一种新型大环环肽化合物的合成方法与流程
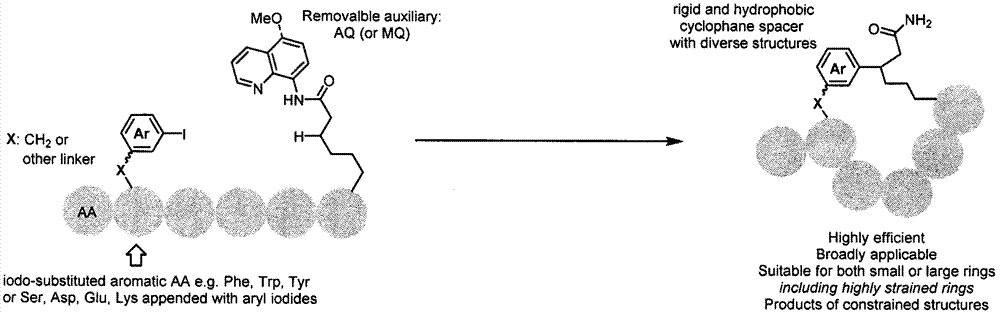
本发明涉及一类从线性肽前体出发,制备一种新型苯环支撑的环肽化合物的合成方法,属于金属催化应用技术领域。
背景技术:
大环化反应是自然界将简单线性前体化合物构建为复杂环状化合物的一种重要方法。在具有大环结构的天然产物中,大环环肽化合物在结构上更加灵活多变,往往表现出很好的生物活性。与小分子药物相比,环肽通常具有较大的体积以及表面积,这使得它们有希望应用于更具有挑战性的生物目标,如蛋白质-蛋白质相互作用,根据lipinski规则,这类生物活性的调节很难通过小分子药物来实现。然而,与小分子化合物成熟多样的合成方法相比,用于构建具有特定三维结构及药物特性的大环环肽化合物的方法非常有限。自然界中可以使用酶催化的c-h键的官能团化构建复杂的、具有生物活性的天然产物,如vancomycin,celogentin,streptide等(图1)。
目前,大环环肽化合物主要通过以下方法进行合成:
1)各种缩合反应及取代反应:如酸胺缩合,sn2反应(frost,j.r.,scully,c.c.g.&yudin,a.k..nat.chem.2016,3,1105.),snar反应(spokoyny,a.m.etal.j.am.chem.soc.2013,135,5946.)。
2)金属催化的转化:如叠氮一炔烃环加成反应,烯烃关环复分解反应(kimy.-w.,grossmannt.n.&verdine,g.l.nat.protoc.2011,6,761.),这些反应可以用于订书肽的合成。
通过上述现有的大环化方法可以制备一系列环肽化合物,以及用于稳定具有特定氨基酸序列多肽的二级结构,但是现有方法在成环过程中需要依靠氢键等弱相互作用来实现,不能直接控制产物的三维结构。综上所述,环肽作为一类重要的化合物近些年受到学术界和药物化学领域科学家的重点关注,但是现有合成方法的局限性制约了新型大环环肽化合物的合成。
技术实现要素:
本发明的目的之一在于提供一种简单实用的方法,可以从简单的线性多肽前体出发,通过关环反应,制备环肽;目的之二在于制备得到的环肽由苯环支撑,不依靠分子内的氢键,结构新颖。
1.一种通过钯催化的分子内c-h键的芳基化反应,高效制备新型环肽化合物的制备方法,其特征在于该方法的具体步骤(图2)。在反应瓶中依次加入原料、银盐、金属钯催化剂、添加剂(additive)以及溶剂,指定温度下搅拌12小时,冷却至室温,加入乙酸乙酯稀释,硅藻土过滤,浓缩,加入二氯甲烷,饱和碳酸氢钠水溶液洗涤,有机相用无水硫酸钠干燥,柱层析分离得目标产物。
2.本发明所涉及到的金属催化剂是金属pd相关催化剂,可以是pd(oac)2,也可以是其他二价pd金属催化剂。
3.本发明所涉及到的银盐可以是碳酸银,醋酸银,三氟乙酸银,也可以是其他一价银金属催化剂。
4.本发明所涉及到的添加剂(additive)是羧酸相关添加剂,可以是2-苯基苯甲酸,也可以是如图3所示的其他羧酸,催化剂的用量一般为0.5equiv。
5.本发明所用溶剂是叔丁醇,其用量是为每毫摩尔原料对应使用范围为40ml至200ml。
6.本发明所涉及到的反应温度可以在80℃至120℃中的任意温度下进行。
7.本发明所用原料由氨基酸经酸胺缩合得到,但不局限于这些组成片段。
本发明的优点是:
1.本发明所用各种试剂均可商业所得,原料来源广泛,价格低廉,且各种试剂常温常压下能够稳定存在,操作处理方便,无须特殊处理。
2、本发明浓度范围为5-25mm,不需要高度稀释,适合大量生产。
3.本发明操作简便,不需要隔绝空气和水,一步反应即可得到两个产物。对设备要求简单,后处理也无特别要求,大大降低了合成该类化合物的生产成本。
4.本发明所使用的催化剂用量较低,在保持良好催化效果的同时,达到了简化工艺、降低成本、方便后处理工序,溶剂的回收利用便捷,减少环境污染等要求。
具体实施方式
附图说明
图1为自然界中使用酶催化c-h键官能团化构建的天然产物举例;
图2为该方法的具体步骤;
图3为本发明所涉及到的羧酸相关添加剂;
图4为实施例1的具体合成步骤;
图5为实施例2的具体合成步骤;
图6为实施例3的具体合成步骤;
图7为实施例4的具体合成步骤;
图8为实施例5的具体合成步骤;
图9为实施例6的具体合成步骤;
图10为实施例7的具体合成步骤;
图11为实施例8的具体合成步骤;
图12为实施例9的具体合成步骤;
图13为实施例10的具体合成步骤;
图14为实施例11的具体合成步骤;
图15为实施例12的具体合成步骤;
图16为实施例13的具体合成步骤;
图17为实施例14的具体合成步骤;
图18为实施例15的具体合成步骤;
图19为实施例16的具体合成步骤;
图20为实施例17的具体合成步骤;
图21为实施例18的具体合成步骤;
图22为实施例19的具体合成步骤;
图23为实施例20的具体合成步骤;
图24为实施例21的具体合成步骤;
图25为实施例22的具体合成步骤;
图26为实施例23的具体合成步骤;
图27为实施例24的具体合成步骤;
图28为实施例25的具体合成步骤;
下面的实施示例将更好的说明本发明,但需要强调的是本发明决不仅限于这几个实施示例所表示的内容。以下实例显示了本发明的不同侧面。所给出的数据包括具体操作和反应条件及产物。产物纯度通过核磁鉴定。
实施例1(图4):在反应瓶中依次加入原料(76mg,0.1mmol),碳酸银(22mg,0.8eq),醋酸钯(1.1mg,5mol%),o-pba(10mg,0.5equiv)以及叔丁醇(4ml),100℃下搅拌12小时,冷却至室温,加入乙酸乙酯(50ml)稀释,硅藻土过滤,浓缩,加入二氯甲烷,饱和碳酸氢钠水溶液洗涤,有机相用无水硫酸钠干燥,柱层析分离,得产品为白色固体46mg,产率73%。1hnmr(400mhz,cdcl3)δ9.75(s,0.49h),9.67(s,0.45h),8.75-8.74(m,1h),8.71-8.68(m,1h),8.14-8.11(m,1h),7.53-7.41(m,3h),7.25-7.11(m,3h),7.01(d,j=5.8hz,1h),6.93(d,j=7.3hz,0.56h),6.89-6.85(m,1h),6.76(d,j=8.4hz,0.48h),6.22(d,j=7.6hz,0.49h),6.06(d,j=7.7hz,0.46h),4.98-4.89(m,1h),4.42-4.30(m,1h),4.20(dd,j=17.0,7.3hz,0.51h),4.11(dd,j=16.9,7.0hz,0.57h),3.76(s,1.5h),3.75(s,1.5h),3.57-3.43(m,1h),3.25-3.18(m,2h),2.98-2.89(m,1h),2.85-2.74(m,2h),2.27-2.18(m,1h),2.11-1.99(m,1h),1.81-1.78(m,1h),1.72-1.41(m,6h),1.17-1.05(m,2h),0.93-0.84(m,6h);13cnmr(101mhz,cdcl3)δ173.61,173.56,173.06,172.85,171.86,170.56,170.37,168.77,168.74,148.19,144.12,143.98,138.51,138.35,136.50,136.42,136.08,135.98,134.40,128.82,128.79,128.49,128.40,128.15,128.04,127.98,127.43,127.41,126.41,125.84,121.75,121.70,121.67,121.63,116.98,116.55,53.00,52.74,52.70,52.65,51.98,51.95,46.57,45.71,43.17,43.09,42.94,42.57,40.52,37.63,37.56,36.17,36.04,35.79,35.67,27.05,26.55,25.50,25.14,24.89,23.04,22.98,21.89,21.82.
