(m,2h),-0.05(s,9h);13cnmr(150mhz,cdcl3)δ161.2,152.8,146.2,145.6,138.3,133.7,133.5,129.4,126.6,118.3,112.6,110.3,93.8,62.4,60.3,55.4,19.5,-2.3;esi-lrmsm/z(%):458.2[m+h]+.。
实施例54(s,z)-1-(3,4,5-三甲氧基苯基)-4-(3-羟基-4-甲氧基苯基)-3-亚乙基氮杂环丁烷-2-酮(55)的合成
在100ml茄形瓶中加入化合物53(8mg,0.018mmol),tbaf(7mg,0.026mmol),thf1ml,冰浴反应30分钟;tlc(展开剂:pe/ea=4:1)展开两次,显示原料点(rf=0.2)消失,生成新点(rf=0.1);反应液加水,用ea萃取三次,有机相合并后用饱和食盐水洗一次,na2so4干燥;蒸干,柱层析分离(展开剂:pe/ea=3:1),得到白色固体(55)6mg,收率90%;1hnmr(400mhz,cdcl3)δ6.98(d,j=1.8hz,1h),6.91(dd,j=8.2hz,1.8hz,1h),6.80(d,j=8.2hz,1h),6.71(s,2h),5.62(brs,1h),5.64(q,j=9.2hz,1h),5.27(s,1h),3.89(s,3h),3.76(s,3h),3.70(s,6h),2.07(d,j=9.2hz,2h);esi-lrmsm/z(%):386.2[m+h]+.。
实施例55(s,e)-1-(3,4,5-三甲氧基苯基)-4-(3-羟基-4-甲氧基苯基)-3-亚乙基氮杂环丁烷-2-酮(56)的合成
在100ml茄形瓶中加入化合物54(10mg,0.022mmol),tbaf(9mg,0.034mmol),thf1ml,冰浴反应30分钟;tlc(展开剂:pe/ea=4:1)展开两次,显示原料点(rf=0.2)消失,生成新点(rf=0.1);反应液加水,用ea萃取三次,有机相合并后用饱和食盐水洗一次,na2so4干燥;蒸干,柱层析分离(展开剂:pe/ea=3:1),得到白色固体(56)8mg,收率95%;1hnmr(400mhz,cdcl3)δ7.01(d,j=1.8hz,1h),6.96(d,j=8.2hz,1.8hz,1h),6.82(d,j=8.2hz,1h),6.57(s,2h),6.45(q,j=8.7hz,1h),5.64(brs,1h),5.11(s,1h),3.81(s,3h),3.74(s,3h),3.70(s,6h),2.09(d,j=8.7hz,,2h).13cnmr;esi-lrmsm/z(%):386.2[m+h]+.。
实施例56(s,z)-1-(3,4,5-三甲氧基苯基)-4-(3-羟基-4-甲氧基苯基)-3-(苯亚甲基)氮杂环丁烷-2-酮(57)的合成
向50mlschlenk管中加入化合物1(16mg,0.043mmol),二氯乙烷1ml,通氮气除氧30分钟,加入grubb’s二代催化剂(5mg,0.006mmol),苯乙烯(13μl,0.11mmol),氮气保护下,60℃反应12h;tlc(展开剂:pe/ea=2:1)显示生成新点(rf=0.7);加水,ea萃取,饱和食盐水洗,硫酸钠干燥,柱层析分离(展开剂:pe/ea=4:1),原料回收5mg,回收率31%;同时得到黄色固体(57)10mg,收率51%;1hnmr(400mhz,cdcl3)δ7.99(d,j=7.2hz,2h),7.41-7.31(m,3h),7.02(d,j=2.0hz,1h),6.96(dd,j=8.2,2.0hz,1h),6.86(d,j=8.2hz,1h),6.68(s,2h),6.29(s,1h),5.70(s,1h),5.28(s,1h),3.89(s,3h),3.78(s,3h),3.76(s,6h);esi-lrmsm/z(%):448.2[m+h]+.。
实施例57(s,z)-1-(3,4,5-三甲氧基苯基)-4-(3-羟基-4-甲氧基苯基)-3-[(4-叔丁基苯基)亚甲基]氮杂环丁烷-2-酮(58)的合成
向50mlschlenk管中加入化合物1(10mg,0.027mmol),无水二氯乙烷1ml,通氮气除氧30分钟,加入grubb’s二代催化剂(2mg,0.003mmol),对叔丁基苯乙烯(13μl,0.081mmol),氮气保护下,60℃反应12h;tlc(展开剂:pe/ea=2:1)显示生成新点(rf=0.7);加水,ea萃取,饱和食盐水洗,硫酸钠干燥,柱层析分离(展开剂:pe/ea=4:1),得到黄色固(58)4mg,收率30%;原料回收5mg,回收率50%;mp77–78℃;[α]d20=+131.8(c1.0,chcl3).1hnmr(400mhz,cdcl3):δ7.92(d,j=8.4hz,2h),7.42(d,j=8.4hz,2h),7.01(d,j=1.9hz,1h),6.95(dd,j=8.2,1.9hz,1h),6.85(d,j=8.2hz,1h),6.68(s,2h),6.28(s,1h),5.68(s,1h),5.27(s,1h),3.89(s,3h),3.77(s,3h),3.76(s,6h),1.31(s,9h).13cnmr(150mhz,cdcl3):δ159.9,152.9,152.4,146.4,145.6,139.1,133.8,133.6,130.8,130.0,129.6,129.1,125.0,118.2,112.5,110.3,94.0,76.6,76.4,76.2,61.8,60.4,55.4,55.4,34.2,30.5.esi-ms(m/z):504.2(m+h+).esi-hrms(m/z):calcdforc30h33no6+h+[m+h]+,504.2381;found,504.2376.。
实施例58(3r,4r)-1-(3,4,5-三甲氧基苯基)-4-(3-羟基-4-甲氧基苯基)-3-(2-三甲基硅基乙基)氮杂环丁烷-2-酮(59)的合成
在100ml茄形瓶中加入化合物53和54混合物(38mg,0.083mmol),10%pd/c4mg,甲醇1ml,常压氢气下,室温反应12h,tlc(展开剂:pe/ea=4:1)展开两次显示原料点(rf=0.2)消失,生成新点(rf=0.3);过滤除去pd/c后蒸干,柱层析分离(展开剂:pe/ea=4:1),得到白色固体(59)33mg,收率87%;1hnmr(400mhz,cdcl3)δ6.86-6.82(m,2h),6.77(d,j=8.2hz,1h),6.55(s,2h),5.70(s,1h),5.07(d,j=5.6hz,1h),3.89(s,3h),3.81–3.68(m,10h),3.46(dt,j=9.5,6.0hz,1h),0.91–0.82(m,1h),0.48(td,j=14.0,4.8hz,1h),0.21(td,j=14.0,3.7hz,1h),-0.21(s,9h);esi-lrmsm/z(%):482.2[m+na]+.。
实施例59(3r,4r)-1-(3,4,5-三甲氧基苯基)-4-(3-羟基-4-甲氧基苯基)-3-(4-羟基苄基)氮杂环丁烷-2-酮(60)的合成
向50mlschlenk管中加入化合物1(20mg,0.054mmol),无水甲苯1ml,氮气保护下,加入grubb’s二代催化剂(5mg,0.006mmol),对羟基苯乙烯(41mg,0.34mmol),80℃反应12h;tlc(展开剂:pe/ea=2:1)显示生成新点(rf=0.15);加水,ea萃取,饱和食盐水洗,硫酸钠干燥,蒸干快速柱层析粗分后(展开剂:pe/ea=4:1),加入10%pd/c3mg,甲醇1ml,常压氢气下,室温反应12小时,tlc(展开剂:pe/ea=2:1)显示原料点(rf=0.15)消失,生成新点(rf=0.19);过滤除去pd/c后蒸干,柱层析分离(展开剂:pe/ea=2:1),得到白色固体(60)17mg,收率83%;1hnmr(400mhz,cdcl3)δ6.85–6.69(m,5h),6.65(d,j=8.4hz,2h),6.56(s,2h),5.72(s,1h),5.26(brs,1h),5.07(d,j=5.6hz,1h),3.91(s,3h),3.83–3.74(m,4h),3.71(s,6h),2.84(dd,j=14.8,7.2hz,1h),2.44(dd,j=14.8,8.5hz,1h);esi-lrmsm/z(%):466.2[m+h]+.。
实施例60(3s,4s)-1-(3,4,5-三甲氧基苯基)-3-(4-羟甲基-1h-1,2,3-三氮唑-1-基)-4-(3-羟基-4-甲氧基苯基)氮杂环丁烷-2-酮(61)的合成
向100ml茄形瓶中加入化合物75、炔丙醇(5μl,0.086mmol)、vc-na(2mg,0.009mmol)、水合硫酸铜(1mg,0.004mmol)、乙醇1ml和水0.2ml,室温反应12h;tlc(展开剂:pe/ea=1:3)显示生成新点(rf=0.15);加水,ea萃取,饱和食盐水洗,硫酸钠干燥,快速柱层析粗分后,加入10%pd/c3mg,甲醇1ml,常压氢气下,室温反应12小时,tlc(展开剂:pe/ea=1:3)显示原料点(rf=0.15)消失,生成新点(rf=0.1);过滤除去pd/c后蒸干,柱层析分离(展开剂:pe/ea=1:4),得到白色固体(61)11mg,收率84%;1hnmr(400mhz,cdcl3)δ;1hnmr(400mhz,cdcl3)δ7.75(s,1h),6.98(d,j=1.6hz,1h),6.94(dd,j=8.0,1.6hz,1h),6.89(d,j=8.0hz,1h),6.59(s,2h),5.76(s,1h),5.55(d,j=1.6hz,1h),5.31(d,j=1.6hz,1h),4.85(s,2h),3.92(s,3h),3.79(s,3h),3.74(s,6h);13cnmr(150mhz,cdcl3)δ159.0,153.7,147.6,146.7,135.5,132.5,127.6,121.5,118.4,112.0,111.2,95.65,72.0,71.5,67.92,66.2,65.5,64.1,63.5,61.0,59.9,58.8,58.5,56.7,56.2,56.1,55.9,54.7,50.7.。
实施例61(3r,4r)-1-(3,4,5-三甲氧基苯基)-4-(3-羟基-4-甲氧基苯基)-3-(二甲氨基甲基)氮杂环丁烷-2-酮(62)的合成
在100mlschlenk管中加入化合物1(16mg,0.043mmol),盐酸二甲胺(11mg,0.13mmol),dbu(19μl,0.13mmol)与甲醇1ml,橡胶塞密封,回流反应8小时;tlc(展开剂:pe/ea=1:5)显示原料点(rf=0.99)消失,生成新点(rf=0.1);反应液加水,用ea萃取三次,有机相合并后用饱和食盐水洗一次,na2so4干燥;蒸干,柱层析分离(展开剂:pe/ea=2:1),得到淡黄色固体(62)16mg,收率89%;1hnmr(400mhz,cdcl3)δ6.85-6.83(m,2h),6.77(d,j=7.1hz,1h),6.55(s,2h),5.12(d,j=5.8hz,1h),3.90(s,3h),3.82–3.74(m,4h),3.71(s,6h),2.44(dd,j=13.3,6.8hz,1h),2.25(dd,j=13.3,5.8hz,1h),2.17(s,6h);13cnmr(150mhz,cdcl3)δ166.0,152.8,146.1,145.3,133.8,133.1,126.9,118.4,112.9,110.1,94.3,60.3,57.6,55.4,55.3,53.8,53.0,44.9.。
实施例62(3r,4r)-1-(3,4,5-三甲氧基苯基)-4-(3-羟基-4-甲氧基苯基)-3-(n-苄基氨基甲基)氮杂环丁烷-2-酮(63)的合成