实施例2(图5):在反应瓶中依次加入原料(63mg,0.1mmol),碳酸银(22mg,0.8eq),醋酸钯(1.1mg,5mol%),o-pba(10mg,0.5equiv)以及叔丁醇(20ml),100℃下搅拌12小时,冷却至室温,加入乙酸乙酯(50ml)稀释,硅藻土过滤,浓缩,加入二氯甲烷,饱和碳酸氢钠水溶液洗涤,有机相用无水硫酸钠干燥,柱层析分离,得产品为白色固体36mg,产率73%。异构体a:1hnmr(400mhz,cdcl3)δ9.75(s,1h),8.76-8.72(m,2h),8.13(d,j=8.2hz,1h),7.52-7.42(m,3h),7.28-7.25(m,1h),7.21(d,j=7.8hz,1h),7.08(d,j=7.6hz,1h),6.96(d,j=7.8hz,1h),6.67-6.66(m,1h),4.49(d,j=7.9hz,1h),3.90-3.82(m,1h),3.41(t,j=8.4hz,1h),3.30-3.20(m,2h),3.04-2.81(m,4h),2.76-2.71(m,1h),2.52-2.47(m,1h),2.15-1.93(m,4h),1.90-1.83(m,1h),1.70-1.57(m,3h),1.42-1.30(m,2h),1.19-0.98(m,3h);13cnmr(101mhz,cdcl3)δ173.18,171.26,170.27,148.16,142.74,138.39,137.42,136.48,134.48,131.70,130.08,128.47,128.01,127.52,124.41,121.69,121.58,116.59,59.56,47.30,46.16,42.59,40.75,34.15,33.69,31.35,26.74,25.46,24.99,21.91.异构体b:1hnmr(400mhz,cdcl3)δ9.81(s,1h),8.78-8.72(m,2h),8.14(dd,j=8.2,1.4hz,1h),7.52-7.42(m,3h),7.34(d,j=8.0hz,1h),7.24(d,j=8.4hz,1h),7.18(d,j=6.4hz,1h),6.94(d,j=7.6hz,1h),6.31(s,1h),4.52(d,j=7.8hz,1h),3.85-3.77(m,1h),3.37-3.23(m,3h),3.21-3.14(m,1h),3.00(dd,j=14.8,7.3hz,1h),2.89(dd,j=14.7,7.6hz,1h),2.84-2.76(m,2h),2.46-2.43(m,1h),2.02-1.92(m,5h),1.75-1.65(m,2h),1.59-1.50(m,1h),1.32-1.11(m,3h),1.02-0.92(m,1h),0.82-0.72(m,1h);13cnmr(101mhz,cdcl3)δ173.69,171.22,170.36,148.21,142.79,138.41,137.21,136.46,134.51,130.11,129.30,129.17,128.02,127.49,126.57,121.71,121.59,116.56,59.70,47.48,44.72,42.80,40.47,36.34,34.52,34.02,27.37,27.35,24.96,24.91.
实施例3(图6):在反应瓶中依次加入原料(83mg,0.1mmol),碳酸银(22mg,0.8eq),醋酸钯(2.2mg,10mol%),o-pba(10mg,0.5equiv)以及叔丁醇(20ml),100℃下搅拌12小时,冷却至室温,加入乙酸乙酯(50ml)稀释,硅藻土过滤,浓缩,加入二氯甲烷,饱和碳酸氢钠水溶液洗涤,有机相用无水硫酸钠干燥,柱层析分离,得产品为白色固体42mg,产率59%。1hnmr(400mhz,cdcl3)δ9.53(s,1h),8.79-8.75(m,1h),8.60(t,j=7.7hz,1h),8.54(dd,j=8.2,3.4hz,1h),7.41(dd,j=8.3,4.1hz,1h),7.34-7.20(m,6h),7.18-7.05(m,3h),7.00(d,j=7.3hz,0.55h),6.83(d,j=7.5hz,0.59h),6.77(t,j=8.0hz,1h),6.53(d,j=8.7hz,0.42h),6.30(d,j=7.8hz,0.51h),6.14(d,j=7.3hz,0.43h),5.97(d,j=9.4hz,0.56h),4.95-4.82(m,1h),4.57-4.43(m,1h),4.09(d,j=8.3hz,0.54h),4.04-4.03(m,0.49h),3.96(s,1.5h),3.95(s,1.5h),3.76(s,1.5h),3.76(s,1.5h),3.48-3.42(m,1h),3.35-3.28(m,2h),3.14-3.00(m,2h),2.96-2.81(m,3h),2.57(t,j=12.5hz,0.54h),2.46(t,j=12.9hz,0.61h),2.21-1.96(m,3h),1.82-1.40(m,5h);13cnmr(101mhz,cdcl3)δ173.31,172.92,171.91,171.76,171.13,169.68,169.53,169.18,169.13,150.26,150.22,148.58,148.55,141.86,141.20,139.02,136.48,135.98,134.66,134.33,131.27,131.20,131.11,130.90,130.15,129.92,129.84,129.31,129.10,128.93,128.70,128.60,127.89,127.82,127.13,127.09,125.25,124.17,120.72,120.39,120.38,116.75,116.63,104.33,104.28,61.63,61.45,55.80,55.79,54.04,53.43,53.34,52.92,52.71,52.63,46.91,46.76,44.42,43.52,41.58,41.10,39.31,39.23,38.29,36.87,32.69,32.66,32.22,32.02,31.44,22.51,22.08.
实施例4(图7):在反应瓶中依次加入原料(94mg,0.1mmol),碳酸银(22mg,0.8eq),醋酸钯(2.2mg,10mol%),o-pba(10mg,0.5equiv)以及叔丁醇(20ml),100℃下搅拌12小时,冷却至室温,加入乙酸乙酯(50ml)稀释,硅藻土过滤,浓缩,加入二氯甲烷,饱和碳酸氢钠水溶液洗涤,有机相用无水硫酸钠干燥,柱层析分离,得产品为白色固体52mg,产率64%。1hnmr(400mhz,cdcl3)δ9.68(d,j=11.6hz,1h),8.85-8.63(m,2h),8.13(d,j=7.9hz,1h),7.52-7.46(m,2h),7.43(s,1h),7.36(s,0.66h),7.25-7.09(m,3h),7.03-6.82(m,2h),6.75-6.56(m,2h),4.84-4.76(m,4h),4.55-4.50(m,1h),4.46-4.23(m,4h),4.17(d,j=6.7hz,5h),3.75(d,j=16.9hz,3h),3.65(d,j=15.8hz,1h),3.28-3.25(m,1h),3.20-3.16(m,1h),2.96-2.95(m,1h),2.82-2.76(m,2h),2.32-2.23(m,2h),2.14-1.98(m,1h),1.82-1.78(m,2h),1.73-1.60(m,1h),1.48-1.45(m,1h),1.08-1.08(m,1h),0.92-0.88(m,1h);13cnmr(101mhz,cdcl3)δ173.41,172.89,172.37,172.12,171.73,171.50,170.32,170.27,169.49,169.31,168.34,168.13,148.15,142.39,142.32,138.33,136.38,134.41,133.76,133.50,129.81,129.68,129.62,127.95,127.88,127.79,127.43,121.67,121.57,116.51,115.58,71.91,70.38,70.36,70.32,70.01,69.87,69.77,64.24,64.08,53.35,53.30,53.19,52.73,52.67,46.67,46.21,43.42,43.23,42.77,42.44,37.53,37.05,36.41,36.03,35.61,35.06,29.78,29.38,27.33,25.97,25.34,24.93.
实施例5(图8):在反应瓶中依次加入原料(81mg,0.1mmol),碳酸银(22mg,0.8eq),醋酸钯(2.2mg,10mol%),o-pba(10mg,0.5equiv)以及叔丁醇(20ml),100℃下搅拌12小时,冷却至室温,加入乙酸乙酯(50ml)稀释,硅藻土过滤,浓缩,加入二氯甲烷,饱和碳酸氢钠水溶液洗涤,有机相用无水硫酸钠干燥,柱层析分离,得产品为白色固体37mg,产率54%。异构体a:1hnmr(400mhz,dmso)δ10.04(s,1h),8.91(d,j=3.4hz,1h),8.65-8.62(m,1h),8.57(d,j=7.6hz,1h),8.39(d,j=8.2hz,1h),7.86(d,j=7.2hz,1h),7.82(d,j=7.7hz,1h),7.64-7.61(m,2h),7.59-7.51(m,2h),7.21(d,j=7.8hz,2h),7.06(d,j=6.2hz,1h),7.00(d,j=7.8hz,2h),4.58-4.54(m,1h),4.22-4.16(m,1h),4.10-4.03(m,1h),3.77(dd,j=16.4,6.8hz,1h),3.45-3.43(m,1h),3.16-3.07(m,1h),2.99-2.90(m,3h),2.83-2.70(m,3h),1.91(s,3h),1.70-1.62(m,1h),1.54-1.45(m,2h),1.33-1.17(m,4h),1.11-1.01(m,4h),0.89(dd,j=6.3,3.2hz,6h);13cnmr(101mhz,dmso)δ172.33,171.15,170.53,170.05,169.10,168.55,148.89,142.59,138.06,136.67,134.57,134.47,129.64,127.90,127.59,127.04,122.22,121.85,116.62,53.59,51.50,48.66,43.69,42.86,41.96,41.40,37.74,33.74,27.25,24.23,22.86,22.57,22.16,17.25.异构体b:1hnmr(400mhz,dmso)δ10.02(s,1h),8.91(d,j=3.9hz,1h),8.56(d,j=7.6hz,1h),8.42-8.37(m,2h),8.03(d,j=8.0hz,1h),7.92(d,j=7.0hz,1h),7.64-7.60(m,2h),7.53(t,j=7.9hz,1h),7.43-7.40(m,1h),7.20(d,j=7.8hz,2h),7.02(d,j=7.8hz,2h),6.84(d,j=7.9hz,1h),4.40-4.35(m,1h),4.33-4.27(m,1h),4.13-4.06(m,1h),3.83(dd,j=16.5,6.4hz,1h),3.51(dd,j=16.4,3.7hz,1h),3.33-3.26(m,1h),3.23-3.16(m,1h),2.92-2.85(m,3h),2.66(t,j=11.8hz,2h),1.85(s,3h),1.74-1.65(m,1h),1.61-1.55(m,1h),1.52-1.43(m,1h),1.36-1.27(m,3h),1.17(d,j=7.2hz,4h),0.87(dd,j=9.0,6.8hz,6h);13cnmr(101mhz,dmso)δ172.56,171.79,170.60,170.04,169.15,168.57,148.98,142.43,138.12,136.71,134.55,134.49,129.49,127.94,127.75,127.08,122.27,121.97,116.77,54.92,50.91,48.73,44.13,43.04,41.54,41.05,38.49,38.21,32.46,26.93,24.35,22.91,22.66,22.18,17.43.
实施例6(图9):在反应瓶中依次加入原料(89mg,0.1mmol),碳酸银(22mg,0.8eq),醋酸钯(2.2mg,10mol%),o-pba(10mg,0.5equiv)以及叔丁醇(20ml),100℃下搅拌12小时,冷却至室温,加入乙酸乙酯(50ml)稀释,硅藻土过滤,浓缩,加入二氯甲烷,饱和碳酸氢钠水溶液洗涤,有机相用无水硫酸钠干燥,柱层析分离,得产品为白色固体45mg,产率63%。异构体a:1hnmr(400mhz,cdcl3)δ9.82(s,1h),8.82(dd,j=4.2,1.6hz,1h),8.60-8.55(m,1h),8.12(dd,j=8.3,1.6hz,1h),8.02(d,j=8.6hz,1h),7.65(s,1h),7.56-7.52(m,1h),7.49-7.42(m,3h),6.86-6.75(m,2h),6.58(d,j=8.4hz,1h),5.05-5.01(m,1h),4.79-4.76(m,1h),4.66(t,j=7.5hz,1h),4.57-4.51(m,2h),4.01(d,j=9.1hz,1h),3.88-3.84(m,1h),3.72-3.61(m,2h),3.56-3.54(m,5h),3.35(t,j=12.6hz,1h),3.06-2.98(m,2h),2.79(dd,j=13.8,5.3hz,1h),2.41-2.37(m,1h),2.21-2.10(m,3h),1.95-1.75(m,4h),1.61-1.49(m,2h),1.39(d,j=7.3hz,3h),1.18-1.11(m,1h),0.93(d,j=6.7hz,3h),0.79(t,j=7.3hz,3h);13cnmr(101mhz,cdcl3)δ173.41,172.89,172.28,172.25,171.14,170.79,156.17,148.19,138.41,136.35,134.73,131.19,130.59,127.93,127.74,127.41,127.22,121.65,121.27,116.41,110.37,63.51,59.46,55.32,54.61,53.64,52.10,49.58,42.53,37.18,36.23,35.79,34.86,34.78,25.33,25.33,24.85,24.41,18.15,15.52,10.83.异构体b:1hnmr(400mhz,cdcl3)δ9.72(s,1h),8.75-8.69(m,2h),8.12(d,j=8.3hz,1h),8.04-7.85(m,3h),7.49-7.41(m,3h),7.10(s,1h),6.74(d,j=8.2hz,1h),6.65(d,j=8.5hz,1h),4.75-4.74(m,1h),4.38-4.24(m,3h),4.03-4.01(m,1h),3.75(s,5h),3.52(s,3h),2.97-2.85(m,3h),2.67-2.59(m,1h),2.31-2.15(m,5h),1.89-1.81(m,1h),1.74-1.62(m,4h),1.42(d,j=7.0hz,3h),1.12(d,j=4.5hz,4h),0.90(t,j=6.7hz,3h);13cnmr(101mhz,cdcl3)δ182.68,175.94,173.80,173.37,171.62,170.97,169.89,156.69,148.11,138.41,136.35,134.67,131.37,128.21,128.19,128.03,127.69,127.41,121.64,121.34,116.40,110.74,62.03,60.13,56.98,55.46,54.06,52.07,51.62,45.33,37.34,36.27,34.13,33.90,26.70,25.33,24.91,17.17,15.74,10.24.
实施例7(图10):在反应瓶中依次加入原料(87mg,0.1mmol),碳酸银(22mg,0.8eq),醋酸钯(1.1mg,5mol%),o-pba(10mg,0.5equiv)以及叔丁醇(20ml),100℃下搅拌12小时,冷却至室温,加入乙酸乙酯(50ml)稀释,硅藻土过滤,浓缩,加入二氯甲烷,饱和碳酸氢钠水溶液洗涤,有机相用无水硫酸钠干燥,柱层析分离,得产品为白色固体53mg,产率71%。异构体a:1hnmr(400mhz,cdcl3)δ9.83(s,1h),9.75(s,1h),8.70(dd,j=4.2,1.6hz,1h),8.62(dd,j=5.6,3.4hz,1h),8.09(dd,j=8.3,1.6hz,1h),7.75-7.72(m,1h),7.44-7.38(m,3h),7.33-7.30(m,2h),7.10-7.00(m,4h),6.23(s,1h),4.93-4.88(m,1h),4.47-4.41(m,1h),4.18(dd,j=16.7,7.2hz,1h),4.02(dd,j=16.7,6.6hz,1h),3.85-3.77(m,1h),3.67(s,3h),3.59(s,3h),3.49-3.37(m,2h),3.31-3.18(m,2h),3.03-2.93(m,2h),2.54-2.46(m,1h),2.40-2.33(m,1h),2.25-2.16(m,1h),2.12-2.03(m,2h),1.95-1.91(m,1h),1.78-1.69(m,1h),1.67-1.59(m,1h),1.54-1.38(m,2h);13cnmr(101mhz,cdcl3)δ174.76,174.50,172.69,172.17,171.36,170.56,168.90,148.24,138.30,136.42,135.59,134.28,127.96,127.81,127.53,127.33,124.33,121.77,121.72,120.02,118.94,116.59,116.50,109.46,52.68,52.62,52.44,52.41,44.31,43.33,43.23,36.35,35.55,35.35,30.11,27.75,25.48,23.95.异构体b:1hnmr(400mhz,cdcl3)δ9.79(s,1h),9.64(s,1h),8.71-8.65(m,2h),8.13-8.11(m,1h),7.55-7.37(m,5h),7.12-7.03(m,4h),6.87(d,j=7.4hz,1h),6.18(s,1h),4.95-4.93(m,1h),4.53-4.49(m,1h),4.16-4.06(m,2h),3.65-3.50(m,8h),3.28-3.21(m,2h),3.12-3.02(m,2h),2.56-2.48(m,1h),2.36-2.28(m,1h),2.19-1.95(m,5h),1.84-1.76(m,1h),1.63-1.55(m,1h),1.48-1.41(m,1h);13cnmr(101mhz,cdcl3)δ175.02,174.52,172.82,172.08,171.33,170.75,168.65,148.24,138.44,136.46,135.00,134.34,128.14,128.04,127.40,127.11,124.14,121.87,121.74,121.17,120.03,117.24,116.82,109.97,52.63,52.58,52.55,52.43,43.79,43.60,43.34,34.68,32.86,30.04,28.18,25.09,23.34.