在100mlschlenk管中加入化合物1(0.5g,1.35mmol),苄胺(0.18ml,1.63mmol)与甲醇12ml,回流反应12小时;tlc(展开剂:pe/ea=1:1)显示生成新点(rf=0.1);反应液加水,用ea萃取三次,有机相合并后用饱和食盐水洗一次,na2so4干燥;蒸干,柱层析分离(展开剂:pe/ea=2:1),回收原料0.22g;同时得到淡黄色固体(63)0.15g,收率23%;1hnmr(400mhz,cdcl3)δ7.29–7.16(m,3h),7.05(d,j=6.6hz,2h),6.85(d,j=1.4hz,1h),6.78(q,j=8.3hz,2h),6.53(s,2h),5.10(d,j=5.6hz,1h),3.90(s,3h),3.84–3.74(m,4h),3.71(s,6h),3.63(d,j=13.2hz,1h),3.48(d,j=13.2hz,1h),2.79(dd,j=12.2,6.3hz,1h),2.60(dd,j=12.2,9.2hz,1h);;13cnmr(150mhz,cdcl3)δ165.7,152.9,146.1,145.4,138.8,133.8,133.0,127.7,127.3,126.6,126.3,118.0,112.4,110.2,94.3,60.3,56.9,55.5,55.30,54.1,53.2,44.0.。
实施例63(3r,4s)-1-(3,4,5-三甲氧基苯基)-4-(3-羟基-4-甲氧基苯基)-3-(3-羧基丙酰氧基)氮杂环丁烷-2-酮(64)的合成
冰浴下,在100ml茄形瓶中加入化合物72(23mg,0.049mmol),丁二酸酐(6mg,0.006mmol),dipea(15μl,mmol),dmap1mg与无水dcm1ml,室温反应6小时;tlc(展开剂:pe/ea=1:2)显示原料点(rf=0.4)消失,生成新点(rf=0.03);反应液加水,用ea萃取三次,有机相合并后用饱和食盐水洗一次,na2so4干燥;蒸干,加入10%pd/c3mg,甲醇1ml,常压氢气下,室温反应12小时,tlc(展开剂:dcm/meoh=10:1)显示原料点(rf=0.4)消失,生成新点(rf=0.3);过滤除去pd/c后蒸干,柱层析分离(展开剂:dcm/meoh=10:1),得到白色固体(64)18mg,收率77%;1hnmr(400mhz,cdcl3)δ6.86(d,j=1.7hz,1h),6.84–6.78(m,2h),6.57(s,2h),5.95(d,j=4.9hz,1h),5.25(d,j=4.9hz,1h),3.88(s,3h),3.77(s,3h),3.73(s,6h),2.54–2.43(m,1h),2.42–2.35(m,2h),2.29–2.18(m,1h).。
实施例64(3r,4s)-1-(3,4,5-三甲氧基苯基)-4-(3-羟基-4-甲氧基苯基)-3-(乙氧羰基甲氧基)氮杂环丁烷-2-酮(65)的合成
冰浴下,在100ml茄形瓶中加入化合物72(15mg,0.032mmol),溴乙酸乙酯(6μl,0.05mmol),nah(2mg,0.05mmol)与无水thf1ml,室温反应1小时;tlc(展开剂:pe/ea=1:1)显示原料点(rf=0.6)消失,生成新点(rf=0.8);反应液加水,用ea萃取三次,有机相合并后用饱和食盐水洗一次,na2so4干燥;蒸干,加入10%pd/c3mg,甲醇1ml,常压氢气下,室温反应24小时,tlc(展开剂:pe/ea=1:1)显示原料点(rf=0.6)消失,生成新点(rf=0.5);过滤除去pd/c后蒸干,柱层析分离(展开剂:pe/ea=2:1),得到白色固体(65)7mg,收率47%;1hnmr(400mhz,cdcl3)δ7.01(d,j=1.8hz,1h),6.93(dd,j=8.2,1.8hz,1h),6.85(d,j=8.2hz,1h),6.57(s,2h),5.67(s,1h),5.11(dd,j=11.4,5.0hz,2h),4.14(d,j=16.5hz,1h),4.03(d,j=16.5hz,1h),3.89(s,3h),3.76(s,3h),3.72(s,6h),1.25(t,j=7.1hz,6h);13cnmr(150mhz,cdcl3)δ168.6,163.1,152.9,146.3,145.1,134.2,132.5,125.4,119.4,113.7,109.9,94.7,82.0,66.5,60.9,60.4,60.3,55.5,55.3,29.1,13.5.。
实施例65(3s,4s)-1-(3,4,5-三甲氧基苯基)-4-(3-羟基-4-甲氧基苯基)-3-溴氮杂环丁烷-2-酮(66)和(r)-1-(3,4,5-三甲氧基苯基)-4-(3-羟基-4-甲氧基苯基)氮杂环丁烷-2-酮(67)的合成
在5ml微波反应管中加入化合物73(50mg,0.092mmol),tbab(59mg,0.18mmol)和dmf1ml,微波加热170℃反应3h.tlc(展开剂:pe/ea=2:1)显示原料点(rf=0.15)消失,生成新点(rf=0.7);反应液加水,用ea萃取三次,有机相合并后用饱和食盐水洗一次,na2so4干燥;蒸干,快速柱层析分离粗分后直接加入加入10%pd/c3mg,甲醇1ml,常压氢气下,室温反应12小时,tlc(展开剂:pe/ea=2:1)显示原料点(rf=0.7)消失,生成两个新点(rf=0.4,0.5);过滤除去pd/c后蒸干,柱层析分离(展开剂:pe/ea=3:1),得到rf=0.5的脱苄基产物为白色固体(66)10mg,收率53%;1hnmr(400mhz,cdcl3)δ6.93(d,j=1.9hz,1h),6.90(dd,j=8.2,1.9hz,1h),6.86(d,j=8.2hz,1h),6.54(s,2h),5.73(s,1h),4.87(d,j=1.9hz,1h),4.59(d,j=1.9hz,1h),3.91(s,3h),3.76(s,3h),3.72(s,6h);rf=0.4脱苄基同时得到溴被还原产物为白色固体(67)5mg,收率32%;1hnmr(400mhz,cdcl3)δ6.96(d,j=2.1hz,1h),6.89(dd,j=8.3,2.1hz,1h),6.84(d,j=8.3hz,1h),6.55(s,2h),5.67(s,1h),4.87(dd,j=5.6,2.6hz,1h),3.89(s,3h),3.76(s,3h),3.72(s,6h),3.51(dd,j=15.2,5.6hz,1h),2.93(dd,j=15.2,2.6hz,1h).。
实施例66(3s,4r)-1-(3,4,5-三甲氧基苯基)-4-(3-羟基-4-甲氧基苯基)-3-羟甲基氮杂环丁烷-2-酮(68)和(3r,4r)-1-(3,4,5-三甲氧基苯基)-4-(3-羟基-4-甲氧基苯基)-3-羟甲基氮杂环丁烷-2-酮(69)的合成
氮气保护下下,在50mlschlenk中加入化合物1(11mg,0.030mmol),联硼酸频哪醇酯(10mg,0.039mmol),cucl(0.3mg,0.03mmol),三苯基膦(1mg,0.038mmol),甲醇(1.5μl,0.037mmol),叔丁醇锂(0.3mg,0.037mmol)与无水thf1ml,室温反应12小时;tlc(展开剂:pe/ea=1:2)显示仅原料点(rf=0.4)等高处有点;反应液加水,用ea萃取三次,有机相合并后用饱和食盐水洗一次,na2so4干燥;蒸干,加入nabo3·4h2o(23mg,0.15mmol),thf1ml和水1ml,室温反应12h,tlc(展开剂:pe/ea=1:3)显示原料点(rf=0.9)消失,生成两新点(rf=0.17,0.22);反应液加水,用ea萃取三次,有机相合并后用饱和食盐水洗一次,na2so4干燥;蒸干,柱层析分离(展开剂:pe/ea=1:1),得到反式产物为白色固体(69)4.5mg,收率39%;1hnmr(400mhz,cdcl3)δ6.97(d,j=1.5hz,1h),6.91(dd,j=8.2hz,1.5hz,1h),6.84(d,j=8.2hz,1h),6.56(s,2h),5.70(s,1h),4.91(d,j=1.8hz,1h),4.15(dd,j=12.1,4.5hz,1h),4.00(dd,j=12.1,3.7hz,1h),3.90(s,3h),3.79–3.75(m,4h),3.73(s,6h),3.28(brd,j=2.3hz,1h);13cnmr(151mhz,cdcl3)δ165.60,153.50,146.78,146.32,134.57,133.72,130.77,117.94,112.16,110.99,94.91,62.14,60.93,58.89,57.49,56.06;得到顺式产物(68)为白色固体7mg,收率61%;1hnmr(400mhz,cdcl3)δ6.90(d,j=1.2hz,1h),6.87–6.81(m,2h),6.55(s,2h),5.75(s,1h),5.16(d,j=5.3hz,1h),3.90(s,3h),3.86–3.75(m,5h),3.73(s,6h),3.64(dd,j=11.3,7.8hz,1h);13cnmr(151mhz,cdcl3)δ165.18,153.56,146.73,146.13,134.68,133.60,127.13,118.47,112.90,110.93,95.06,60.96,58.15,57.27,56.72,56.15,55.98.。
实施例67(3r,4r)-1-(3,4,5-三甲氧基苯基)-4-(3-羟基-4-甲氧基苯基)-3-甲基氮杂环丁烷-2-酮(70)的合成
在100ml茄形瓶中加入化合物1(15mg,0.04mmol),10%pd/c3mg,甲醇1ml常压氢气下,室温反应12小时,tlc(展开剂:pe/ea=2:1)显示原料点(rf=0.4)消失,生成新点(rf=0.33);过滤除去pd/c后蒸干,柱层析分离(展开剂:pe/ea=2:1),得到白色固体(70)15mg,收率99%;1hnmr(400mhz,cdcl3)δ6.86–6.77(m,2h),6.72(d,j=8.3hz,1h),6.56(s,2h),5.71(s,1h),5.06(d,j=5.8hz,1h),3.89(s,3h),3.77(s,3h),3.72(s,6h),3.66-3.59(m,1h),0.91(d,j=7.6hz,3h).。
实施例68(s)-1-(3,4,5-三甲氧基苯基)-4-(3-苄氧基-4-甲氧基苯基)氮杂环丁烷-2,3-二酮(71)的合成
在100ml茄形瓶中加入化合物84(0.2g,0.41mmol),高碘酸钠(0.13g,0.62mmol),甲醇4ml与水1ml,室温反应3小时;tlc(展开剂:pe/ea=1:1)显示原料点(rf=0)消失,生成新点(rf=0.2);反应液加水,用ea萃取三次,有机相合并后用饱和食盐水洗一次,na2so4干燥;柱层析分离(展开剂:pe/ea=2:1),得到黄色固体(71)0.15g,收率79%;1hnmr(400mhz,cdcl3)δ7.32–7.18(m,3h),6.90(s,2h),6.56(d,j=6.8hz,1h),6.05(s,1h),5.26(s,1h),4.95(d,j=6.8hz,1h),4.26(s,3h),4.13(s,3h),4.07(s,6h),2.44(s,3h).。
实施例69(3r,4s)-1-(3,4,5-三甲氧基苯基)-3-羟基-4-(3-苄氧基-4-甲氧基苯基)氮杂环丁烷-2-酮(72)的合成
在100ml茄形瓶中加入化合物71(0.18g,0.39mmol),甲醇4ml,冰浴下加入硼氢化钠(18mg,0.47mmol),保持冰浴反应1小时;tlc(展开剂:pe/ea=2:1,2次)显示原料点(rf=0.2)消失,生成新点(rf=0.1);反应液加水,用ea萃取三次,有机相合并后用饱和食盐水洗一次,na2so4干燥;柱层析分离(展开剂:pe/ea=1:1),得到白色固体(72)0.35g,收率90%;1hnmr(400mhz,cdcl3)δ7.40–7.28(m,5h),6.94(d,j=8.3hz,1h),6.91(dd,j=8.3,1.6hz,1h),6.83(brd,j=1.6hz,1h),6.54(s,2h),5.16(s,2h),5.14(d,j=5.2hz,1h),5.10((brd,j=5.2hz,1h),3.91(s,3h),3.80(s,3h),3.70(s,6h).。
实施例70(3r,4s)-1-(3,4,5-三甲氧基苯基)-3-甲磺酰氧基-4-(3-苄氧基-4-甲氧基苯基)氮杂环丁烷-2-酮(73)的合成