实施例8(图11):在反应瓶中依次加入原料(128mg,0.1mmol),碳酸银(22mg,0.8eq),醋酸钯(1.1mg,5mol%),o-pba(10mg,0.5equiv)以及叔丁醇(20ml),100℃下搅拌12小时,冷却至室温,加入乙酸乙酯(50ml)稀释,硅藻土过滤,浓缩,加入二氯甲烷,饱和碳酸氢钠水溶液洗涤,有机相用无水硫酸钠干燥,柱层析分离,得产品为白色固体70mg,产率61%。1hnmr(400mhz,dmso)δ10.01(d,j=10.6hz,1h),8.93-8.90(m,1h),8.62-8.58(m,1h),8.38(d,j=8.0hz,1h),8.33-8.27(m,1h),8.23-8.14(m,1h),8.11-8.08(m,1h),7.91(d,j=7.7hz,0.64h),7.84(d,j=7.3hz,0.63h),7.63-7.60(m,2h),7.56-7.53(m,1h),7.21-7.09(m,5h),6.84-6.72(m,1h),6.54-6.37(m,1h),4.75-4.72(m,0.50h),4.67-4.54(m,1h),4.50-4.48(m,1.47h),4.30-4.28(m,1.48h),4.25-4.21(m,0.52h),4.02-3.96(m,1h),3.68-3.62(m,4h),3.57(d,j=4.6hz,3h),3.38-3.30(m,6h),3.06-3.01(m,3h),2.96-2.88(m,4h),2.53-2.48(m,6h),2.45-2.40(m,4h),2.03-1.96(m,4h),1.79(s,3h),1.64-1.58(m,4h),1.48-1.36(m,9h);13cnmr(101mhz,dmso)δ173.10,173.02,172.68,172.60,172.25,172.15,172.07,171.95,171.76,171.72,171.62,171.56,171.32,170.49,170.47,170.36,170.19,170.14,170.08,168.45,168.37,168.35,168.31,168.23,157.41,156.04,148.71,142.29,142.23,137.93,137.25,136.49,134.69,134.64,134.51,134.47,134.16,131.40,129.33,128.92,128.66,127.75,127.59,127.49,126.89,124.28,122.03,121.68,118.76,116.43,116.24,115.17,86.25,79.15,62.98,62.86,60.59,59.79,59.54,54.21,53.33,52.99,52.86,52.73,52.60,52.06,51.53,49.25,49.11,48.62,46.81,46.75,46.31,44.52,44.40,43.96,43.79,42.42,41.46,41.37,41.29,35.72,35.59,35.34,35.15,33.48,33.38,33.15,32.89,31.56,31.26,29.75,29.23,28.88,28.61,28.24,27.98,27.19,26.26,26.00,25.57,25.33,24.51,24.45,24.21,23.92,23.12,22.98,22.59,22.33,18.93,17.58.
实施例9(图12):在反应瓶中依次加入原料(133mg,0.1mmol),碳酸银(22mg,0.8eq),醋酸钯(2.2mg,10mol%),o-pba(10mg,0.5equiv)以及叔丁醇(20ml),100℃下搅拌12小时,冷却至室温,加入乙酸乙酯(50ml)稀释,硅藻土过滤,浓缩,加入二氯甲烷,饱和碳酸氢钠水溶液洗涤,有机相用无水硫酸钠干燥,柱层析分离,得产品为白色固体88mg,产率66%。1hnmr(400mhz,dmso)δ10.82(s,1h),9.72(s,0.5h),9.71(s,0.5h),8.90(s,1h),8.52(d,j=8.4hz,1h),8.43(d,j=8.6hz,1h),8.19-7.93(m,3h),7.82-7.54(m,4h),7.36-7.22(m,7h),7.19-7.06(m,9h),7.01-6.96(m,4h),5.08-4.99(m,2h),4.77(dd,j=18.0,5.0hz,1h),4.64-4.40(m,2h),4.22-4.18(m,3h),3.93(s,4h),3.64(s,3h),3.11-2.97(m,5h),2.89-2.72(m,4h),2.64-2.60(m,1h),2.08-1.96(m,1h),1.90-1.82(m,1h),1.65-1.51(m,4h),1.39(d,j=9.7hz,4h),1.24-1.15(m,4h),1.04(d,j=5.8hz,3h);13cnmr(101mhz,dmso)δ172.63,172.61,171.96,171.94,171.29,171.24,171.20,171.09,171.02,156.10,149.73,149.69,149.18,142.87,142.78,138.91,138.84,137.91,137.29,136.16,134.77,134.68,130.69,128.80,128.33,128.10,127.82,127.71,127.48,127.39,126.23,123.51,121.27,120.94,119.70,118.63,118.52,118.22,117.36,117.22,111.29,110.31,110.25,104.65,66.68,66.64,65.14,57.72,57.60,55.87,55.32,55.27,54.93,53.40,53.14,53.04,52.85,52.73,52.07,52.04,43.92,43.88,41.60,41.59,36.84,36.75,35.70,35.50,35.32,35.23,31.53,31.34,29.77,29.22,28.85,28.42,27.06,26.73,25.24,25.00,22.60,22.48,19.82,19.7.
实施例10(图13):在反应瓶中依次加入原料(146mg,0.1mmol),碳酸银(22mg,0.8eq),醋酸钯(2.2mg,10mol%),o-pba(10mg,0.5equiv)以及叔丁醇(20ml),100℃下搅拌12小时,冷却至室温,加入乙酸乙酯(50ml)稀释,硅藻土过滤,浓缩,加入二氯甲烷,饱和碳酸氢钠水溶液洗涤,有机相用无水硫酸钠干燥,柱层析分离,得产品为白色固体76mg,产率57%。1hnmr(400mhz,meod)δ8.77(s,1h),8.53(d,j=5.0hz,1h),8.18(d,j=8.0hz,1h),7.49-7.45(m,3h),7.35-7.20(m,10h),7.18-7.14(m,2h),7.09-7.05(m,2h),5.18-5.08(m,4h),4.68-4.61(m,3h),4.44-4.38(m,1h),4.32-4.17(m,1h),4.07(dd,j=36.9,23.2hz,2h),3.76(s,1h),3.64(t,j=22.7hz,4h),3.35(s,0.57h),3.24-2.98(m,4h),2.97-2.69(m,6.59h),2.48-2.42(m,3.56h),2.30-2.28(m,2h),2.15-2.08(m,3h),2.02-1.96(m,5.45h),1.75-1.20(m,15h),0.92(dd,j=16.0,4.9hz,6h);13cnmr(101mhz,meod)δ176.30,176.27,175.82,175.77,175.03,174.92,174.63,174.54,174.47,174.27,173.98,173.87,173.66,173.63,173.01,172.95,172.91,172.84,172.76,172.72,171.81,171.71,171.67,171.46,171.42,158.10,149.75,143.74,139.66,138.04,137.57,137.04,135.98,135.29,130.64,129.56,129.49,129.37,129.30,129.16,129.14,128.98,128.86,128.80,128.74,127.92,123.31,122.95,118.09,68.03,67.60,56.59,56.26,56.09,54.64,54.24,54.19,54.05,53.89,52.86,52.77,49.85,46.09,45.93,43.83,43.41,41.68,41.13,40.11,37.79,37.72,36.82,36.65,36.60,36.55,32.98,32.80,32.54,31.50,30.67,30.32,30.12,29.83,29.56,28.23,28.10,27.79,27.59,26.60,25.80,24.05,23.30,22.25,22.21,15.39,15.32,14.44.