在100ml茄形瓶中加入化合物72(0.2g,0.43mmol),三乙胺(0.09ml,0.64mmol),二氯甲烷4ml,冰浴下加入甲烷磺酰氯(0.05ml,0.64mmol),逐渐升至室温反应1小时;tlc(展开剂:pe/ea=2:1,两次)显示原料点(rf=0.1)消失,生成新点(rf=0.2);反应液加水,用ea萃取三次,有机相合并后用饱和食盐水洗一次,na2so4干燥;柱层析分离(展开剂:pe/ea=5:1),得到白色固体(73)0.2g,收率86%;1hnmr(400mhz,cdcl3)δ7.36(d,j=7.7hz,2h),7.33–7.20(m,3h),6.99–6.84(m,3h),6.47(s,2h),5.76(d,j=5.0hz,1h),5.21(d,j=5.0hz,1h),5.13(s,2h),3.88(s,3h),3.77(s,3h),3.67(s,6h),2.66(s,3h);13cnmr(101mhz,cdcl3)δ160.3,153.7,150.8,148.3,136.8,135.5,132.6,128.8,128.2,127.5,123.8,121.5,113.7,111.9,95.4,79.4,71.2,61.7,61.2,56.28,56.2,38.9.。
实施例71(3r,4s)-1-(3,4,5-三甲氧基苯基)-3-甲烷磺酰氧基-4-(3-羟基-4-甲氧基苯基)氮杂环丁烷-2-酮(74)的合成
在100ml茄形瓶中加入化合物73(11mg,0.02mmol),10%pd/c3mg,甲醇1ml常压氢气下,室温反应12小时,tlc(展开剂:pe/ea=1:1)显示原料点(rf=0.4)消失,生成新点(rf=0.1);过滤除去pd/c后蒸干,柱层析分离(展开剂:pe/ea=1:1),得到白色固(74)9mg,收率98%;1hnmr(400mhz,cdcl3)δ6.96–6.85(m,3h),6.56(s,2h),5.80(d,j=5.1hz,1h),5.70(s,1h),5.28(d,j=5.1hz,1h),3.90(s,3h),3.77(s,3h),3.73(s,6h),2.91(s,3h);13cnmr(151mhz,cdcl3)δ159.5,153.0,146.8,145.4,131.8,123.8,119.5,113.3,110.1,94.8,78.6,60.7,60.3,55.5,55.3,38.4.。
实施例72(3s,4s)-1-(3,4,5-三甲氧基苯基)-3-叠氮基-4-(3-苄氧基-4-甲氧基苯基)氮杂环丁烷-2-酮(75)的合成
在100ml茄形瓶中加入化合物73(0.5g,0.92mmol),叠氮化钠(90mg,1.38mmol),dmf10ml,100℃反应48小时;tlc(展开剂:pe/ea=2:1)显示原料点(rf=0.2)消失,生成新点(rf=0.4);反应液加水,用ea萃取三次,有机相合并后用饱和食盐水洗一次,na2so4干燥;柱层析分离(展开剂:pe/ea=5:1),得到黄色液体(75)0.24g,收率53%;[α]d20=-50.4(c1.0,chcl3).1hnmr(400mhz,cdcl3):δ7.38–7.23(m,5h),6.94–6.88(m,2h),6.80(brs,1h),6.43(s,2h),5.12(s,2h),4.67(d,j=1.4hz,1h),4.39(d,j=1.4hz,1h),3.89(s,3h),3.76(s,3h),3.65(s,6h).13cnmr(150mhz,cdcl3):δ160.7,152.9,150.0,148.1,135.8,134.5,132.2,127.9,127.4,126.8,126.6,118.8,111.5,111.0,94.5,71.7,70.5,62.5,60.3,55.5,55.4.esi-ms(m/z):491.1(m+h+).esi-hrms(m/z):calcdforc26h26n4o6+h+[m+h]+,491.1925;found,491.1924.。
实施例73(3s,4s)-1-(3,4,5-三甲氧基苯基)-3-氨基-4-(3-苄氧基-4-甲氧基苯基)氮杂环丁烷-2-酮(76)的合成
在100ml茄形瓶中加入化合物75(0.23g,0.47mmol),氯化亚锡(0.28g,1.4mmol),甲醇8ml,9%盐酸2ml,回流反应1小时;tlc(展开剂:pe/ea=2:1)显示原料点(rf=0.4)消失,生成新点(rf=0);反应液加碳酸氢钠,用ea萃取三次,有机相合并后用饱和食盐水洗一次,na2so4干燥;柱层析分离(展开剂:pe/ea=5:1),得到白色固体(76)0.14g,收率64%;mp64–65℃;[α]d20=+7.9(c1.0,chcl3).1hnmr(400mhz,cdcl3):δ7.39–7.21(m,5h),6.97–6.82(m,3h),6.47(s,2h),5.12(s,2h),4.50(s,1h),3.98(s,1h),3.89(s,3h),3.77(s,3h),3.66(s,6h).13cnmr(150mhz,cdcl3):δ167.9,153.5,150.1,148.6,136.6,134.6,133.6,129.2,128.5,128.0,127.3,119.2,112.1,111.6,95.0,71.1,69.7,66.8,60.9,56.1,56.0.esi-ms(m/z):465.1(m+h+).esi-hrms(m/z):calcdforc26h28n2o6+h+[m+h]+,465.2020;found,465.2035.。
实施例74(3s,4s)-1-(3,4,5-三甲氧基苯基)-3-乙酰氨基-4-(3-羟基-4-甲氧基苯基)氮杂环丁烷-2-酮(77)的合成
在100ml茄形瓶中加入化合物76(29mg,0.062mmol),dipea(16μl,0.093mmol),醋酐(8μl,0.08mmol)与二氯甲烷2ml,室温反应1h;tlc(展开剂:pe/ea=1:5)显示原料点(rf=0.1)消失,生成新点(rf=0.3);反应液加饱和nahco3,用dcm萃取三次,有机相合并后用饱和食盐水洗一次,na2so4干燥;整个,加入10%pd/c3mg,甲醇1ml常压氢气下,室温反应12小时,tlc(展开剂:pe/ea=1:5)显示原料点(rf=0.3)消失,生成新点(rf=0.25);过滤除去pd/c后蒸干,柱层析分离(展开剂:pe/ea=2:1),得到白色固体(77)15mg,收率58%;1hnmr(400mhz,cdcl3)δ7.32–7.18(m,3h),6.90(s,2h),6.56(d,j=6.8hz,1h),6.05(s,1h),5.26(s,1h),4.95(d,j=6.8hz,1h),4.26(s,3h),4.13(s,3h),4.07(s,6h),2.44(s,3h).。
实施例75(3s,4s)-1-(3,4,5-三甲氧基苯基)-3-苯甲酰氨基-4-(3-羟基-4-甲氧基苯基)氮杂环丁烷-2-酮(78)的合成
在100ml茄形瓶中加入化合物76(32mg,0.069mmol),dipea(19μl,0.11mmol),苯甲酸酐(23mg,0.1mmol)与二氯甲烷5ml,室温反应4h;tlc(展开剂:pe/ea=1:5)显示原料点(rf=0.1)消失,生成新点(rf=0.4);反应液加饱和nahco3,用dcm萃取三次,有机相合并后用饱和食盐水洗一次,na2so4干燥;蒸干,加入10%pd/c3mg,甲醇1ml,常压氢气下,室温反应12小时,tlc(展开剂:pe/ea=1:5)显示原料点(rf=0.4)消失,生成新点(rf=0.33);过滤除去pd/c后蒸干,柱层析分离(展开剂:pe/ea=2:1),得到白色固体(78)26mg,收率80%;1hnmr(400mhz,cdcl3)δ7.82(d,j=7.2hz,2h),7.53(t,j=7.3hz,1h),7.44(t,j=7.6hz,2h),7.00(d,j=6.7hz,1h),6.97(d,j=2.0hz,1h),6.93(dd,j=8.2,2.0hz,1h),6.85(d,j=8.2hz,1h),6.55(s,2h),5.69(s,1h),5.00(d,j=2.1hz,1h),4.77(dd,j=6.7,2.1hz,1h),3.89(s,3h),3.75(s,3h),3.70(s,6h).。
实施例76(3s,4s)-1-(3,4,5-三甲氧基苯基)-3-甲磺酰氨基-4-(3-羟基-4-甲氧基苯基)氮杂环丁烷-2-酮(79)的合成
在100ml茄形瓶中加入化合物76(12mg,0.026mmol),dipea(5μl,0.031mmol),甲磺酰氯(2μl,0.028mmol)与二氯甲烷1ml,室温反应2h;tlc(展开剂:pe/ea=1:5)显示原料点(rf=0.1)消失,生成新点(rf=0.3);反应液加饱和nahco3,用dcm萃取三次,有机相合并后用饱和食盐水洗一次,na2so4干燥;蒸干,加入10%pd/c3mg,甲醇1ml,常压氢气下,室温反应12小时,tlc(展开剂:pe/ea=1:5)显示原料点(rf=0.3)消失,生成新点(rf=0.26);过滤除去pd/c后蒸干,柱层析分离(展开剂:pe/ea=2:1),得到白色固体(79)15mg,收率99%;1hnmr(400mhz,cdcl3)δ6.75–6.65(m,2h),6.62(d,j=1.5hz,1h),6.14(s,2h),5.62(t,j=8.0hz,1h),4.92(d,j=2.8hz,2h),4.53(d,j=2.0hz,1h),4.17(dd,j=8.8,2.0hz,1h),3.70(s,3h),3.59(s,3h),3.45(s,6h),2.92(s,3h).。
实施例77(3r,4s)-1-(3,4,5-三甲氧基苯基)-4-(3-苄氧基-4-甲氧基苯基)-3-羟基-3-羟甲基氮杂环丁烷-2-酮(84)的合成的合成
在100ml茄形瓶中加入化合物47(0.93g,2mmol),锇酸钾(10mg,0.03mmol),nmo(0.62ml,3mmol)与丙酮5ml,水0.2ml,室温反应8小时;tlc(展开剂:pe/ea=2:1)显示原料点(rf=0.6)消失,生成新点(rf=0);反应液加水,用ea萃取三次,有机相合并后用饱和食盐水洗一次,na2so4干燥;柱层析分离(展开剂:pe/ea=1:4),得到白色固体840.91g,收率92%;mp123–124℃;[α]d20=+61.8(c1.0,chcl3).1hnmr(400mhz,cdcl3):δ7.34–7.21(m,5h),6.90–6.77(m,2h),6.72(s,1h),6.43(s,2h),5.10(s,2h),4.94(s,1h),4.53(brd,j=30.9hz,1h),3.88(s,3h),3.77(s,3h),3.63(s,6h),3.53(dd,j=12.3,4.3hz,1h),3.27(dd,j=12.3,5.5hz,1h).13cnmr(150mhz,cdcl3):δ166.5,152.8,149.5,147.4,135.9,134.3,132.3,127.9,127.4,126.6,124.3,119.1,112.0,111.3,95.0,85.7,70.4,66.9,61.6,60.3,55.4,55.4.esi-ms(m/z):496.1(m+h+).esi-hrms(m/z):calcdforc27h29no8+h+[m+h]+,496.1966;found,496.1978..。
实施例78(3r,4s)-1-(3,4,5-三甲氧基苯基)-4-(3-羟基-4-甲氧基苯基)-3-羟基-3-羟甲基氮杂环丁烷-2-酮(80)的合成的合成
在100ml茄形瓶中加入化合物84(11mg,0.022mmol),10%pd/c3mg,甲醇1ml常压氢气下,室温反应12小时,tlc(展开剂:pe/ea=1:2)显示原料点(rf=0.6)消失,生成新点(rf=0.3);过滤除去pd/c后蒸干,柱层析分离(展开剂:pe/ea=1:2),得到白色固体(80)8mg,收率89%;1hnmr(400mhz,cdcl3)δ6.84-6.82(m,2h),6.76(brd,j=8.1hz,1h),6.52(s,2h),5.80(s,1h),5.01(s,1h),4.53(brs,1h),3.89(s,3h),3.82–3.62(m,10h),3.45(d,j=12.3hz,1h),2.49(brs,1h).13cnmr(151mhz,cdcl3)δ166.6,152.9,146.2,145.4,134.3,132.3,125.2,117.8,112.1,110.3,95.1,85.7,76.6,76.4,76.2,66.8,61.6,60.3,55.5,55.3.。