实施例11(图14):在反应瓶中依次加入原料(84mg,0.1mmol),碳酸银(22mg,0.8eq),醋酸钯(2.2mg,10mol%),o-pba(10mg,0.5equiv)以及叔丁醇(20ml),100℃下搅拌12小时,冷却至室温,加入乙酸乙酯(50ml)稀释,硅藻土过滤,浓缩,加入二氯甲烷,饱和碳酸氢钠水溶液洗涤,有机相用无水硫酸钠干燥,柱层析分离,得产品为白色固体76mg,产率73%。1hnmr(400mhz,cdcl3)δ9.72(d,j=9.5hz,1h),8.76(d,j=3.9hz,1h),8.69(dd,j=6.2,2.7hz,1h),8.13(d,j=8.3hz,1h),7.98(d,j=8.2hz,1h),7.93(d,j=8.2hz,1h),7.53-7.37(m,5.50h),7.33-7.18(m,3.50h),7.08(d,j=6.9hz,1h),7.03(d,j=6.9hz,1h),6.07-6.03(m,1.53h),5.90(t,j=6.0hz,0.49h),4.98-4.93(m,1h),4.72-4.68(m,1h),4.58-4.54(m,1h),4.48-4.43(m,1h),3.96(dd,j=15.4,5.8hz,0.54h),3.90-3.77(m,3.57h),3.65(dd,j=15.7,6.7hz,0.64h),3.53-3.48(m,1h),3.38-3.34(m,0.56h),2.93-2.81(m,2.49h),2.75(dd,j=13.6,5.0hz,0.60h),2.19-2.16(m,1h),2.09-1.97(m,2h),1.90-1.85(m,2h),1.73-1.51(m,2h),1.39-1.28(m,1h),1.20-1.15(m,1h);13cnmr(101mhz,cdcl3)δ172.43,172.31,171.99,171.92,169.81,169.67,169.59,168.74,168.65,165.83,165.78,150.48,150.00,148.26,148.23,138.34,136.52,136.47,134.30,134.28,130.59,130.38,129.29,129.22,129.04,129.01,128.12,128.03,128.01,127.73,127.48,127.46,121.75,116.69,116.63,64.17,63.91,54.63,53.22,51.53,51.42,45.99,45.17,44.52,44.29,43.18,42.41,39.48,39.39,36.32,35.49,35.12,34.97,26.41,26.11,24.96,24.54.
实施例12(图15):在反应瓶中依次加入原料(95mg,0.1mmol),碳酸银(22mg,0.8eq),醋酸钯(2.2mg,10mol%),o-pba(10mg,0.5equiv)以及叔丁醇(20ml),100℃下搅拌12小时,冷却至室温,加入乙酸乙酯(50ml)稀释,硅藻土过滤,浓缩,加入二氯甲烷,饱和碳酸氢钠水溶液洗涤,有机相用无水硫酸钠干燥,柱层析分离,得产品为白色固体58mg,产率71%。1hnmr(400mhz,cdcl3)δ9.72(d,j=5.1hz,1h),8.74-8.64(m,2h),8.13-8.10(m,1h),8.00(s,0.46h),7.93(s,0.55h),7.88-7.76(m,1h),7.55-7.32(m,7h),7.23-7.08(m,6h),6.94(d,j=7.6hz,0.53h),6.74(d,j=6.9hz,0.54h),5.00-4.93(m,1h),4.83-4.75(m,1h),4.68-4.63(m,0.53h),4.61-4.42(m,1.5h),4.28-4.03(m,2h),3.77-3.55(m,4h),3.52-3.43(m,0.55h),3.39-3.36(m,1h),3.16-2.99(m,2h),2.91(dd,j=14.5,7.9hz,0.55h),2.84-2.77(m,1.55h),2.26-2.11(m,2h),1.90-1.65(m,4h),1.60-1.52(m,1h),1.50-1.29(m,2h),1.18-1.05(m,2h),0.87-0.76(m,6h);13cnmr(101mhz,cdcl3)δ174.24,173.95,172.65,172.47,171.83,170.26,170.20,169.86,169.71,168.22,166.70,166.66,148.31,148.25,144.30,144.16,138.40,138.32,136.46,136.16,136.13,134.31,134.26,132.25,131.91,129.98,129.94,129.87,129.61,129.31,129.23,129.01,128.94,128.69,128.62,128.25,128.00,127.38,127.35,127.13,121.81,121.78,121.72,116.80,116.62,64.92,64.48,58.35,53.92,52.95,52.82,52.46,52.44,45.42,45.22,43.52,43.38,42.14,41.29,37.84,36.67,36.51,35.74,35.19,34.87,34.20,29.79,25.75,25.08,25.03,24.96,24.74,15.73,11.42.
实施例13(图16):在反应瓶中依次加入原料(85mg,0.1mmol),碳酸银(22mg,0.8eq),醋酸钯(2.2mg,10mol%),o-pba(10mg,0.5equiv)以及叔丁醇(20ml),100℃下搅拌12小时,冷却至室温,加入乙酸乙酯(50ml)稀释,硅藻土过滤,浓缩,加入二氯甲烷,饱和碳酸氢钠水溶液洗涤,有机相用无水硫酸钠干燥,柱层析分离,得产品为白色固体58mg,产率33%。异构体a:1hnmr(400mhz,cdcl3)δ9.85(s,1h),8.72-8.68(m,3h),8.10(t,j=8.8hz,2h),8.01(d,j=8.3hz,1h),7.86-7.84(m,2h),7.74(d,j=8.5hz,1h),7.61(d,j=8.3hz,1h),7.52-7.35(m,3h),7.30(s,1h),5.06-4.94(m,1h),4.81(d,j=8.7hz,1h),4.67(d,j=13.4hz,1h),4.46(d,j=10.9hz,1h),4.23(dd,j=14.7,5.2hz,1h),4.00-3.76(m,4h),3.58-3.52(m,1h),3.33(dd,j=14.8,5.7hz,1h),3.17(dd,j=14.8,7.6hz,1h),3.03(dd,j=14.8,7.2hz,1h),2.17-2.05(m,1h),1.90(s,1h),1.81-1.53(m,3h),1.49-1.35(m,1h),1.00-0.98(m,1h),0.70-0.68(m,1h),0.53(t,j=6.7hz,3h),0.35-0.26(m,6h);13cnmr(101mhz,cdcl3)δ172.71,172.25,169.99,169.89,169.62,166.00,148.17,145.22,138.33,136.47,136.04,134.32,132.79,131.46,130.04,127.98,127.92,127.46,125.87,121.73,116.60,64.48,55.94,53.19,50.96,44.97,44.16,43.58,38.40,37.73,35.72,27.13,26.10,24.69,14.72,10.95.异构体b:1hnmr(400mhz,cdcl3)δ9.75(s,1h),8.79-8.58(m,3h),8.25(d,j=8.5hz,1h),8.11(d,j=7.8hz,1h),7.99(d,j=8.2hz,1h),7.85-7.67(m,4h),7.50-7.37(m,3h),7.29(s,1h),5.02-4.98(m,1h),4.80(d,j=9.0hz,1h),4.66(d,j=11.0hz,1h),4.42(dd,j=10.9,3.6hz,1h),4.31(dd,j=14.7,5.6hz,1h),3.97-3.79(m,4h),3.54-3.42(m,1h),3.32(dd,j=14.8,5.7hz,1h),3.08-2.91(m,2h),2.02-1.89(m,1h),1.89-1.69(m,3h),1.58-1.48(m,1h),1.14-0.94(m,3h),0.55-0.53(m,1h),0.39(t,j=7.0hz,3h),0.19(d,j=6.6hz,4h),-0.24(t,j=13.1hz,1h);13cnmr(101mhz,cdcl3)δ172.83,172.79,170.05,169.75,169.72,165.83,148.18,145.01,138.35,136.46,136.03,134.32,133.25,131.52,130.72,128.08,127.99,127.46,125.89,125.84,125.06,121.71,116.64,64.58,56.02,53.20,51.00,46.17,45.05,43.94,38.28,36.28,33.75,26.88,25.14,24.55,14.63,10.71.
实施例14(图17):在反应瓶中依次加入原料(87mg,0.1mmol),碳酸银(22mg,0.8eq),醋酸钯(2.2mg,10mol%),o-pba(10mg,0.5equiv)以及叔丁醇(20ml),100℃下搅拌12小时,冷却至室温,加入乙酸乙酯(50ml)稀释,硅藻土过滤,浓缩,加入二氯甲烷,饱和碳酸氢钠水溶液洗涤,有机相用无水硫酸钠干燥,柱层析分离,得产品为白色固体50mg,产率68%。1hnmr(400mhz,dmso)δ9.99(d,j=9.9hz,1h),8.90(d,j=3.1hz,1h),8.66(dd,j=24.8,8.1hz,1h),8.56-8.53(m,1h),8.41(dd,j=27.9,7.9hz,2h),7.62-7.60(m,2h),7.52(t,j=7.9hz,1h),7.38-7.35(m,5h),7.26-7.04(m,3h),6.75(t,j=7.2hz,2h),5.21-5.05(m,2h),4.90-4.78(m,1h),4.67-4.59(m,0.68h),4.58-4.52(m,0.47h),4.15-4.08(m,2h),3.76-3.59(m,4h),3.17-2.94(m,2h),2.92-2.81(m,2h),2.70-2.54(m,2h),2.15-2.08(m,1h),1.95-1.90(m,1h),1.68-1.42(m,4h),1.29-1.12(m,1h),0.83-0.78(m,1h);13cnmr(101mhz,dmso)δ172.15,171.88,170.86,170.82,170.46,170.41,169.77,168.95,168.85,168.55,168.53,156.55,156.36,148.75,137.97,136.65,136.55,136.27,135.83,134.47,128.99,128.84,128.46,128.44,128.06,128.03,127.79,127.66,126.93,122.08,121.69,116.47,114.34,114.26,67.88,66.23,66.18,52.36,51.81,51.71,49.92,44.57,43.76,41.25,41.22,40.94,37.26,37.06,35.29,34.75,34.50,33.41,31.29,24.84,24.80,24.51,24.22.
实施例15(图18):在反应瓶中依次加入原料(89mg,0.1mmol),碳酸银(22mg,0.8eq),醋酸钯(2.2mg,10mol%),o-pba(10mg,0.5equiv)以及叔丁醇(20ml),100℃下搅拌12小时,冷却至室温,加入乙酸乙酯(50ml)稀释,硅藻土过滤,浓缩,加入二氯甲烷,饱和碳酸氢钠水溶液洗涤,有机相用无水硫酸钠干燥,柱层析分离,得产品为白色固体62mg,产率81%。1hnmr(400mhz,cdcl3)δ9.72(d,j=6.8hz,1h),8.82-8.66(m,2h),8.13(d,j=8.2hz,1h),7.74(dd,j=15.3,7.9hz,1h),7.54-7.41(m,3.40h),7.39-7.27(m,6.49h),7.23-7.18(m,1h),7.10-7.06(m,1.50h),7.00-6.98(m,0.45h),5.29-5.07(m,3h),4.98(dd,j=28.6,12.5hz,1h),4.72-4.55(m,1h),4.29-4.10(m,2h),3.73-3.60(m,1h),3.60-3.47(m,1h),3.46-3.24(m,2h),2.91-2.73(m,2h),2.46-2.42(m,2h),2.32-1.97(m,8h),1.93-1.80(m,2h),1.73-1.52(m,2h),1.46-1.43(m,1h),1.21-1.10(m,1h);13cnmr(101mhz,cdcl3)δ173.21,173.03,172.59,172.49,172.42,172.39,171.75,171.74,170.34,170.31,169.79,169.72,148.25,144.11,144.01,138.35,136.77,136.63,136.42,135.52,134.45,128.80,128.74,128.66,128.64,128141,128.38,128.28,127.99,127.79,127.53,127.39,126.93,126.63,126.14,126.09,121.74,121.63,116.47,67.22,66.13,60.95,52.25,52.08,47.86,47.71,45.97,45.73,43.01,41.65,41.52,34.74,34.59,34.39,31.29,31.00,28.96,27.06,26.86,26.27,26.04,25.28,25.27,24.08,23.80.
实施例16(图19):在反应瓶中依次加入原料(85mg,0.1mmol),碳酸银(22mg,0.8eq),醋酸钯(2.2mg,10mol%),o-pba(10mg,0.5equiv)以及叔丁醇(20ml),100℃下搅拌12小时,冷却至室温,加入乙酸乙酯(50ml)稀释,硅藻土过滤,浓缩,加入二氯甲烷,饱和碳酸氢钠水溶液洗涤,有机相用无水硫酸钠干燥,柱层析分离,得产品为白色固体47mg,产率65%。1hnmr(400mhz,dmso)δ9.99(d,j=10.8hz,1h),9.81(d,j=21.7hz,1h),8.90(s,1h),8.56(d,j=7.7hz,1h),8.38(d,j=7.9hz,1h),8.21(d,j=8.0hz,0.58h),8.04(d,j=8.4hz,0.48h),7.95(d,j=7.8hz,0.51h),7.88(d,j=7.6hz,0.54h),7.68-7.36(m,5h),7.31-7.10(m,6h),4.63-4.02(m,2h),3.72-3.46(m,4h),3.18-2.62(m,5h),2.38-2.34(m,2h),2.13-1.77(m,4h),1.64-1.47(m,2h),1.28-1.20(m,2h),0.99-0.78(m,2h);13cnmr(101mhz,dmso)δ172.58,172.09,172.01,171.32,171.22,170.44,170.39,170.33,168.63,168.31,148.75,138.30,138.21,138.08,137.95,137.34,137.10,136.53,134.44,128.97,128.06,127.76,127.71,127.60,126.91,126.18,122.06,121.68,119.16,119.02,116.48,116.42,55.00,54.50,51.98,51.67,51.49,44.69,44.02,42.64,41.40,40.92,36.89,36.79,36.17,35.49,34.94,34.49,33.61,33.38,26.99,26.31,26.19,25.74,25.19,24.10.
实施例17(图20):在反应瓶中依次加入原料(83mg,0.1mmol),碳酸银(22mg,0.8eq),醋酸钯(2.2mg,10mol%),o-pba(10mg,0.5equiv)以及叔丁醇(20ml),100℃下搅拌12小时,冷却至室温,加入乙酸乙酯(50ml)稀释,硅藻土过滤,浓缩,加入二氯甲烷,饱和碳酸氢钠水溶液洗涤,有机相用无水硫酸钠干燥,柱层析分离,得产品为白色固体47mg,产率67%。1hnmr(400mhz,cdcl3)δ9.72(d,j=9.0hz,1h),8.80-8.72(m,1h),8.72-8.63(m,1h),8.18-8.06(m,1h),7.78(s,0.44h),7.69(dd,j=6.0,1.6hz,1h),7.64(d,j=7.5hz,0.42h),7.56(dd,j=12.7,6.6hz,0.56h),7.51-7.31(m,5.50h),7.20-7.08(m,1h),6.98-6.95(m,1h),4.68-4.54(m,1h),4.55-4.42(m,1h),3.94(dd,j=16.5,6.2hz,1h),3.76-3.65(m,3.54h),3.57-3.19(m,5h),3.03(dd,j=16.9,5.6hz,0.44h),2.89-2.80(m,2h),2.27-2.11(m,5h),1.98-1.55(m,10h),1.47-1.37(m,2h),1.09-0.95(m,1h);13cnmr(101mhz,cdcl3)δ173.63,173.56,172.85,172.74,172.57,172.47,170.30,170,15,169.89,169.43,168.29,168.27,148.32,144.17,143.95,138.33,136.45,136.39,135.32,135.24,134.38,134.35,131.58,129.72,129.10,128.69,127.99,127.97,127.40,127.35,126.94,126.21,126.00,125.74,121.75,121.72,121.67,116.57,116.49,59.92,59.68,53.89,52.65,51.78,50.95,47.76,47.65,45.83,43.18,42.77,42.59,42.08,38.99,38.94,35.94,35.18,34.33,34.00,31.43,30.89,29.37,27.94,27.79,27.41,27.24,27.09,26.42,25.03,24.94,24.59,24.34,21.85,21.60.