实施例79(3r,4s)-1-(3,4,5-三甲氧基苯基)-4-(3-羟基-4-甲氧基苯基)-3-甲氧基-3-甲氧甲基氮杂环丁烷-2-酮(81)的合成
在冰浴下,在100ml茄形瓶中加入化合物84(15mg,0.03mmol),硫酸二甲酯(15μl,0.15mmol),nah(3mg,0.075mmol)与无水thf1ml,室温反应1小时;tlc(展开剂:pe/ea=1:2)显示原料点(rf=0.4)消失,生成新点(rf=0.9);反应液加水,用ea萃取三次,有机相合并后用饱和食盐水洗一次,na2so4干燥;蒸干,加入10%pd/c3mg,甲醇1ml,常压氢气下,室温反应12小时,tlc(展开剂:pe/ea=1:1)显示原料点(rf=0.6)消失,生成新点(rf=0.5);过滤除去pd/c后蒸干,柱层析分离(展开剂:pe/ea=2:1),得到白色固体(81)11mg,收率84%;1hnmr(400mhz,cdcl3)δ6.85(d,j=1.4hz,1h),6.82(d,j=8.2hz,1h),6.77(dd,j=8.2,1.4hz,1h),6.58(s,2h),5.06(s,1h),3.89(s,3h),3.78(s,3h),3.72(s,6h),3.63(s,3h),3.51(d,j=11.0hz,1h),3.37(d,j=11.0hz,1h),3.03(s,3h);13cnmr(150mhz,cdcl3)δ163.7,152.9,145.9,145.0,134.3,132.3,125.7,118.4,112.6,109.8,95.1,91.8,68.8,63.1,60.3,58.7,55.5,55.3,53.1.。
实施例80(3r,4s)-1-(3,4,5-三甲氧基苯基)-4-(3-乙酰氧基-4-甲氧基苯基)-3-羟基-3-羟甲基氮杂环丁烷-2-酮(82)的合成
在100ml茄形瓶中加入化合物46(96mg,0.22mmol),锇酸钾(3mg,0.01mmol),nmo(0.1ml,0.48mmol)与丙酮5ml,水0.2ml,室温反应8小时;tlc(展开剂:pe/ea=2:1)显示原料点(rf=0.6)消失,生成新点(rf=0);反应液加水,用ea萃取三次,有机相合并后用饱和食盐水洗一次,na2so4干燥;柱层析分离(展开剂:pe/ea=1:3),得到白色固体(82)93mg,收率95%;1hnmr(400mhz,cdcl3)δ7.10(d,j=8.2hz,1h),6.98(s,1h),6.95(d,j=8.2hz,1h),6.52(s,2h),5.04(s,1h),3.82(s,3h),3.78(s,3h),3.71-3.69(m,7h),3.46(d,j=12.2hz,1h),2.28(s,3h).esi-lrmsm/z(%):470.1[m+na]+.。
实施例81(3r,4s)-1-(3,4,5-三甲氧基苯基)-4-(3-乙酰氧基-4-甲氧基苯基)-3-乙酰氧基-3-乙酰氧甲基氮杂环丁烷-2-酮(83)的合成
在100ml茄形瓶中加入化合物82(6.5mg,0.015mmol),4-二甲氨基吡啶0.5mg,三乙胺(5μl,0.03mmol)与二氯甲烷5ml,醋酸酐(3.5μl,0.03mmol),室温反应30分钟;tlc(展开剂:pe/ea=1:4)显示原料点(rf=0.2)消失,生成新点(rf=0.8);反应液加饱和nahco3,用ea萃取三次,有机相合并后用饱和食盐水洗一次,na2so4干燥;柱层析分离(展开剂:pe/ea=1:1),得到白色固体(83)6mg,收率85%;1hnmr(400mhz,cdcl3)δ7.24(d,j=8.6hz,1h),7.13(s,1h),6.95(d,j=8.6hz,1h),6.54(s,2h),5.29(s,1h),4.31(d,j=12.7hz,1h),4.24(d,j=12.7hz,1h),3.83(s,3h),3.78(s,3h),3.71(s,6h),2.28(s,3h),2.21(s,3h),1.99(s,3h);esi-lrmsm/z(%):532.1[m+h]+.。
实施例82(s)-1-(3,4,5-三甲氧基苯基)-4-(3-羟基苯基)-3-亚甲基氮杂环丁烷-2-酮(85)的合成
化合物85的合成路线与化合物1类似:
82.13-叔丁基二甲基硅氧基苯甲醛(85b)的合成
500ml三口瓶中加入3-羟基苯甲醛(85a)16g(0.13mol),dmap(320mg,2.6mmol),无水dcm(150ml),无水三乙胺(23ml,0.182mol),冷至冰浴下,tbscl(20g,0.156mol)溶于无水dcm(50ml)中,由滴液漏斗缓慢加入;滴完后撤去冰浴,室温搅拌两小时;加入饱和碳酸氢钠水溶液淬灭反应,分出有机相,水相用dcm萃取,无水硫酸钠干燥3h,湿法上柱,分离纯化,收集对应的洗脱液,蒸干溶剂得无色油状化合物(85b)30g,收率97%;1hnmr(400mhz,cdcl3)δ9.95(s,1h),7.47(d,j=7.6hz,1h),7.40(t,j=7.6hz,1h),7.32(s,1h),7.12–7.08(m,1h),0.99(s,9h),0.22(s,6h);esi-lrmsm/z(%):237.1[m+h]+.
82.22-[1-(3-叔丁基二甲基硅氧基苯基)-1-羟基甲基]丙烯酸乙酯(85c)的合成
在50ml茄形瓶中加入化合物85b(30g,0.114mol),丙烯酸乙酯(13.7g,0.136mol),dabco(15.3g,0.136mol),室温搅拌反应20d后,tlc(展开剂:石油醚/乙酸乙酯=10:1)显示原料(rf=0.6)明显减少,有大量新点生成(rf=0.2),停止反应,用较粗的柱子,直接将反应液倒到硅胶柱上,用少量甲苯洗涤反应瓶再倒到柱子上,然后用pe及pe/ea10/1先除去丙烯酸乙酯,继而进行梯度洗脱,收集对应的洗脱液,回收原料8.3g,回收率28%,得无色油状产物(85c)17g,扣除回收原料,收率55%;1hnmr(400mhz,cdcl3)δ7.11(t,j=7.8hz,1h),6.89(d,j=7.6hz,1h),6.81(s,1h),6.69(dd,j=7.8,2.0hz,1h),6.24(s,1h),6.24(s,1h),5.79(s,1h),5.42(d,j=5.2hz,1h),4.05(q,j=7.1hz,2h),3.76(d,j=5.2hz,1h),1.14(t,j=7.1hz,3h),0.95(s,9h),0.15(s,6h);esi-lrmsm/z(%):337.2[m+h]+.
82.32-[1-(3-叔丁基二甲基硅氧基苯基)-1-乙酰氧基甲基]丙烯酸乙酯(85d)的合成
化合物85c(16.6g,0.049mol),dmap(600mg,0.0049mol)和tea(9.9g,0.098mmol)溶于50ml无水dcm中,0℃搅拌条件下,缓慢滴加乙酸酐(10g,0.098mmol),加完后在冰浴中继续搅拌;10min后,tlc(展开剂:石油醚/乙酸乙酯=5:1)显示原料(rf=0.3)完全消失,有新点(rf=0.5)生成,缓慢加入饱和碳酸氢钠水溶液淬灭反应;分液,水层用dcm(3x50ml)萃取,合并有机相,无水硫酸钠干燥3h,拌硅胶柱层析分离纯化,收集对应的洗脱液,减压蒸干溶剂得无色油状化合物(85d)8.9g,收率47%;1hnmr(400mhz,cdcl3)δ7.17(t,j=7.9hz,1h),6.95(d,j=7.6hz,1h),6.83(s,1h),6.78–6.73(m,1h),6.62(s,1h),6.37(s,1h),5.77(s,1h),4.14(q,j=7.1hz,2h),2.07(s,3h),1.20(t,j=7.1hz,3h),0.96(s,9h),0.17(s,6h);13cnmr(150mhz,cdcl3)δ182.1,177.8,168.5,152.9,152.2,142.3,142.2,138.4,133.4,132.8,132.2,85.7,73.7,38.5,33.9,33.8,31.1,26.9,8.4;esi-lrmsm/z(%):401.2[m+na]+;esi-hrmsm/z(%):calcdforc20h34no5si[m+h]+396.2201,found396.2201.
82.4(s)-2-[1-(3-叔丁基二甲基硅氧基苯基)-1-(3,4,5-三甲氧基苯基氨基)甲基]丙烯酸乙酯(85e)的合成
氮气氛围下,三(二亚苄基丙酮)二钯(22mg,0.023mmol)和1e(44mg,0.060mmol)分别加入一schlenk管中,加入无水ch2cl2(5ml),室温下搅拌10分钟后,先后加入底物85d(900mg,2.38mmol),k2co3(1.0m水溶液,7.14ml,7.14mmol)和3,4,5-三甲氧基苯胺(653mg,3.57mmol);室温搅拌,过夜反应后,tlc(展开剂:石油醚/乙酸乙酯=5:1)显示原料(rf=0.5)完全消失,有新点(rf=0.2)生成,用二氯甲烷萃取(3×50ml),无水硫酸钠干燥,过滤浓缩后,柱层析纯化,得淡黄色油状物(85e)1.12g,收率94%;[α]d20=+27.0(c0.16,chcl3);1hnmr(400mhz,cdcl3)δ7.19(t,j=7.9hz,1h),6.96(d,j=7.7hz,1h),6.83(t,j=2.0hz,1h),6.75(dd,j=7.7,2.0hz,1h),6.37(s,1h),5.91(s,1h),5.82(s,2h),5.33(s,1h),4.21–4.12(m,2h),3.76(s,6h),3.75(s,3h),1.23(t,j=7.1hz,3h),0.95(d,j=4.9hz,9h),0.15(s,6h);13cnmr(150mhz,cdcl3)δ166.2,156.0,153.8,143.5,142.1,140.8,130.5,129.7,126.0,120.4,119.4,119.2,91.4,61.1,60.9,59.2,55.9,25.7,18.2,14.1,-4.4;esi-lrmsm/z(%):502.2[m+h]+;esi-hrmsm/z(%):calcdforc27h40no6si[m+h]+502.2620,found502.2619.
以0.005当量的三苯基膦替换1e做配体,其他操作完全相同,可得相应外消旋体。
82.5(s)-1-(3,4,5-三甲氧基苯基)-4-(3-叔丁基二甲基硅氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(85f)的合成
化合物85e(1.094g)和sn[n(tms)2]2(183mg,0.40mmol)加入一schlenk管中,加入无水甲苯(10ml),加热回流6小时后,tlc(展开剂:石油醚/乙酸乙酯=5:1)显示产物与原料极性相近,反应液冷却至室温后,浓缩,柱层析纯化,得无色油状化合物(85f)753mg,收率76%;[α]d20=+44.3(c0.24,chcl3);1hnmr(400mhz,cdcl3)δ7.27–7.20(m,1h),6.99(d,j=7.7hz,1h),6.83–6.78(m,2h),6.57(s,2h),5.81(t,j=1.7hz,1h),5.30(s,1h),5.16–5.15(m,1h),3.75(s,3h),3.71(s,6h),0.91(s,9h),0.11(s,3h),0.10(s,3h);13cnmr(150mhz,cdcl3)δ160.7,156.4,153.6,149.6,138.0,134.8,133.7,130.1,120.7,119.8,118.2,110.7,95.0,63.7,60.9,56.0,25.6,18.2,-4.4,-4.5;esi-lrmsm/z(%):456.1[m+h]+;esi-hrmsm/z(%):calcdforc25h34no5si[m+h]+456.2201,found456.2201.
82.6(s)-1-(3,4,5-三甲氧基苯基)-4-(3-羟基苯基)-3-亚甲基氮杂环丁烷-2-酮(85)的合成
化合物85f(753mg)溶于10mlthf,冷至冰浴下,tbaf(863mg,3.3mmol)溶于3mlthf,由分液漏斗缓慢滴加,滴完后继续搅拌15min后,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料(rf=0.8)完全消失,有新点(rf=0.5)生成,减压旋干,10ml乙酸乙酯溶解,水洗(5mlx2),饱和食盐水洗(5ml),无水硫酸钠干燥3h,过滤浓缩后,柱层析纯化,得淡黄色固体(85)489mg,收率86%;mp150-151℃;[α]d20=+101.5(c0.13,chcl3),98%ee[determinedbyhplcanalysisusingachiralcelad-hcolumn;n-hex/i-proh=80:20,1.0ml/min,254nm;tr(minor)=12.09min;tr(major)=8.43min];1hnmr(400mhz,cdcl3)δ7.59(s,1h),7.22–7.21(m,1h),6.94(d,j=7.6hz,1h),6.88–6.86(m,2h),6.56(s,2h),5.73((t,j=1.7hz,1h),5.31(s,1h),5.14(dd,j=1.7,1.2hz,1h),3.74(s,3h),3.66(s,6h);13cnmr(100mhz,cdcl3)δ161.4,157.4,153.5,148.7,137.7,133.5,130.3,119.1,116.6,113.0,111.5,95.1,64.1,60.9,56.0;esi-lrmsm/z(%):342.1[m+h]+;esi-hrmsm/z(%):calcdforc19h20no5[m+h]+342.1336,found342.1337.。
实施例83(s)-1-(3,4,5-三甲氧基苯基)-4-(3-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(86)的合成
20ml茄型瓶中加入化合物85(20mg,0.059mmol),碳酸钾(9.7mg,0.071mmol)和丙酮(3ml),搅拌条件下慢慢加入硫酸二甲酯(9.6mg,0.077mmol),回流反应0.5h后,tlc(展开剂:石油醚/乙酸乙酯=2:1)显示原料(rf=0.2)完全消失,有新点生成(rf=0.4),停止反应,过滤出去反应液中固体成分,直接拌硅胶加压蒸干,柱层析分离纯化,收集对应的洗脱液,蒸干溶剂得白色固体(86)20mg,收率96%;mp122-123℃;[α]d20=+64.3(c0.29,chcl3);1hnmr(400mhz,cdcl3)δ7.30(t,j=7.7hz,1h),6.99(d,j=7.7hz,1h),6.89(m,2h),6.59(s,2h),5.83(s,1h),5.33(s,1h),5.17(s,1h),3.78(s,3h),3.76(s,3h),3.73(s,6h);13cnmr(150mhz,cdcl3)δ160.2,159.6,152.9,148.8,137.5,134.1,133.2,129.6,118.6,113.6,111.6,110.3,94.2,63.3,60.3,55.4,54.7;esi-lrmsm/z(%):356.0[m+h]+;esi-hrmsm/z(%):calcdforc20h22no5[m+h]+356.1492,found356.1494.。
实施例84(s)-1-(3,4,5-三甲氧基苯基)-4-(4-甲基苯基)-3-亚甲基氮杂环丁烷-2-酮(87)的合成
化合物84的合成与化合物1的合成类似:
84.12-[1-(4-甲基苯基)-1-羟基甲基]丙烯酸乙酯(84b)的合成
在50ml茄形瓶中加入化合物对甲苯甲醛84a(5g,0.042mol),丙烯酸乙酯(4.17g,0.042mol),dabco(4.67g,0.042mol),室温搅拌反应12d后,tlc(展开剂:石油醚/乙酸乙酯=10:1)显示原料(rf=0.5)明显减少,有大量新点生成(rf=0.2),停止反应,用较粗的柱子,直接将反应液倒到硅胶柱上,用少量甲苯洗涤反应瓶再倒到柱子上,然后用pe及pe/ea10/1先除去丙烯酸乙酯,继而进行梯度洗脱,收集对应的洗脱液,蒸干溶剂得无色油状物质(84b)6.7g,收率73%;1hnmr(400mhz,cdcl3)δ7.25(d,j=8.0hz,2h),7.15(d,j=8.0hz,2h),6.32(s,1h),5.83(s,1h),5.52(d,j=5.4hz,1h),4.16(q,j=7.1hz,2h),3.07(d,j=5.4hz,1h),2.33(s,3h),1.24(t,j=7.1hz,3h);esi-lrmsm/z(%):221.1[m+h]+.
84.22-[1-(4-甲基苯基)-1-乙酰氧基甲基]丙烯酸乙酯(84c)的合成
化合物84b(2g,8.93mmol),dmap(108mg,0.893mmol)和tea(1.8g,17.86mmol)溶于15ml无水dcm中,0℃搅拌条件下,缓慢滴加乙酸酐(1.8g,17.86mmol),加完后在冰浴中继续搅拌;10min后,tlc(展开剂:石油醚/乙酸乙酯=10:1)显示原料(rf=0.2)完全消失,有新点(rf=0.4)生成,缓慢加入饱和碳酸氢钠水溶液淬灭反应;分液,水层用dcm(3x15ml)萃取,合并有机相,无水硫酸钠干燥3h,拌硅胶柱层析分离纯化,收集对应的洗脱液,减压蒸干溶剂得无色油状化合物(84c)1.95g,收率83%;1hnmr(400mhz,cdcl3)δ7.28(d,j=7.9hz,2h),7.15(d,j=7.9hz,2h),6.67(s,1h),6.39(s,1h),5.84(s,1h),4.15(q,j=6.6hz,2h),2.33(s,3h),2.09(s,3h),1.22(t,j=7.1hz,3h);esi-lrmsm/z(%):263.1[m+h]+.
84.3(s)-2-[1-(4-甲基苯基)-1-(3,4,5-三甲氧基苯基氨基)甲基]丙烯酸乙酯(84d)的合成
氮气氛围下,三(二亚苄基丙酮)二钯(6mg,0.0064mmol)和1e(12mg,0.016mmol)分别加入一schlenk管中,加入无水ch2cl2(5ml),室温下搅拌10分钟后,先后加入底物84c(167mg,0.64mmol),k2co3(1.0m水溶液,2ml,2mmol)和3,4,5-三甲氧基苯胺(175mg,0.96mmol);室温下搅拌4小时后,tlc(展开剂:石油醚/乙酸乙酯=2:1)显示原料(rf=0.7)完全消失,有新点(rf=0.5)生成,用二氯甲烷萃取(3×5ml),无水硫酸钠干燥,过滤浓缩后,柱层析纯化,得淡黄色油状液体(84d)127mg,收率52%;[α]d20=+88.9(c0.10,chcl3);1hnmr(400mhz,cdcl3)δ7.26(d,j=7.9hz,2h),7.14(d,j=7.9hz,2h),6.38(s,1h),5.95(s,1h),5.82(s,2h),5.35(s,1h),4.21–4.05(m,3h),3.76(s,6h),3.75(s,3h),2.33(s,3h),1.22(t,j=7.1hz,3h);13cnmr(150mhz,cdcl3)δ166.3,153.8,143.6,140.8,137.7,137.5,130.5,129.4,127.3,125.7,91.2,61.1,60.8,59.1,55.9,21.1,14.1;esi-lrmsm/z(%):386.2[m+h]+;esi-hrmsm/z(%):calcdforc22h28no5[m+h]+386.1962,found386.1964.
以0.005当量的三苯基膦替换1e做配体,其他操作完全相同,可得相应外消旋体。
84.4(s)-1-(3,4,5-三甲氧基苯基)-4-(4-甲基苯基)-3-亚甲基氮杂环丁烷-2-酮(84)的合成
化合物84d(127mg,0.33mmol)和sn[n(tms)2]2(218mg,0.50mmol)加入一schlenk管中,加入无水甲苯(5ml),加热回流6小时后,tlc(展开剂:石油醚/乙酸乙酯=2:1)显示原料(rf=0.5)完全消失,有新点(rf=0.45)生成,反应液冷却至室温后,浓缩,柱层析纯化,得无色油状化合物(84)101mg,收率89%;[α]d20=+87.5(c0.12,chcl3),97%ee[determinedbyhplcanalysisusingachiralcelad-hcolumn;n-hex/i-proh=85:15,1.0ml/min,254nm;tr(minor)=9.04min;tr(major)=11.43min];1hnmr(400mhz,cdcl3)δ7.29(d,j=8.0hz,2h),7.19(d,j=8.0hz,2h),6.58(s,2h),5.83(t,j=1.7hz,1h),5.33(s,1h),5.16–5.12(m,1h),3.76(s,3h),3.72(s,6h),2.35(s,3h);13cnmr(150mhz,cdcl3)δ160.9,153.5,149.9,138.9,134.7,133.8,133.5,129.8,126.8,110.7,94.9,63.9,60.9,56.1,21.2;esi-lrmsm/z(%):340.1[m+h]+;esi-hrmsm/z(%):calcdforc20h22no4[m+h]+340.1543,found340.1550.。
实施例85(s)-1-(3,4,5-三甲氧基苯基)-4-(4-异丙基苯基)-3-亚甲基氮杂环丁烷-2-酮(88)的合成
化合物88的合成与化合物1的合成类似:
85.12-[1-(4-异丙基苯基)-1-羟基甲基]丙烯酸乙酯(88b)的合成
在50ml茄形瓶中加入化合物4-异丙基苯甲醛88a(2g,13.5mmol),丙烯酸乙酯(1.35g,13.5mmol),dabco(1.5g,13.5mmol),室温搅拌反应13d后,tlc(展开剂:石油醚/乙酸乙酯=10:1)显示原料(rf=0.7)明显减少,有大量新点生成(rf=0.2),停止反应,用较粗的柱子,直接将反应液倒到硅胶柱上,用少量甲苯洗涤反应瓶再倒到柱子上,然后用pe及pe/ea10/1先除去丙烯酸乙酯,继而进行梯度洗脱,收集对应的洗脱液,回收原料0.6g,回收率30%,得无色油状产物(88b)1.1g,扣除回收原料,收率47%;1hnmr(400mhz,cdcl3)δ7.29(d,j=8.2hz,2h),7.20(d,j=8.2hz,2h),6.33(s,1h),5.83(t,j=1.1hz,1h),5.54(s,1h),4.17(q,j=7.1hz,2h),2.99(s,1h),2.89(dt,j=13.8,6.9hz,1h),1.26–1.23(m,9h);esi-lrmsm/z(%):249.1[m+h]+.
85.22-[1-(4-异丙基苯基)-1-乙酰氧基甲基]丙烯酸乙酯(88c)的合成
化合物88b(1.1g,4.44mmol),dmap(54mg,0.444mmol)和tea(0.9g,8.88mmol)溶于15ml无水dcm中,0℃搅拌条件下,缓慢滴加乙酸酐(0.9g,8.88mmol),加完后在冰浴中继续搅拌;10min后,tlc(展开剂:石油醚/乙酸乙酯=10:1)显示原料(rf=0.2)完全消失,有新点(rf=0.4)生成,缓慢加入饱和碳酸氢钠水溶液淬灭反应;分液,水层用dcm(3x15ml)萃取,合并有机相,无水硫酸钠干燥3h,拌硅胶柱层析分离纯化,收集对应的洗脱液,减压蒸干溶剂得无色油状化合物(88c)946mg,收率74%;1hnmr(400mhz,cdcl3)δ7.28(d,j=7.9hz,2h),7.15(d,j=7.9hz,2h),6.67(s,1h),6.39(s,1h),5.84(s,1h),4.25–4.00(m,2h),2.33(s,3h),2.09(s,3h),1.22(t,j=7.1hz,3h);esi-lrmsm/z(%):291.1[m+h]+.
85.3(s)-2-[1-(4-异丙基基苯基)-1-(3,4,5-三甲氧基苯基氨基)甲基]丙烯酸乙酯(88d)的合成
氮气氛围下,三(二亚苄基丙酮)二钯(4.7mg,0.0052mmol)和1e(9.3mg,0.0129mmol)分别加入一schlenk管中,加入无水ch2cl2(5ml),室温下搅拌10分钟后,先后加入底物88c(150mg,0.52mmol),k2co3(1.0m水溶液,1.5ml,1.5mmol)和3,4,5-三甲氧基苯胺(143mg,0.78mmol);室温下搅拌24小时后,tlc(展开剂:石油醚/乙酸乙酯=5:1)显示原料(rf=0.7)完全消失,有新点(rf=0.3)生成,用二氯甲烷萃取(3×5ml),无水硫酸钠干燥,过滤浓缩后,柱层析纯化,得淡黄色油状物(88d)210mg,收率98%;[α]d20=+67.5(c0.17,chcl3);1hnmr(400mhz,cdcl3)δ7.28(d,j=8.2hz,2h),7.19(d,j=8.2hz,2h),6.38(s,1h),5.97(s,1h),5.82(s,2h),5.35(s,1h),4.19–4.13(m,2h),3.76(s,6h),3.75(s,3h),2.95–2.82(m,1h),1.26–1.19(m,9h);13cnmr(150mhz,cdcl3)δ166.4,153.8,148.5,143.6,140.8,138.0,130.4,127.4,126.8,125.6,91.2,61.1,60.8,59.1,55.9,33.8,24.0,14.1;esi-lrmsm/z(%):414.1[m+h]+.