实施例18(图21):在反应瓶中依次加入原料(59mg,0.1mmol),碳酸银(22mg,0.8eq),醋酸钯(2.2mg,10mol%),o-pba(10mg,0.5equiv)以及叔丁醇(20ml),100℃下搅拌12小时,冷却至室温,加入乙酸乙酯(50ml)稀释,硅藻土过滤,浓缩,加入二氯甲烷,饱和碳酸氢钠水溶液洗涤,有机相用无水硫酸钠干燥,柱层析分离,得产品为白色固体47mg,产率62%。异构体a:1hnmr(400mhz,cdcl3)δ9.94(s,1h),8.81(d,j=3.6hz,1h),8.77(d,j=13.7hz,1h),8.15(d,j=8.2hz,1h),7.57-7.41(m,3h),7.02(d,j=7.9hz,1h),5.00(d,j=10.1hz,1h),4.94-4.87(m,1h),3.77(s,3h),3.68-3.62(m,1h),3.55(dd,j=13.3,6.1hz,1h),3.04(dd,j=14.6,7.0hz,1h),2.86(dd,j=14.7,8.3hz,1h),2.60(t,j=12.4hz,1h),1.91-1.88(m,1h),1.67-1.58(m,1h),1.52-1.40(m,2h),1.19-0.74(m,4h);13cnmr(101mhz,cdcl3)δ172.70,172.59,170.48,148.35,142.28,138.46,136.45,135.00,134.56,130.75,130.08,128.86,128.05,127.63,127.51,121.77,121.61,116.57,51.90,40.85,40.48,38.57,34.82,34.11,25.04,21.36.异构体b:1hnmr(400mhz,cdcl3)δ9.89(s,1h),8.84-8.67(m,2h),8.13(d,j=8.1hz,1h),7.53-7.40(m,3h),7.32(d,j=7.8hz,1h),7.23-7.18(m,2h),7.06(d,j=7.8hz,1h),5.08(d,j=10.3hz,1h),5.00-4.96(m,1h),3.76(s,3h),3.54(dd,j=13.2,5.7hz,1h),3.20-3.06(m,2h),2.94-2.90(m,1h),2.56(t,j=12.5hz,1h),2.08-1.98(m,1h),1.78-1.68(m,3h),1.45-1.32(m,2h),0.93-0.85(m,1h),0.78-0.68(m,1h);13cnmr(101mhz,cdcl3)δ172.77,172.21,170.30,148.18,142.03,138.46,136.46,135.22,134.55,133.06,131.17,128.71,128.03,127.52,125.53,121.69,121.55,116.62,51.61,43.66,42.89,38.81,36.16,34.32,26.36,23.22.
实施例19(图22):在反应瓶中依次加入原料(57mg,0.1mmol),碳酸银(22mg,0.8eq),醋酸钯(2.2mg,10mol%),o-pba(10mg,0.5equiv)以及叔丁醇(20ml),100℃下搅拌12小时,冷却至室温,加入乙酸乙酯(50ml)稀释,硅藻土过滤,浓缩,加入二氯甲烷,饱和碳酸氢钠水溶液洗涤,有机相用无水硫酸钠干燥,柱层析分离,得产品为白色固体10mg,产率23%。1hnmr(400mhz,cdcl3)δ9.90(s,1h),8.79-8.72(m,2h),8.14(dd,j=8.2,1.5hz,1h),7.52-7.41(m,3h),7.36(d,j=7.8hz,1h),7.27-7.25(m,1h),7.16(d,j=7.8hz,1h),7.05(d,j=8.0hz,1h),4.90-4.69(m,1h),4.56(d,j=10.9hz,1h),3.75(s,3h),3.56(dd,j=13.0,7.0hz,1h),3.27-3.16(m,1h),3.11(dd,j=14.7,8.1hz,1h),2.93(dd,j=14.7,6.8hz,1h),2.56(dd,j=12.8,11.0hz,1h),2.33-2.12(m,2h),1.74-1.69(m,2h),1.45-1.40(m,1h),1.22-1.13(m,1h);13cnmr(101mhz,cdcl3)δ173.58,172.57,170.14,148.21,141.65,138.40,136.49,135.91,134.46,132.89,131.59,130.41,128.01,127.51,125.43,121.71,121.61,116.60,52.50,52.19,43.75,43.60,40.29,39.05,37.79,19.66.
实施例20(图23):在反应瓶中依次加入原料(52mg,0.1mmol),碳酸银(22mg,0.8eq),醋酸钯(2.2mg,10mol%),o-pba(10mg,0.5equiv)以及叔丁醇(20ml),100℃下搅拌12小时,冷却至室温,加入乙酸乙酯(50ml)稀释,硅藻土过滤,浓缩,加入二氯甲烷,饱和碳酸氢钠水溶液洗涤,有机相用无水硫酸钠干燥,柱层析分离,得产品为白色固体8mg,产率21%。1hnmr(400mhz,cdcl3)δ9.92(s,1h),8.79-8.76(m,2h),8.15(d,j=8.1hz,1h),7.54-7.42(m,3h),7.40-7.26(m,2h),7.20(t,j=6.8hz,2h),3.68(s,1h),3.24-3.22(m,1h),3.15-3.11(m,1h),3.06-2.87(m,4h),2.40-2.38(m,1h),2.28-2.15(m,1h),2.10-1.98(m,2h),1.76-1.62(m,1h),1.26-1.22(m,1h),0.92-0.83(m,1h);13cnmr(101mhz,cdcl3)δ171.99,170.10,148.23,141.52,140.94,138.38,136.44,134.45,131.29,130.43,128.37,128.00,127.44,126.52,121.71,121.63,116.57,43.01,42.76,42.69,42.09,38.43,32.78,24.26.
实施例21(图24):在反应瓶中依次加入原料(66mg,0.1mmol),碳酸银(22mg,0.8eq),醋酸钯(2.2mg,10mol%),o-pba(10mg,0.5equiv)以及叔丁醇(20ml),100℃下搅拌12小时,冷却至室温,加入乙酸乙酯(50ml)稀释,硅藻土过滤,浓缩,加入二氯甲烷,饱和碳酸氢钠水溶液洗涤,有机相用无水硫酸钠干燥,柱层析分离,得产品为白色固体34mg,产率64%。1hnmr(400mhz,cdcl3)δ9.90(s,0.69h),9.79(s,0.22h),8.86-8.69(m,2h),8.21-8.08(m,1h),7.93-7.79(m,2h),7.77-7.66(m,2h),7.58-7.41(m,3h),7.36(d,j=9.1hz,1h),7.32-7.28(m,1h),7.24-7.13(m,2h),5.02(dd,j=11.3,6.4hz,0.73h),4.72(dd,j=12.5,5.0hz,0.23h),4.55(t,j=11.2hz,0.23h),4.42-4.38(m,0.73h),4.23-4.08(m,1h),3.69-3.41(m,1.84h),3.25(dd,j=13.2,6.4hz,1h),3.10(dd,j=14.7,6.7hz,1h),2.99-2.90(m,0.27h),2.88-2.83(m,1h),2.20-2.16(m,1h),1.90-1.69(m,2h),1.65-1.56(m,1h),1.50-1.46(m,1h);13cnmr(101mhz,cdcl3)δ170.27,170.13,169.86,169.50,167.95,167.86,148.27,142.61,138.37,136.91,136.48,136.36,134.43,134.33,131.85,131.80,131.25,130.70,129.20,128.00,127.49,127.26,125.39,123.61,121.74,121.67,116.61,116.51,67.36,66.55,56.91,55.77,45.83,43.06,42.49,41.14,36.56,35.41,34.83,34.19,24.69.