以0.005当量的三苯基膦替换1e做配体,其他操作完全相同,可得相应外消旋体。
85.4(s)-1-(3,4,5-三甲氧基苯基)-4-(4-异丙基苯基)-3-亚甲基氮杂环丁烷-2-酮(88)的合成
化合物88d(110mg)和sn[n(tms)2]2(140mg,0.32mmol)加入一schlenk管中,加入无水甲苯(5ml),加热回流3.5小时后,tlc(展开剂:石油醚/乙酸乙酯=3:1)显示原料(rf=0.5)完全消失,有新点(rf=0.45)生成,反应液冷却至室温后,浓缩,柱层析纯化,得白色固体(88)21mg,收率21%;mp99-100℃;[α]d20=+47.7(c0.11,chcl3);92%ee[determinedbyhplcanalysisusingachiralcelad-hcolumn;n-hex/i-proh=75:25,1.0ml/min,254nm;tr(minor)=5.30min;tr(major)=6.59min];1hnmr(400mhz,cdcl3)δ7.32(d,j=8.2hz,2h),7.23(d,j=8.2hz,2h),6.58(s,2h),5.83(t,j=1.7hz,1h),5.34(s,1h),5.19–5.15(m,1h),3.76(s,3h),3.71(s,6h),2.89(dq,j=13.7,6.9hz,1h),1.24(s,3h),1.22(s,3h);13cnmr(150mhz,cdcl3)δ161.0,153.5,149.9,149.8,134.7,133.9,133.8,127.2,126.9,110.7,95.0,64.0,60.9,56.0,33.9,23.9;esi-lrmsm/z(%):368.2[m+h]+;esi-hrmsm/z(%):calcdforc20h26no4[m+h]+368.1856,found368.1859.。
实施例86(3r,4s)-1-(3,4,5-三甲氧基苯基)-4-(3-丙烯酰氧基-4-甲氧基苯基)-3-丙烯酰氧基-3-丙烯酰氧甲基氮杂环丁烷-2-酮(89)的合成
在100ml茄形瓶中加入化合物80(21mg,0.052mmol),丙烯酰氯(42μl,0.52mmol),三乙胺(70μl,0.52mmol),dmap1mg与无水dcm1ml,室温反应0.5小时;tlc(展开剂:pe/ea=1:2)显示原料点(rf=0.3)消失,生成新点(rf=0.95);反应液加水,用ea萃取三次,有机相合并后用饱和食盐水洗一次,na2so4干燥;蒸干,柱层析分离(展开剂:pe/ea=2:1),得到白色固体(89)14mg,收率48%;1hnmr(400mhz,cdcl3)δ7.28(d,j=10.4hz,1h),7.20(s,1h),6.98(d,j=8.5hz,1h),6.65–6.51(m,4h),6.43–6.17(m,3h),6.13–5.96(m,3h),5.84(d,j=10.5hz,1h),5.38(s,1h),4.42(s,2h),3.83(s,3h),3.79(s,3h),3.73(s,6h);;13cnmr(150mhz,cdcl3)δ164.5,164.3,163.0,160.7,153.0,151.1,139.2,134.5,133.0,132.3,132.2,131.2,126.9,126.8,126.5,125.2,123.6,121.8,112.0,94.9,88.0,64.1,60.3,55.5,55.4.。
实施例87(3s,4s)-1-(3,4,5-三甲氧基苯基)-4-(3-烯丙酰氧基-4-甲氧基苯基)-3-烯丙酰氨基氮杂环丁烷-2-酮(90)的合成
在100ml茄形瓶中加入化合物76(17mg,0.045mmol),丙烯酰氯(11μl,0.13mmol),三乙胺(19μl,0.13mmol),dmap1mg与无水dcm1ml,室温反应3小时;tlc(展开剂:pe/ea=1:3)显示原料点(rf=0.2)消失,生成新点(rf=0.95);反应液加水,用ea萃取三次,有机相合并后用饱和食盐水洗一次,na2so4干燥;蒸干,柱层析分离(展开剂:pe/ea=2:1),得到白色固体(90)15mg,收率69%;1hnmr(400mhz,cdcl3)δ7.24(dd,j=8.5hz,2.1hz,1h),7.12(d,j=2.1hz,1h),6.99(d,j=8.5hz,1h),6.71(d,j=6.8hz,1h),6.59(d,j=17.3hz,1h),6.51(s,2h),6.40–6.25(m,2h),6.15(dd,j=17.0,10.3hz,1h),6.02(d,j=10.4hz,1h),5.72(d,j=10.4hz,1h),5.02(d,j=2.2hz,1h),4.57(dd,j=6.8,2.2hz,1h),3.82(s,3h),3.76(s,3h),3.70(s,6h);13cnmr(150mhz,cdcl3)δ165.8,163.9,163.7,153.5,151.6,140.1,134.8,133.4,132.9,129.4,128.8,128.3,127.4,124.7,121.1,113.1,95.2,66.2,62.5,60.9,56.1.。
实施例88(s)-1-(3,4,5-三甲氧基苯基)-2-(3-羟基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷(91)的合成
向50mlschlenk管中加入无水thf3ml,冰浴,氮气保护,缓缓加入三氯化铝(36mg,0.27mmol),四氢锂铝(10mg,0.27mmol),氮气保护下逐渐升至室温反应1小时;加入化合物1(10mg,0.027mmol),室温反应3h后,tlc(展开剂:pe/ea=2:1)显示生成新点(rf=0.46);反应液加饱和氯化铵,用乙酸乙酯萃取三次,有机相合并后用饱和食盐水洗一次,na2so4干燥;柱层析分离(展开剂:pe/ea=5:1),得到白色固体(91)5mg,收率51%;1hnmr(400mhz,cdcl3)δ6.79(d,j=1.1hz1h),6.73(dd,j=8.1,1.1hz,1h),6.63(d,j=8.1hz,1h),5.94(s,2h),5.19(s,1h),4.92(s,1h),4.73(s,1h),3.89–3.74(m,14h).。
实施例89(3s,4r)-2-(3,4,5-三甲氧基苯基)-3-(3-乙酰氧基-4-甲氧基苯基)-2-氮杂-5,7-二氧杂螺[3.4]辛烷-1,6-二酮(92)的合成
在100ml茄形瓶中加入化合物82(18mg,0.04mmol),4-二甲氨基吡啶0.5mg,三乙胺(14μl,0.1mmol)与二氯甲烷5ml,草酰氯(5μl,0.058mmol),室温反应3小时;tlc(展开剂:pe/ea=1:4)显示原料点(rf=0.2)消失,生成新点(rf=0.9);反应液加饱和nahco3,用ea萃取三次,有机相合并后用饱和食盐水洗一次,na2so4干燥;柱层析分离(展开剂:pe/ea=1:1),得到白色固体(92)12mg,收率63%;1hnmr(400mhz,cdcl3)δ7.26(d,j=1.0hz,1h),7.15–7.00(m,2h),6.53(s,2h),5.33(s,1h),4.55(d,j=9.9hz,1h),4.05(d,j=9.9hz,1h),3.86(s,3h),3.78(s,3h),3.72(s,6h),2.30(s,3h);13cnmr(101mhz,cdcl3)δ168.8,160.4,153.9,152.8,152.7,141.0,135.9,132.1,123.4,113.7,96.0,90.3,67.1,64.5,61.2,56.4,56.3.。
实施例90(s)-1-(3,4,5-三甲氧基苯基)-4-(3-乙酰氧基-4-甲氧基苯基)氮杂环丁烷-2,3-二酮(93)的合成
在100ml茄形瓶中加入化合物82(0.25g,0.56mmol),高碘酸钠(0.18g,0.84mmol),甲醇4ml与水1ml,室温反应3小时;tlc(展开剂:pe/ea=1:1)显示原料点(rf=0)消失,生成新点(rf=0.2);反应液加水,用ea萃取三次,有机相合并后用饱和食盐水洗一次,na2so4干燥;柱层析分离(展开剂:pe/ea=1:1),得到黄色固体(93)0.18g,收率80%;1hnmr(400mhz,cdcl3)δ7.17(dd,j=8.5,2.2hz,1h),7.04(d,j=2.2hz,1h),6.99(d,j=8.5hz,1h),6.74(s,2h),5.49(s,1h),3.83(s,3h),3.81(s,3h),3.76(s,6h),2.30(s,3h);13cnmr(101mhz,dmso)δ209.3,191.0,168.8,160.5,153.9,152.4,140.6,136.6,132.6,125.13,124.2,121.7,113.3,96.1,74.7,61.2,56.4,56.3,20.9.。
实施例91(3r,4s)-1-(3,4,5-三甲氧基苯基)-3-甲基-3-羟基-4-(3-乙酰氧基-4-甲氧基苯基)氮杂环丁烷-2-酮(94)的合成
向50mlschlenk管中加入化合物93(10mg,0.024mmol),抽换三次氮气,加入无水thf1ml,冰浴下加入mgcl(3minthf)(1.2μl,0.036mmol),氮气保护下,室温反应1h;tlc(展开剂:pe/ea=1:2)显示生成新点(rf=0.2);加水,ea萃取,饱和食盐水洗,硫酸钠干燥,柱层析分离(展开剂:pe/ea=1:1),得到白色固体(94)6mg,收率58%;1hnmr(400mhz,cdcl3)δ7.17(dd,j=8.5,2.1hz,1h),7.02-7.00(m,2h),6.59(s,2h),4.90(s,1h),3.84(s,3h),3.78(s,3h),3.73(s,6h),2.30(s,3h),1.70(s,3h).。
实施例92(3r,4s)-1-(3,4,5-三甲氧基苯基)-3-甲基-3-羟基-4-(3-羟基-4-甲氧基苯基)氮杂环丁烷-2-酮(95)的合成
在100ml茄形瓶中加入化合物94(6mg,0.014mmol),70%水合肼(1.5μl,0.03mmol),meoh1ml,室温反应1小时;tlc(展开剂:pe/ea=1:3)显示原料点(rf=0.4)消失,生成新点(rf=0.3);反应液加水,用ea萃取三次,有机相合并后用饱和食盐水洗一次,na2so4干燥;柱层析分离(展开剂:pe/ea=1:2),得到白色固体(95)5mg,收率96%;1hnmr(400mhz,cdcl3)δ6.84(d,j=1.5hz,1h),6.83(d,j=8.3hz,1h),6.76(dd,j=8.3,1.5hz,1h),6.57(s,2h),5.61(brs,1h),4.78(s,1h),3.89(s,3h),3.78(s,3h),3.72(s,6h),1.69(s,3h).;esi-lrmsm/z(%):390.1[m+h]+.。
实施例93(s)-1-(3,4,5-三甲氧基苯基)-4-(3-对甲氧基苯甲酰氧基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(96)的合成
化合物1(20mg,0.054mmol)溶于2mldcm中,加入tea(15μl,0.108mmol),对甲氧基苯甲酸(17mg,0.108mmol),edci(21mg,0.108mmol),dmap(1mg,0.008mmol),室温反应0.5h,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料1(rf=0.3)完全消失,有新点生成(rf=0.4),停止反应(1ml水淬灭),加入10mldcm,以水(2x10ml)洗涤,饱和食盐水(10ml)洗,无水硫酸钠干燥3h,拌硅胶柱层析分离纯化,收集对应的洗脱液,蒸干溶剂得无色油状液体(96)25mg,收率93%;[α]d20=+31.7(c1.0,chcl3);1hnmr(cdcl3,400mhz)δ:7.95(d,j=8.7hz,1h),7.11~7.05(m,3h),6.84~6.78(m,3h),6.43(s,2h),5.68(s,1h),5.15(s,1h),5.04(s,1h),3.71(s,3h),3.63(s,3h),3.59~3.58(m,9h);13cnmr(cdcl3,150mhz)δ:163.5,163.3,160.2,153.0,151.3,149.0,139.9,134.1,133.1,131.8,128.3,124.5,121.4,120.8,113.2,112.3,110.4,94.2,62.8,60.3,55.5,55.4,54.9.ms(esi)m/z(%):506.1[m+h]+;hrms(esi)calcdforc28h27nnao8[m+na]+528.1629,found528.1631.。
实施例94(3s,4r)-1-(3,4,5-三甲氧基苯基)-4-(3-羟基-4-甲氧基苯基)-3-甲基氮杂环丁烷-2-酮(97)的合成
化合物69(210mg,0.54mmol)溶于5mlmecn中,加入碳酸钾(112mg,0.81mmol),氯化苄(75μl,0.65mmol),回流反应8h,tlc(展开剂:pe/ea=1:3)显示原料点(rf=0.22)消失,生成新点(rf=0.5);反应液加水,用ea萃取三次,有机相合并后用饱和食盐水洗一次,na2so4干燥;蒸干,溶于5ml无水thf中,加入四溴化碳(537mg,1.62mmol),三苯基膦(425mg,1.62mmol),室温反应4h,tlc(展开剂:pe/ea=1:1)显示原料点(rf=0.1)消失,生成新点(rf=0.5);反应液加水,用ea萃取三次,有机相合并后用饱和食盐水洗一次,na2so4干燥;蒸干,溶于5ml乙醇中,加入无水醋酸钠(132mg,1.62mmol),10%钯碳(20mg),常压通氢气室温反应8h,tlc(展开剂:pe/ea=1:1)显示原料点(rf=0.5)消失,生成新点(rf=0.4);反应液过滤除去钯碳,拌硅胶柱层析分离纯化,收集对应的洗脱液,蒸干得到白色固体64mg,收率32%;熔点:54–55℃;[α]d20=+12.8(c0.5,chcl3).1hnmr(400mhz,cdcl3)δ6.93(d,j=1.5hz,1h),6.87(dd,j=8.3,1.5hz,1h),6.83(d,j=8.3hz,1h),6.54(s,2h),5.69(s,1h),4.44(d,j=2.2hz,1h),3.89(s,3h),3.76(s,3h),3.72(s,6h),3.11(qd,j=7.3,2.2hz,1h),1.45(d,j=7.3hz,3h).13cnmr(150mhz,cdcl3)δ167.7,152.9,146.1,145.6,133.7,133.5,130.5,117.1,111.4,110.3,94.1,62.2,60.3,55.4,54.5,12.4.esi-ms(m/z):374.0(m+h+).。
实施例95化合物(r)-1-(3,4,5-三甲氧基苯基)-4-(3-羟基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(98)的合成
95.1(r)-2-[1-(3-叔丁基二甲基硅氧基-4-甲氧基苯基)-1-(3,4,5-三甲氧基苯基氨基)甲基]丙烯酸乙酯(98b)的合成
氮气氛围下,三(二亚苄基丙酮)二钯(11mg,0.012mmol)和98a(21mg,0.031mmol)分别加入一schlenk管中,加入无水ch2cl2(5ml),室温下搅拌10分钟后,先后加入底物1d(0.5g,1.23mmol),k2co3(1.0m水溶液,2.1ml,2.1mmol)和3,4,5-三氧基苯胺(0.34g,0.26mmol);室温下搅拌2h后,tlc(展开剂:石油醚/乙酸乙酯=9:1)显示原料(rf=0.5)完全消失,有新点(rf=0.2)生成,用二氯甲烷萃取(3×10ml),无水硫酸钠干燥,过滤浓缩后,柱层析纯化,得黄色油状物(98b)0.62g,收率95%.
95.2(r)-1-(3,4,5-三甲氧基苯基)-4-(3-叔丁基二甲基硅氧基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(98c)的合成
化合物98b(0.62g)和sn[n(tms)2]2(0.76g,1.77mmol)加入一schlenk管中,加入无水甲苯(20ml),加热回流8小时后,tlc(展开剂:石油醚/乙酸乙酯=3:1)显示原料(rf=0.8)消失,有新点(rf=0.5)生成,反应液冷却至室温后,浓缩,柱层析纯化,得无色油状化合物(98c)0.52g,收率93%.
95.3(r)-1-(3,4,5-三甲氧基苯基)-4-(3-羟基-4-甲氧基苯基)-3-亚甲基氮杂环丁烷-2-酮(98)的合成
化合物98c(0.52g,1.1mmol)溶于15mlthf,冷至冰浴下,tbaf(0.42g,1.6mmol)溶于5mlthf,缓慢滴加,滴完后继续搅拌15min后,tlc(展开剂:石油醚/乙酸乙酯=1:1)显示原料(rf=0.7)完全消失,有新点(rf=0.5)生成,减压旋干,20ml乙酸乙酯溶解,水洗(20mlx2),饱和食盐水洗(20ml),无水硫酸钠干燥。过滤浓缩后,柱层析纯化,得无色液体(98)0.29g,收率73%。[α]d20=-37.9(c0.77,chcl3);1hnmr(400mhz,cdcl3):δ6.93(d,j=1.7hz,1h),6.87(dd,j=8.2,1.7hz,1h),6.82(d,j=8.2hz,1h),6.58(s,2h),5.79(s,1h),5.26(s,1h),5.13(s,1h),3.84(s,3h),3.73(s,3h),3.71(s,6h).13cnmr(150mhz,cdcl3):δ160.3,152.9,149.2,146.5,145.6,134.1,133.2,128.9,118.1,112.3,110.3,110.0,94.31,63.0,60.3,55.5,55.4.esi-ms(m/z):372.1(m+h+).esi-hrms(m/z):calcdforc20h21no6+h+[m+h]+,372.1442;found,372.1441.。
实施例96(3s,4s)-1-(3,4,5-三甲氧基苯基)-4-(3-羟基-4-甲氧基苯基)-3-羟甲基氮杂环丁烷-2-酮(99)和(3s,4r)-1-(3,4,5-三甲氧基苯基)-4-(3-羟基-4-甲氧基苯基)-3-羟甲基氮杂环丁烷-2-酮(100)的合成
氮气保护下下,在50mlschlenk中加入化合物98(220mg,0.6mmol),联硼酸频哪醇酯(0.2g,0.78mmol),cucl(6mg,0.06mmol),三苯基膦(20mg,0.076mmol),甲醇(30μl,0.74mmol),叔丁醇锂(6mg,0.074mmol)与无水thf3ml,室温反应12小时;tlc(展开剂:pe/ea=1:2)显示仅原料点(rf=0.4)等高处有点;反应液加水,用ea萃取三次,有机相合并后用饱和食盐水洗一次,na2so4干燥;蒸干,加入nabo3·4h2o(0.46g,3mmol),thf2ml和水1ml,室温反应2h,tlc(展开剂:pe/ea=1:3)显示原料点(rf=0.9)消失,生成两新点(rf=0.17,0.22);反应液加水,用ea萃取三次,有机相合并后用饱和食盐水洗一次,na2so4干燥;蒸干,柱层析分离(展开剂:pe/ea=1:1),得到反式产物为无色液体(99)48mg,收率21%;[α]d20=-80.1(c1.68,chcl3);1hnmr(400mhz,cdcl3)δ6.97(d,j=1.5hz,1h),6.91(dd,j=8.2hz,1.5hz,1h),6.84(d,j=8.2hz,1h),6.56(s,2h),5.70(s,1h),4.91(d,j=1.8hz,1h),4.15(dd,j=12.1,4.5hz,1h),4.00(dd,j=12.1,3.7hz,1h),3.90(s,3h),3.79–3.75(m,4h),3.73(s,6h),3.28(brd,j=2.3hz,1h);13cnmr(151mhz,cdcl3)δ165.60,153.50,146.78,146.32,134.57,133.72,130.77,117.94,112.16,110.99,94.91,62.14,60.93,58.89,57.49,56.06;得到顺式产物(100)为白色固体95mg,收率41%;[α]d20=-116.9(c1.0,chcl3);1hnmr(400mhz,cdcl3)δ6.90(d,j=1.2hz,1h),6.87–6.81(m,2h),6.55(s,2h),5.75(s,1h),5.16(d,j=5.3hz,1h),3.90(s,3h),3.86–3.75(m,5h),3.73(s,6h),3.64(dd,j=11.3,7.8hz,1h);13cnmr(151mhz,cdcl3)δ165.18,153.56,146.73,146.13,134.68,133.60,127.13,118.47,112.90,110.93,95.06,60.96,58.15,57.27,56.72,56.15,55.98.。
实施例97(3s,4s)-1-(3,4,5-三甲氧基苯基)-4-(3-羟基-4-甲氧基苯基)-3-甲基氮杂环丁烷-2-酮(101)的合成
在100ml茄形瓶中加入化合物981(15mg,0.04mmol),10%pd/c3mg,甲醇1ml常压氢气下,室温反应12小时,tlc(展开剂:pe/ea=2:1)显示原料点(rf=0.4)消失,生成新点(rf=0.33);过滤除去pd/c后蒸干,柱层析分离(展开剂:pe/ea=2:1),得到白色固体(101)13mg,收率95%;[α]d20=-102.8(c1.64,chcl3);1hnmr(400mhz,cdcl3)δ6.86–6.77(m,2h),6.72(d,j=8.3hz,1h),6.56(s,2h),5.71(s,1h),5.06(d,j=5.8hz,1h),3.89(s,3h),3.77(s,3h),3.72(s,6h),3.66-3.59(m,1h),0.91(d,j=7.6hz,3h).。
实施例98(3r,4s)-1-(3,4,5-三甲氧基苯基)-4-(3-羟基-4-甲氧基苯基)-3-甲基氮杂环丁烷-2-酮(102)的合成
化合物99(30mg,0.078mmol)溶于2mlmecn中,加入碳酸钾(16mg,0.12mmol),氯化苄(10μl,0.09mmol),回流反应8h,tlc(展开剂:pe/ea=1:3)显示原料点(rf=0.22)消失,生成新点(rf=0.5);反应液加水,用ea萃取三次,有机相合并后用饱和食盐水洗一次,na2so4干燥;蒸干,溶于2ml无水thf中,加入四溴化碳(77mg,0.23mmol),三苯基膦(61mg,0.23mmol),室温反应4h,tlc(展开剂:pe/ea=1:1)显示原料点(rf=0.1)消失,生成新点(rf=0.5);反应液加水,用ea萃取三次,有机相合并后用饱和食盐水洗一次,na2so4干燥;蒸干,溶于5ml乙醇中,加入无水醋酸钠(19mg,0.23mmol),10%钯碳(3mg),常压通氢气室温反应8h,tlc(展开剂:pe/ea=1:1)显示原料点(rf=0.5)消失,生成新点(rf=0.4);反应液过滤除去钯碳,拌硅胶柱层析分离纯化,收集对应的洗脱液,蒸干得到白色固体9mg,收率32%;熔点:54–55℃;[α]d20=+14.5(c0.65,chcl3).1hnmr(400mhz,cdcl3)δ6.93(d,j=1.5hz,1h),6.87(dd,j=8.3,1.5hz,1h),6.83(d,j=8.3hz,1h),6.54(s,2h),5.69(s,1h),4.44(d,j=2.2hz,1h),3.89(s,3h),3.76(s,3h),3.72(s,6h),3.11(qd,j=7.3,2.2hz,1h),1.45(d,j=7.3hz,3h).13cnmr(150mhz,cdcl3)δ167.7,152.9,146.1,145.6,133.7,133.5,130.5,117.1,111.4,110.3,94.1,62.2,60.3,55.4,54.5,12.4.esi-ms(m/z):374.0(m+h+).。
实施例99(3r,4r)-1-(3,4,5-三甲氧基苯基)-4-(3-氨基-4-甲氧基苯基)-3-甲基氮杂环丁烷-2-酮(103)的合成
在100ml茄形瓶中加入化合物41(20mg,0.05mmol),10%pd/c3mg,甲醇1ml常压氢气下,室温反应12小时,tlc(展开剂:pe/ea=2:1)显示原料点(rf=0.4)消失,生成新点(rf=0.3);过滤除去pd/c后蒸干,柱层析分离(展开剂:pe/ea=2:1),得到白色固体(103)17mg,收率85%;熔点:75–76℃;[α]d20=+120.7(c1.0,chcl3).1hnmr(400mhz,cdcl3)δ6.74(d,j=8.1hz,1h),6.61–6.52(m,4h),5.01(d,j=5.8hz,1h),3.84(s,3h),3.76(s,3h),3.71(s,6h),3.59(dq,j=7.6,5.8hz,1h),0.91(d,j=7.6hz,3h).13cnmr(100mhz,cdcl3):δ168.8,153.9,146.5,146.2,134.0,132.2,128.2,118.6,113.5,110.0,95.1,61.3,58.8,56.9,56.4,49.5,29.8,9.7.esi-ms(m/z):373.1(m+h+).。
实施例100(3s,4r)-1-(3,4,5-三甲氧基苯基)-4-(3-氨基-4-甲氧基苯基)-3-甲基氮杂环丁烷-2-酮(104)的合成
在100ml茄形瓶中加入化合物41f(100mg,0.19mmol),(s)-ir-phox10mg,二氯甲烷5ml常压氢气下,室温反应12小时;反应液加水,用二氯甲烷萃取3次,有机相合并后用饱和食盐水洗一次,na2so4干燥,蒸干后和sn[n(tms)2]2(100mg,0.33mmol)加入schlenk管中,加入无水甲苯(5ml),加热回流3小时,待反应液冷却至室温后,柱层析纯化,得无色固体(104)46mg,收率65%;熔点:62–64℃;[α]d20=-19.1(c1.0,chcl3).1hnmr(400mhz,cdcl3)δ6.82(d,j=8.0hz,1h),6.65–6.55(m,4h),4.50(d,j=2.1hz,1h),3.88(s,3h),3.79(s,3h),3.70(s,6h),3.32(qd,j=7.1,2.1hz,1h),1.41(d,j=7.1hz,3h).13cnmr(150mhz,cdcl3)δ167.8,152.8,146.0,145.6,133.0,133.8,130.3,117.0,111.5,110.4,94.2,62.1,60.5,55.5,54.1,12.3.esi-ms(m/z):373.1(m+h+).。
实施例101(s)-5-(3,4,5-三甲氧基苯基)-6-(3-羟基-4-甲氧基苯基)-5-氮杂螺[2.3]己烷-4-酮(105)的合成