实施例22(图25):在反应瓶中依次加入原料(67mg,0.1mmol),碳酸银(22mg,0.8eq),醋酸钯(2.2mg,10mol%),o-pba(10mg,0.5equiv)以及叔丁醇(20ml),100℃下搅拌12小时,冷却至室温,加入乙酸乙酯(50ml)稀释,硅藻土过滤,浓缩,加入二氯甲烷,饱和碳酸氢钠水溶液洗涤,有机相用无水硫酸钠干燥,柱层析分离,得产品为白色固体28mg,产率52%。1hnmr(400mhz,cdcl3)δ9.96(s,0.80h),9.87(s,0.18h),8.89-8.68(m,2h),8.16-8.13(m,1h),7.82(dd,j=5.3,3.0hz,2h),7.68(dd,j=5.4,2.9hz,2h),7.55-7.43(m,3h),7.41-7.30(m,4h),4.66-4.51(m,1h),4.47-4.40(m,0.18h),4.33-4.25(m,1.85h),3.81-3.67(m,0.85h),3.26-3.22(m,1h),3.15-3.03(m,1h),2.97-2.72(m,2h),2.69-2.46(m,1h),1.75-1.69(m,1h),1.60-1.54(m,1h),1.20-1.14(m,2h),1.05-0.91(m,1h),0.75-0.59(m,1h);13cnmr(101mhz,cdcl3)δ170.19,170.13,168.35,168.13,148.27,148.21,142.36,141.91,138.35,136.50,136.41,136.37,136.10,134.41,134.15,132.68,131.85,131.46,128.00,127.64,127.50,127.41,127.30,127.23,123.44,121.74,121.67,121.61,116.60,58.65,58.55,43.92,42.77,40.84,39.94,37.63,34.82,33.18,27.59,21.42,18.82.
实施例23(图26):在反应瓶中依次加入原料(65mg,0.1mmol),碳酸银(22mg,0.8eq),醋酸钯(2.2mg,10mol%),o-pba(10mg,0.5equiv)以及叔丁醇(20ml),100℃下搅拌12小时,冷却至室温,加入乙酸乙酯(50ml)稀释,硅藻土过滤,浓缩,加入二氯甲烷,饱和碳酸氢钠水溶液洗涤,有机相用无水硫酸钠干燥,柱层析分离,得产品为白色固体33mg,产率63%。1hnmr(400mhz,cdcl3)δ9.93(s,0.58h),9.86(s,0.42h),8.84-8.69(m,2h),8.14(td,j=8.2,1.6hz,1h),7.56-7.39(m,3h),7.36-7.23(m,3h),7.10(dd,j=20.9,7.8hz,1h),5.52-5.34(m,1h),4.57-4.56(m,0.49h),4.48-4.42(m,0.63h),4.07-3.92(m,1h),3.82-3.55(m,1h),3.34(dd,j=12.3,6.1hz,1h),3.21-2.96(m,2h),2.96-2.57(m,3.50h),2.41-2.29(m,0.59h),2.05-2.01(m,1h),1.73-1.55(m,1h),1.44-1.42(m,10h),1.12-1.08(m,1h),0.70-0.68(m,1h);13cnmr(101mhz,cdcl3)δ171.01,170.95,170.32,170.25,155.25,148.28,148.22,141.94,138.40,136.55,136.48,136.17,134.43,132.49,131.55,131.01,128.05,128.01,127.53,127.48,127.40,127.17,127.09,121.76,121.72,121.65,116.68,116.62,80.04,58.04,43.93,42.75,40.28,37.60,37.55,34.88,28.45,21.35.
实施例24(图27):在反应瓶中依次加入原料(57mg,0.1mmol),碳酸银(22mg,0.8eq),醋酸钯(2.2mg,10mol%),o-pba(10mg,0.5equiv)以及叔丁醇(20ml),100℃下搅拌12小时,冷却至室温,加入乙酸乙酯(50ml)稀释,硅藻土过滤,浓缩,加入二氯甲烷,饱和碳酸氢钠水溶液洗涤,有机相用无水硫酸钠干燥,柱层析分离,得产品为白色固体33mg,产率75%。异构体a:1hnmr(400mhz,cdcl3)δ9.75(s,1h),8.86-8.69(m,2h),8.13(dd,j=8.2,1.1hz,1h),7.51-7.44(m,3h),7.22-7.06(m,3h),6.75(d,j=3.9hz,1h),5.35(d,j=7.0hz,1h),4.79-4.74(m,1h),3.82(s,3h),3.60-3.55(m,1h),3.30-3.25(m,1h),3.06(dd,j=13.4,2.6hz,1h),2.89(dd,j=14.6,7.0hz,1h),2.76(dd,j=14.6,7.7hz,1h),2.28-2.11(m,2h),2.03(td,j=13.3,2.4hz,1h),1.97-1.86(m,1h),1.7-1.75(m,2h),0.94-0.85(m,1h);13cnmr(101mhz,cdcl3)δ173.11,172.41,170.27,148.29,142.86,138.37,136.40,135.51,134.46,130.62,128.88,128.61,127.99,127.45,125.76,121.73,121.63,116.54,53.00,52.87,47.19,45.94,41.41,38.19,36.54,33.09,23.27.异构体b:1hnmr(400mhz,cdcl3)δ9.74(s,1h),8.75-8.71(m,2h),8.11(dd,j=8.2,1.4hz,1h),7.56-7.44(m,2h),7.40(dd,j=8.2,4.2hz,1h),7.23-7.14(m,2h),7.14-7.00(m,2h),6.38(d,j=9.7hz,1h),4.66-4.60(m,1h),3.82(s,3h),3.31-3.23(m,2h),2.84(dd,j=14.4,7.2hz,1h),2.73(dd,j=14.4,7.6hz,1h),2.57(t,j=12.0hz,1h),2.39-2.26(m,1h),2.23-2.12(m,1h),1.92-1.80(m,2h),1.79-1.69(m,1h),1.32-1.25(m,1h);13cnmr(101mhz,cdcl3)δ172.64,172.53,170.10,148.11,142.04,138.28,136.39,136.35,134.35,129.21,128.91,127.93,127.41,127.31,121.64,121.59,116.55,53.74,52.65,46.50,43.02,40.06,39.41,36.32,22.01.
实施例25(图28):在反应瓶中依次加入原料(61mg,0.1mmol),碳酸银(22mg,0.8eq),醋酸钯(2.2mg,10mol%),o-pba(10mg,0.5equiv)以及叔丁醇(20ml),100℃下搅拌12小时,冷却至室温,加入乙酸乙酯(50ml)稀释,硅藻土过滤,浓缩,加入二氯甲烷,饱和碳酸氢钠水溶液洗涤,有机相用无水硫酸钠干燥,柱层析分离,得产品为白色固体42mg,产率87%。异构体a:1hnmr(400mhz,cdcl3)δ9.75(s,1h),8.81(dd,j=7.4,1.1hz,1h),8.70(dd,j=4.2,1.6hz,1h),8.24(s,1h),8.11(dd,j=8.3,1.5hz,1h),7.55-7.45(m,3h),7.39(dd,j=8.3,4.2hz,1h),7.16(d,j=8.3hz,1h),7.07(dd,j=8.3,1.3hz,1h),6.82(d,j=2.1hz,1h),5.80(d,j=6.8hz,1h),5.02-4.93(m,1h),3.83(dd,j=14.6,5.2hz,1h),3.75(s,3h),3.46-3.29(m,1h),3.23(dd,j=14.7,2.2hz,1h),2.92(dd,j=14.4,6.3hz,1h),2.73(dd,j=14.4,8.2hz,1h),2.20-2.07(m,4h),1.81-1.71(m,1h),1.20-1.10(m,1h);13cnmr(101mhz,cdcl3)δ172.98,172.03,170.87,148.24,138.35,136.30,134.57,134.50,133.26,129.46,127.94,127.38,124.28,121.77,121.66,121.58,118.26,116.47,111.05,110.42,55.12,52.53,48.08,40.82,37.33,31.69,26.52,22.64.异构体b:1hnmr(400mhz,cdcl3)δ9.87(s,1h),8.79(d,j=7.3hz,1h),8.77-8.68(m,1h),8.17(s,1h),8.13(d,j=8.3hz,1h),7.58(s,1h),7.56-7.45(m,2h),7.45-7.37(m,1h),7.20(d,j=8.3hz,1h),7.07(d,j=8.4hz,1h),6.88(s,1h),5.80(s,1h),4.64(s,1h),3.78(s,3h),3.69-3.49(m,2h),3.28(dd,j=14.5,5.0hz,1h),3.10(dd,j=14.5,6.3hz,1h),2.85-2.70(m,1h),2.32-2.17(m,2h),1.87-1.56(m,4h);13cnmr(101mhz,cdcl3)δ173.55,172.09,170.92,148.27,138.45,136.41,134.87,134.58,129.10,128.02,127.50,122.18,121.70,121.58,119.57,116.61,111.47,55.40,53.90,52.57,37.14,29.82,29.44,27.09,21.28。
- 还没有人留言评论。精彩留言会获得点赞!