在100ml茄形瓶中加入化合物1g(390mg,0.81mmol),tmsch2n2(1.2ml,2minhexane,2.4mmol),二氯甲烷5ml,室温反应12小时;反应液加水,用二氯甲烷萃取3次,有机相合并后用饱和食盐水洗一次,na2so4干燥,蒸干后加入thf5ml,tbaf(210mg,0.81mmol),室温反应3小时;反应液加水,用乙酸乙酯萃取3次,有机相合并后用饱和食盐水洗一次,na2so4干燥,柱层析纯化,得黄色固体(105)160mg,收率53%;熔点:120–122℃;[α]d20=+63.2(c1.0,chcl3).1hnmr(400mhz,cdcl3)δ6.92(dd,j=8.1,2.0hz,1h),6.88(d,j=8.1hz,1h),6.77(d,j=2.0hz,1h),4.72(s,1h),3.85(s,3h),3.76(s,3h),3.68(s,6h),2.15–2.08(m,2h),1.45–1.38(m,2h).13cnmr(150mhz,cdcl3)δ167.6,152.6,146.2,145.8,133.1,133.8,130.4,117.5,111.0,110.5,94.1,62.2,60.4,55.5,54.0,22.9,18.1,16.3.esi-ms(m/z):386.1(m+h+).。
实施例102(3r,4r)-1-(3,4,5-三甲氧基苯基)-4-(3-羟基-4-甲氧基苯基)-3-乙基氮杂环丁烷-2-酮(106)的合成
在100ml茄形瓶中加入化合物105(20mg,0.05mmol),10%pd/c3mg,甲醇1ml,常压氢气下,室温反应12小时;过滤除去pd/c后蒸干,柱层析分离(展开剂:pe/ea=2:1),得到白色固体(106)15mg,收率75%;熔点:85–87℃;[α]d20=+130.3(c1.0,chcl3).1hnmr(400mhz,cdcl3)δ6.85–6.78(m,2h),6.70(d,j=8.1hz,1h),6.60(s,2h),5.01(d,j=5.7hz,1h),3.88(s,3h),3.76(s,3h),3.70–3.63(m,7h),1.24–1.20(m,1h),0.87(d,j=7.5hz,3h)..13cnmr(150mhz,cdcl3)δ167.6,152.8,147.5,146.4,133.4,133.6,130.1,117.5,111.2,110.4,94.0,62.3,61.4,55.5,54.2,22.7,19.0,18.2,16.6.esi-ms(m/z):388.1(m+h+).。
实施例103目标化合物体外抑制人肿瘤细胞增殖活性检测实验
肿瘤细胞接种于96孔板中于37℃,5%co2的环境下培养24小时,然后加入6个不同浓度的样品,并以阳性样品紫杉醇、ca-4为阳性对照。继续培养48小时后,加入mtt,4小时后弃去上清液,加入dmso溶解紫色结晶,在酶标仪上540nm处测得od值,并计算抑制率;化合物半数抑制浓度ic50数值,根据6个浓度的抑制率计算所得。
表1.二芳基-β-内酰胺类目标化合物抑制肿瘤细胞增殖活性(ic50,μm)
其中:抗肿瘤活性由mtt法测定,数据均是三次测量的平均值;a2780和skov-3是人卵巢癌细胞株;mda-mb-231是人乳腺癌细胞株;hela是人宫颈癌细胞株。
实施例104抑制微管蛋白聚集实验:体外微管蛋白自组装实验
采用浊度法检测受试化合物69、70和97对体外微管聚集的抑制作用,检测试剂盒购自美国cytoskeleton,inc.。按如下步骤,微管聚集体系内含0.1mpipes,ph=6.6,10mmmgcl2,1mmgtp,1mmegta,3.4m甘油,反应液先在冰上预孵育,加入不同浓度的受试化合物,并设置dmso(4%,v/v)组作为阴性对照组,colchicine处理组作为阳性对照,加入10mm微管蛋白后,立刻置于37℃进行聚集反应,保持37℃,并用分光光度计(synergyh4hybrid)在340nm下每隔1min测定吸光度,共测30min,根据吸光度绘制曲线图(如图1所示);结果显示化合物69、70和97能明显抑制微管聚集,ic50分别为3.5、1.7和1.6μm。
表2为化合物69、70和97的微管聚集抑制活性(ic50,μm)
表2
实施例105抑制微管蛋白聚集实验:免疫印迹分析
hela细胞用不同浓度的受试化合物处理6小时,并设置dmso组作为阴性对照组,收集细胞并用pbs洗涤2次后用含有微管稳定剂的细胞裂解液消化(含有100mmpipes,ph6.8,1mmmgcl2,2mmegta,0.5%np-40,2mglycerol,5μm紫杉醇和蛋白激酶抑制剂),消化液用15000转/分离心15分钟,吸出上清液,并将沉淀溶解于sds消化缓冲液;分别取等量上清和沉淀的蛋白样品经sds-page电泳后转移至pvdf膜,用含5%bsa的tbst缓冲液固定后,加入α-微管蛋白一抗,随后加入hrp标记的二抗进行孵育,最后通过化学发光试剂进行显色,结果显示化合物1、69、70和97能明显抑制微管聚集,使微管蛋白维持解聚状态(如图2所示)。
实施例106抑制微管蛋白聚集实验:免疫荧光检测微管蛋白形态实验
培养肿瘤细胞,将细胞接种到预先处理(2mnaoh浸泡2h,75%乙醇浸泡30min)过的盖玻片上,待细胞贴壁后,将盖玻片置于24孔板中,加入培养基,常规培养24h后,加入不同浓度的受试化合物处理24h,同时设置dmso处理组作为阴性对照;弃去培养基,细胞用pbs洗涤2次,加入甲醇固定15min,pbs洗涤3次,用0.1%triton通透15min,pbs洗涤3次,用5%bsa室温封闭1h,加入一抗4℃孵育过夜,pbst漂洗3次,每次5min;加入荧光二抗,室温避光孵育1h,pbst漂洗3次,每次5min。滴加封片剂一滴(含dapi)封片,用共聚焦显微镜观察微管蛋白的形态,检测受试化合物对微管结构的影响,并拍摄相关照片,结果显示化合物1、69、70和97能明显抑制微管的聚集(如图3所示)。
实施例107抑制血管生成实验
将细胞外基质胶matrigel(bdbiosciences,美国)用pbs按1:1稀释混匀后,加入24孔板。置于37℃、5%co2培养箱中孵育1小时,待凝胶形成后,将huvec细胞按浓度3×104个/孔接种到凝胶上,加入含10%pbs的dmem培养基,并加入测试样品,同时设置ca-4为阳性对照,dmso为阴性对照,于37℃、5%co2培养箱中培养12h,用倒置相差显微镜下观察毛细血管形成情况,并拍摄相关照片,结果显示化合物69、70和97能明显抑制huvec细胞生成毛细血管样结构(如图4所示)。
实施例108基质胶塞实验
在含有100ng/ml人源重组vegf-a165的基质胶中,在4℃加入测试化合物,同时设置dmso处理组作为阴性对照。然后将此混合物按0.5ml/只,在6周大的balb/c裸鼠(n=4)的背部皮下注射以形成基质胶塞。2周后处死老鼠,回收基质胶塞,拍摄照片,结果显示化合物69、70和97能明显抑制由vegf介导的新生血管生成(如图5所示)。
实施例109菌落抑制实验
6孔板中,每孔种1000个细胞,待细胞贴壁后,加入不同浓度的受试样品处理细胞48h,并设阳性对照组(ca-4)、阴性对照组(空白溶剂),更换新鲜培养基继续培养7-10天后,弃去培养基,细胞用甲醇固定,并用giemsa或结晶紫染料染色,显微镜下计数大于50个细胞形成的集落数,结果显示化合物69、70和97能明显抑制肿瘤细胞菌落的形成(如图6所示)。
实施例110体外细胞周期实验
6孔板中,按2×105个/孔的数量接种细胞,待细胞贴壁后,用不同浓度的受试样品处理细胞24h,同时设阳性对照组(ca-4)、阴性对照组(空白溶剂),收集细胞,用pbs冲洗两次,再用75%乙醇-20℃固定过夜后,用pi染色后采用流式细胞仪进行测试,结果显示化合物69、70和97能明显将细胞阻滞于g2/m期(如图7所示)。
实施例111体外细胞周期相关蛋白检测实验
a.westernblot实验:选择不同浓度化合物处理肿瘤细胞后(同时设计dmso为阴性对照),收集并用裂解液裂解细胞。蛋白样品经加热变性后,电泳分离,转膜,封闭,依次经一抗反应、二抗反应后,曝光显色;
b.荧光定量pcr实验:用不同浓度受试样品处理细胞后,使用定量pcr试剂盒(takara)以及荧光定量pcr仪,检测细胞内相关蛋白mrna水平;
结果显示化合物69、70和97能明显促进磷酸化组蛋白h3、细胞周期蛋白b1、有丝分裂检验点蛋白bubr1表达(如图8所示)。
实施例112体外细胞凋亡实验
6孔板中,按2×105个/孔的数量接种细胞,待细胞贴壁后,用不同浓度的受试样品处理细胞24h,同时设阳性对照组(ca-4)、阴性对照组(空白溶剂)。收集细胞,用pbs冲洗两次,再用75%乙醇-20℃固定过夜后,用pi和annexinv双染色,细胞染色后采用流式细胞仪进行测试,结果显示化合物69、70和97能明显促进细胞凋亡(如图9所示)。
实施例113体外凋亡相关蛋白检测实验
a.westernblot实验:选择不同浓度化合物处理肿瘤细胞后(同时设计dmso为阴性对照),收集并用裂解液裂解细胞。蛋白样品经加热变性后,电泳分离,转膜,封闭,依次经一抗反应、二抗反应后,曝光显色;
b.荧光定量pcr实验:用不同浓度受试样品处理细胞后,使用定量pcr试剂盒(takara)以及荧光定量pcr仪,检测细胞内相关蛋白mrna水平;
结果显示化合物69、70和97能明显促进促凋亡蛋白bax、抑癌基因p53、剪切的dna修复酶的表达(如图10所示)。
实施例114急性毒性测试实验
icr小鼠单独饲养于无病原笼子内。每组小鼠(10只,5雌5雄)腹腔注射待测药物69(95,70,50,35和25mg/kg)、70(500,425,350,275和200mg/kg)或97(275,200,150,125和100mg/kg),或安慰剂(8.3%蓖麻油与8.3%乙醇的pbs溶液)。70、69溶解于蓖麻油乙醇(1:1,v/v)中,然后用pbs(1:5,v/v)稀释,每天记录小鼠死亡情况,持续14天,结果显示化合物69的ld50为61.5mg/kg,化合物70的ld50大于500mg/kg,化合物97的ld50为136.5mg/kg。表3是化合物69、70和97对小鼠的急性毒性结果。
表3.
69:ld50=61.5mg/kg;70:ld50>500mg/kg;97:ld50=136.5mg/kg.。
实施例115动物水平和组织水平的肿瘤治疗作用和机理研究实验
培养卵巢癌细胞系a2780,当细胞处于生长旺盛期进行接种,按每只2×106个细胞腹腔注射接种于6周龄balb/c裸鼠,建立裸鼠肿瘤转移模型,spf条件下生长。待裸鼠皮下移植瘤长至体积约100mm3,将已经建成的荷瘤小鼠随机分成4组,每组10只,经腹腔注射,分别给予不同浓度的7.5mg/kg的69、12.5mg/kg的70、4mg/kg和8mg/kg的97、5mg/kg或10mg/kg紫杉醇和空白对照(与急性毒性测试相同),记录瘤生长大小。肿瘤体积按以下公式计算:肿瘤体积(mm3)=a×b2×0.52(这里a代表最长的直径,b代表最短的直径,0.52是经验系数)。当空白对照组肿瘤体积平均体积达到2000mm3后,处死裸鼠,剥离瘤组织,称取瘤质量,计算抑瘤率:
抑瘤率=(1-实验组平均瘤质量/对照组平均瘤质量)×100%
裸鼠内脏组织用h&e染色进行进一步观察;
结果显示(如图11所示)化合物70、69和97在体内能明显抑制肿瘤生长,并且对小鼠体重无明显影响,组织染色显示出化合物70、69和97能够使肿瘤组织产生坏死区(图中箭头标示),并且对肝、肾、脾的组织染色并未观察到非正常区域。
- 还没有人留言评论。精彩留言会获得点赞!